| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 16, Number 5, May 2024, pages 232-242
Immune Checkpoints Receptors Expression of Macrophage/Monocytes in Response to Acute Viral Respiratory Infection
Asmaa Zahrana, Hosni A. Husseinb, Ali A. Thabetc, Mohamed R. Izzaldind, Ahmed A. Wardanyb, Ali Sobhyd, Mohamed A. Bashird, Magdy M. Afifie, Wageeh A. Alif, Amal Rayang, Khaled Saadh, k , Mohammad Gamal Khalafi, Mahmoud Elsaeed Ahmedj, Noha G. Sayeda
aDepartment of Clinical Pathology, South Egypt Cancer Institute, Assiut University, Assiut, Egypt
bDepartment of Botany and Microbiology, Faculty of Science, Al-Azhar University, Assiut 71524, Egypt
cDepartment of Zoology, Faculty of Science, Al-Azhar University, Assiut 71524, Egypt
dDepartment of Clinical Pathology, Faculty of Medicine, Al-Azhar University, Assiut 71524, Egypt
eDepartment of Botany and Microbiology, Faculty of Science, Al-Azhar University, Nasr City 11884, Cairo, Egypt
fDiagnostic and Interventional Radiology Department, Faculty of Medicine, Assiut University, Assiut, Egypt
gDepartment of Clinical Oncology, Faculty of Medicine, Assiut University, Assiut, Egypt
hDepartment of Pediatrics, Faculty of Medicine, Assiut University, Assiut, Egypt
iDepartment of Chest Diseases, Faculty of Medicine, Assiut University, Assiut, Egypt
jDepartment of Chest Diseases, Faculty of Medicine, Al-Azhar University, Cairo, Egypt
kCorresponding Author: Khaled Saad, Department of Pediatrics, Assiut University Children’s Hospital, Assiut 71111, Egypt
Manuscript submitted December 21, 2023, accepted May 13, 2024, published online May 29, 2024
Short title: Macrophage in Acute Respiratory Viral Infection
doi: https://doi.org/10.14740/jocmr5098
| Abstract | ▴Top |
Background: We aimed to monitor the phenotypic changes in macrophages and their polarization in patients with acute viral respiratory diseases, including coronavirus disease diagnosis, focusing on the variations in the percentages of macrophages and monocytes and their sub-populations in those patients compared to healthy control. Moreover, we defined the correlation between macrophage subtypes and some inflammatory indices.
Methods: Twenty-seven patients with clinical and radiologic diagnosis of acute viral respiratory infection admitted in Al-Azhar and Assiut University hospitals were recruited. Fresh peripheral blood samples were collected from all patients and healthy controls for flow cytometric analysis using BD FACSCanto II analyzer equipped with three lasers.
Results: Compared to healthy controls, accumulation of cluster of differentiation (CD)11B+CD68+ macrophages (M) (P = 0.018), CD274+ M1 (P = 0.01), CD274+ M2 (P < 0.001), and CD80-CD206+ M2 (P = 0.001) was more evident in patients. Moreover, CD273+ M2 (P = 0.03), CD80+CD206- M1 (P = 0.002), and CD80+CD86+ M1 (P = 0.002) were highly expressed in controls compared with patients.
Conclusion: The examination of clinical specimens obtained from patients with signs of acute respiratory viral infection showed the role of the macrophage in the immune response. Dysfunction in macrophages results in heightened immune activity and inflammation, which plays a role in the progression of viral diseases and the emergence of accompanying health issues. This malfunction in macrophages is a common characteristic seen in various viruses, making it a promising focus for antiviral therapies with broad applicability. The immune checkpoint could be a target for immune modulation in patients with severe symptoms.
Keywords: Macrophages; CD274+ M1; CD274+ M2; CD273+ M1; CD273+ M2; Acute respiratory viral infection
| Introduction | ▴Top |
In healthy individuals, the innate immune system serves as the initial defense against most infectious signals. It achieves this by orchestrating a protective inflammatory response that evolves through various stages, starting from initiation and full-blown inflammation to resolution and the restoration of tissue integrity [1]. Within the fully active immune system, macrophages, which originate from monocytes, act as phagocytic cells. They are responsible for targeting and eliminating foreign substances while also regulating the activation and recruitment of lymphocytes. Importantly, macrophages can assume a variety of different phenotypic states [2].
While the activation spectrum of macrophages is intricate, it can be broadly categorized into two main types: classically activated pro-inflammatory macrophages (M1) and alternatively activated anti-inflammatory macrophages (M2). When exposed to factors like interferon-gamma (IFN-γ), tumor necrosis factor-alpha (TNF-α), and lipopolysaccharide (LPS), macrophages can polarize into the M1 phenotype. This phenotype is characterized by the expression of specific markers like CD68, CD86, and CD80 and the secretion of cytokines and chemokines such as interleukin-12 (IL-12), C-X-C motif chemokine ligand 9 (CXCL9), CXCL10, TNF-α, and IL-1β, which promote a pro-inflammatory T-helper 1 (Th1) response. Conversely, when exposed to IL-4 and IL-13, macrophages adopt the M2 phenotype, expressing markers like CD163, CD204, and CD206. These M2 macrophages exert immunomodulatory effects and play a crucial role in dampening endogenous antitumor immune responses [3]. While M2 macrophages have a critical role in normal immune function and homeostasis, such as stimulating Th2 responses, eliminating parasites, immunoregulation, wound healing, and tissue regeneration, certain subsets of M2 macrophages also play a critical role in promoting tumor progression.
The dysregulation of macrophages in the context of viral pathogenesis is an area ripe for exploration in terms of targeting and drug development. This is because it represents a broad target and is a pathogenic trait shared by numerous viruses [4]. In recent times, the pivotal role of monocytes/macrophages has been elucidated in the persistence or dissemination of more than 35 different viruses spanning 13 distinct families. Among these viruses are single-stranded RNA and double-stranded DNA agents, which present multiple challenges in terms of disease management [5, 6]. Following microbial infections, tissue-resident macrophages release cytokines like TNF-α, CXCL1/2, IL-1α, and monocyte chemoattractant protein-1 (MCP-1) to attract neutrophils to the infection site. These neutrophils subsequently produce azurocidin, which upregulates the expression of molecules like E-selectin and vascular cell adhesion molecule-1 (VCAM-1) on endothelial cells, further facilitating the recruitment of monocytes. Additionally, infiltrating neutrophils secrete cytokines such as IL-6, IL-12, and IFN-γ, which contribute to the activation of pro-inflammatory macrophages and the differentiation of T-helper cells [7, 8]. Following the resolution of inflammation, pro-inflammatory macrophages bind to neutrophils via TNF, triggering apoptosis and the clearance of apoptotic neutrophils [9, 10].
Many chronic viral infections result in T-cell exhaustion, which is the main source of host difficulty in eliminating such infections [3, 4]. Immune checkpoint molecules are negative regulatory receptors expressed on immune cells. These molecules have been shown to participate in the mechanism of immune escape by causing T-cell dysfunction in a variety of diseases, such as cancer and infection. As a negative regulatory signal for the activation and proliferation of T cells, the immune checkpoint pathway is involved in the immune escape of many viruses [5, 6].
In general, however, M1 are defined by expression of CD68, CD80 and CD86 and secretion of pro-inflammatory cytokines such as TNF-α, IL-1α, IL-1β, IL-6, IL-12, IL-18, and IL-23 as well as nitric oxide (NO) synthase that contribute to eliminating tumor cells [11-13]. On the contrary, M2 express CD163 and CD206 [14] and secrete IL-10, transforming growth factor-beta (TGF-β), CCL2, CCL5 [11, 12] and IL-13 that maintain the immune-suppressive environment [5].
Regarding specific checkpoint molecules in the pathophysiology of these infections, PD-1 “PD-1 interacts with the ligands PD-L1 (CD274; also called B7-H1) and PD-L2 (CD273; also called B7-DC)” and Tim-3 are already shown to inhibit production of inflammatory IL-12 on monocytes and macrophages. Consequently, increased levels of anti-inflammatory cytokines such as IL-10 further contribute to the “pro-disease” M2 immune profile, ultimately facilitating viral persistence.
In this research, our objective was to observe the alterations in the characteristics of macrophages and their shift in patients who have been diagnosed with viral respiratory illnesses, including those with a coronavirus disease (COVID) diagnosis. Our main emphasis was on examining the differences in the proportions of macrophages and their specific subgroups in these patients when compared to a group of individuals who appeared to be in good health. Furthermore, we sought to establish a connection between the various types of macrophages and certain markers of inflammation.
| Materials and Methods | ▴Top |
Participants
This case-control study was conducted in a sample of 27 hospitalized adults and 27 age- and sex-matched healthy controls. All patients fulfilled the criteria for acute lower respiratory tract infection [15] with no other chronic diseases. The patients included in the study met specific clinical criteria for pneumonia diagnosis, including: 1) detection of a new infiltrating shadow in the lungs via chest radiograph or computed tomography (CT) scan (a mandatory criterion); 2) presence of newly developed or aggravated cough symptoms; and 3) fever (temperature > 37.0 °C) or low body temperature (temperature < 35.6 °C). Additionally, patients diagnosed with viral pneumonia by clinicians exhibited at least one of the following symptoms within 7 days: fever, cough with or without sputum, chest pain, shortness of breath, or nasal congestion. We excluded any patient with other chronic diseases or immune diseases. All participants in the healthy control group exhibited no signs of disease or infection based on their medical history, clinical examination, and complete blood count (CBC).
Methods
Samples were taken at the time of hospital admission to detect viral etiologies by real-time polymerase chain reaction (RT-PCR).
Radiologic diagnosis
Viruses are a common cause of acute respiratory infections, and the causative agents of lower respiratory tract infections vary depending on the patient’s age and immunity. Viral pneumonia often presents as bilateral multifocal ground-glass opacities with patchy consolidations on CT scans, while bacterial pneumonia typically manifests as a diffuse airspace pattern. Adenovirus can cause multifocal consolidation or ground-glass opacities, and diffuse airspace patterns are more frequently seen in bacterial infections. CT patterns of viral pneumonia can be related to the pathogenesis of pulmonary viral infection, and diagnostic tests, including radiologic studies and blood or serologic tests, can help establish the cause of pneumonia, reduce unnecessary antibiotic use, and improve clinical outcomes [16, 17] (Supplementary Material 1, www.jocmr.org).
Flow cytometric detection of macrophage subtypes
1) Specimens
Fresh (0 to 48 h post-collection) peripheral blood samples were collected.
2) Instruments and software
Flow cytometry data were collected using a BD FACSCanto II analyzer equipped with three lasers. We configured the instrument using BD Cytometer Setup and Tracking (CS & T) beads. For data acquisition and analysis, we utilized BD FACSDiva™ software (version 6.1.3). We fine-tuned the photomultiplier tube (PMT) voltages by configuring application settings to optimize the cytometer’s performance.
3) Sample staining
The antibody reagent specified in Table 1 was employed for this purpose. A volume of 100 µL of the sample was added to each tube, and these tubes were then incubated in the dark at room temperature for 20 min. To lyse the red blood cells (RBCs), 1 mL of 1X BD Pharm Lyse lysing buffer was added to each tube, followed by an additional incubation of 10 min in the dark at room temperature. Subsequently, the samples were placed on ice and analyzed within 1 h after the lysis step. The acquisition and analysis of data were performed using FACS Canto flow cytometry from Becton Dickinson Biosciences, located in San Jose, CA. We analyzed a total of 100,000 events, and for each sample, an isotype-matched negative control was utilized.
 Click to view | Table 1. Antibody Reagent |
4) Data analysis
Following the removal of doublets and debris, we conducted our analysis using a sequential gating approach. Table 2 [18-21] shows all markers utilized to recognize macrophages based on their general phenotypic features and to delineate their functional characteristics. First, gating was based on high side scatter (SSC) to identify macrophages and monocytes. The characterization of macrophages depended on the cell subset where CD68+11b+ was used as a general phenotypic marker. Sequentially, cell populations were gated with CD80+CD206- followed by CD80+CD86+ for the characterization of M1 and CD80-CD206+ followed by CD206+CD163+ for the characterization of M2 population. Eventually, the determination of two immune checkpoints, CD273 (PD-L2, also known as B7-DC) and CD274 (PD-L1, also known as B7-H1), concerned the predetermined M1 and M2 subsets. The gating populations are illustrated in Table 2 and Figure 1.
 Click to view | Table 2. Gating Populations |
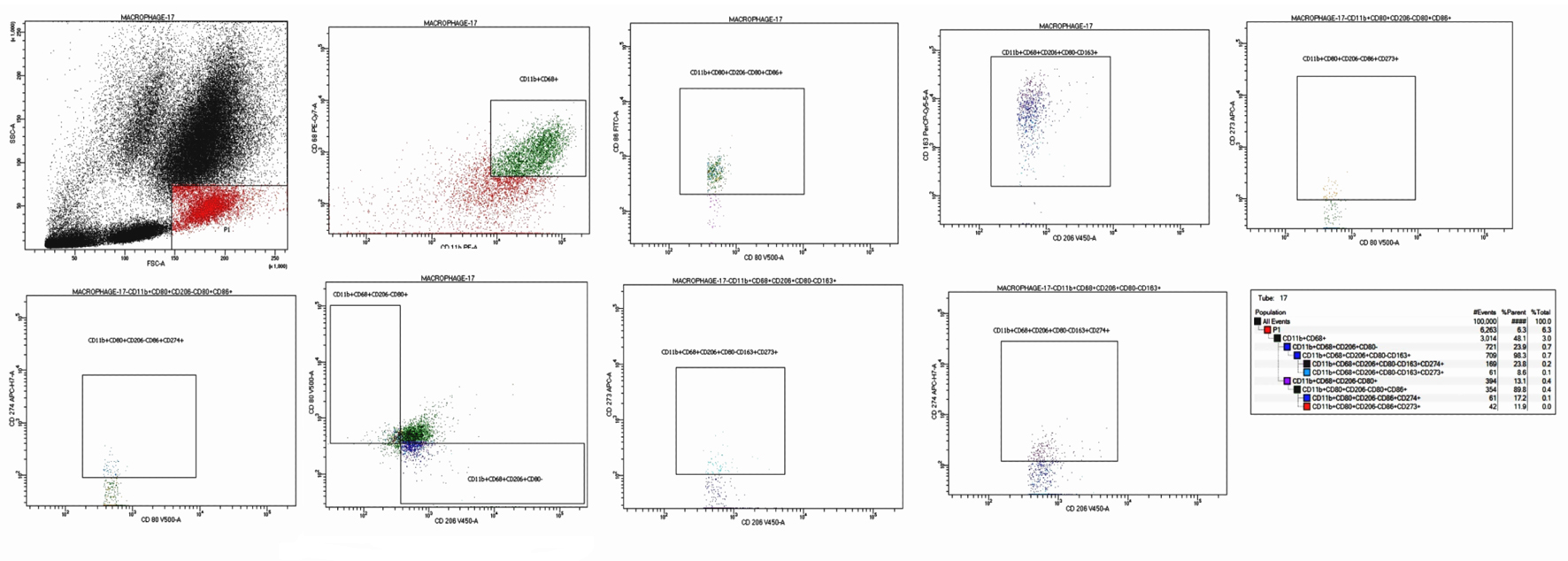 Click for large image | Figure 1. Flow cytometric detection of macrophages subtypes. First blot: FSC and SSC were gating on monocyte and macrophage region (high FSC, low SSC). Second blot: gating on macrophage/monocytes (coexpression of CD68+CD11b+). Third blot: subclassification of the (CD68+CD11b+) into M1 (CD80+CD206-) and M2 (CD80-CD206+). Fourth blot: further characterization of M1 as (CD80+CD86+). Fifth blot: further characterization of M2 as (CD206+CD163+). Sixth blot: expression of the PD-L1 (CD274) on M1 (CD80+CD86+CD274+). Seventh blot: expression of the PD-L2 (CD273) on M1 (CD80+CD86+CD273+). Eighth blot: expression of the PD-L1 (CD274) on M2 (CD206+CD163+CD274+). Ninth blot: expression of the PD-L2 (CD273) on M2 (CD206+CD163+CD273+). FSC: forward scatter; SSC: side scatter. |
Statistical analysis
All data were analyzed using IBM-SPSS ver. 26, and after running the Shapiro-Wilk test, all hematologic indices were normally distributed (P > 0.05) and analyzed by independent sample t-test. However, all macrophage subtypes with the studied markers were not normally distributed (P = 0.02 to < 0.001), so they were compared and analyzed by Mann-Whitney U-test between patients and controls and between each other, Spearman rho test was used for correlation, data were expressed as mean and standard error (SE), and all information created were considered significant at P < 0.05.
Ethics approval
This study received approval from the ethical committees of both the Faculty of Medicine and the Faculty of Science at Al-Azhar University in Assiut (ID: 6/2021). The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
| Results | ▴Top |
Table 3 shows all clinical and demographic data of all participants.
 Click to view | Table 3. Clinical and Demographic Data of All Participants |
Peripheral hemogram characteristics of patients compared to controls
Twenty-seven patients with viral chest infection were found to have significantly reduced hemoglobin (Hb), neutrophilic counts, platelet counts (PCs), and mean platelet volume (MPV) compared to 27 healthy controls. Likewise, neutrophil/lymphocyte ratio (NLR) and PC/MPV ratio were significantly lower in patients compared to controls (Table 4).
 Click to view | Table 4. Blood Characteristics of Patients and Controls |
Differences in the total macrophage percentages between patients and controls
CD11B+CD68+ macrophages (gated from low forward scatter (FSC) high SSC monocytic region) were significantly increased (depending on percent expression ratio of the gated cells) in infected patients compared to healthy controls (mean ± SE: 59.8 ± 3.9 vs. 45.4 ± 3.8, P = 0.018) (Fig. 2).
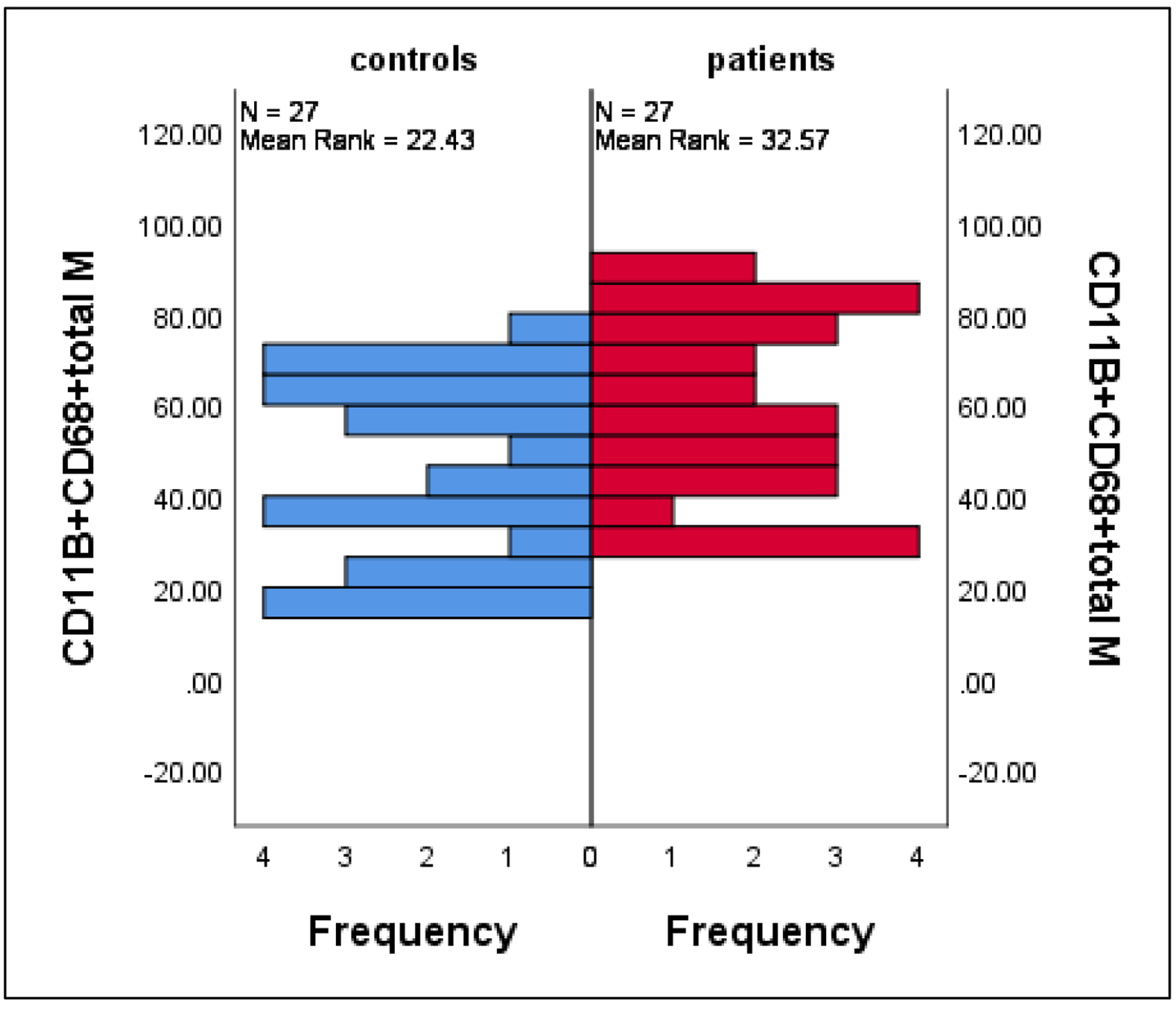 Click for large image | Figure 2. Differences in the mean percentages of macrophage (characterized by CD11B+CD68+ phenotype) between patients and controls. Data are expressed as mean ± SE, Mann-Whitney U-test. SE: standard error of the mean. |
Differential expression of CD273 and CD274 on M1 and M2 subtypes between patients and controls
We detected significantly higher levels of the mean percentages of CD274+ M1 and CD274+ M2 in patients compared with controls (mean ± SE: 37.8 ± 4.4 vs. 22.9 ± 2.3, P = 0.01 and 46.95 ± 3.8 vs. 23.3 ± 2.2, P < 0.001 for M1 and M2, respectively). Conversely, there was an accumulation of CD273+ M2 in controls compared to patients with mean ± SE of 23.3 ± 3.3 vs. 13.1 ± 1.7 (P = 0.03), while no significant differences between patients and controls regarding CD273+ M1 with mean ± SE of 17.7 ± 1.5 vs. 15.9 ± 0.9 (P = 0.4) (Fig. 3).
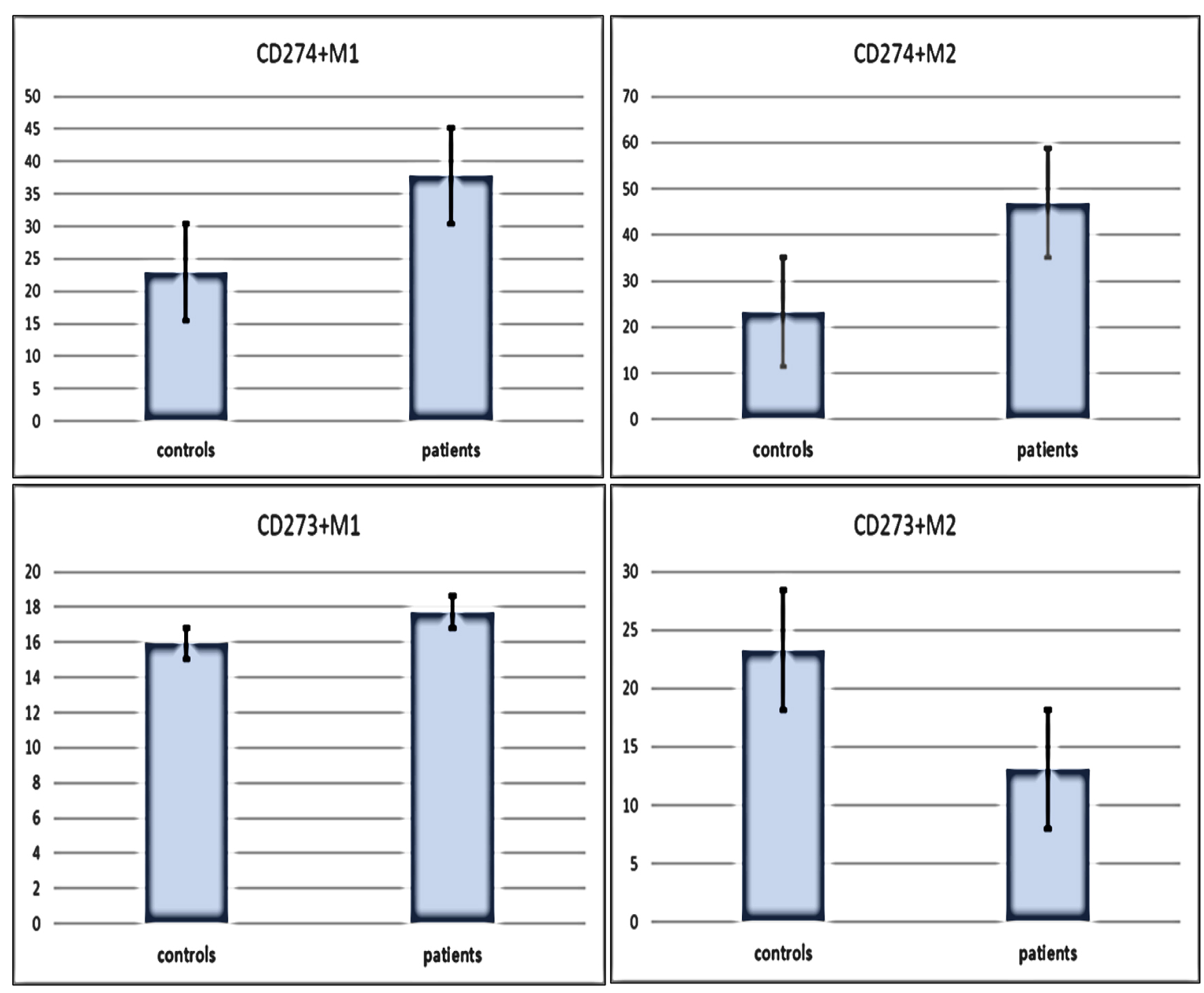 Click for large image | Figure 3. Differences in immune check points (CD273 and CD274) expressions on M1 and M2 macrophages subtypes between patients and controls. Data are expressed as mean ± SE. Mann-Whitney U-test. SE: standard error of the mean. |
Differential expression of CD80, CD86, and CD206 on M1 bewteen patients and controls
As expected, M1 macrophages are resident macrophages responsible for tissue homeostasis and scavenging of dead cells with subsequent immune surveillance. Furthermore, they exhibited high plasticity when switching from classically pro-inflammatory type M1 to actively anti-inflammatory type M2. This phenomenon was clearly defined in the current results with significant accumulations of CD80+CD86+ M1 cells and CD80+CD206- M1 cells in controls compared to patients with mean ± SE of 93.2 ± 0.5 vs. 89.5 ± 1.03 (P = 0.002) and 11.1 ± 1.1 vs. 6.2 ± 1.0 (P = 0.002), respectively (Fig. 4).
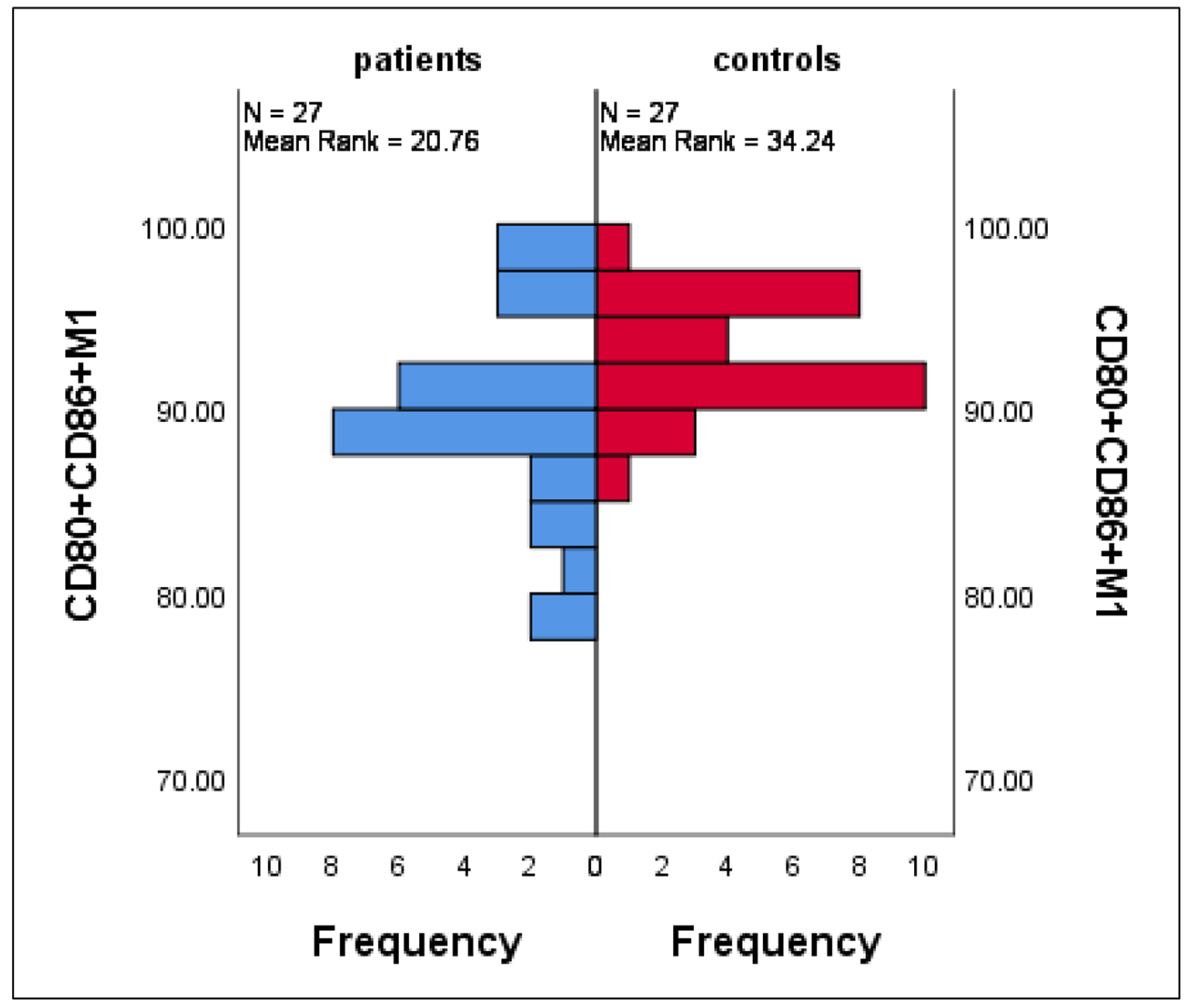 Click for large image | Figure 4. Differences in CD80+CD86+ M1 between patients and controls. Data are expressed as mean ± SE. Mann-Whitney U-test. SE: standard error of the mean. |
Differential expression of CD80-CD206+ M2 and CD206+CD163+ M2 between patients and controls
M2 macrophages activate suppressor T cells reducing tissue inflammation caused by viral infection and promoting their healing and repair, for that CD80-CD206+ M2 were highly expressed in patients compared with controls with mean ± SE of 17.3 ± 2.01 vs. 8.9 ± 1.2 (P = 0.001), respectively; on the other side, there was no significant differences in the mean percentages of CD206+CD163+ M2 between patients and controls with mean ± SE of 83.2 ± 3.04 vs. 89.2 ± 1.8 (P = 0.3) (Fig. 5).
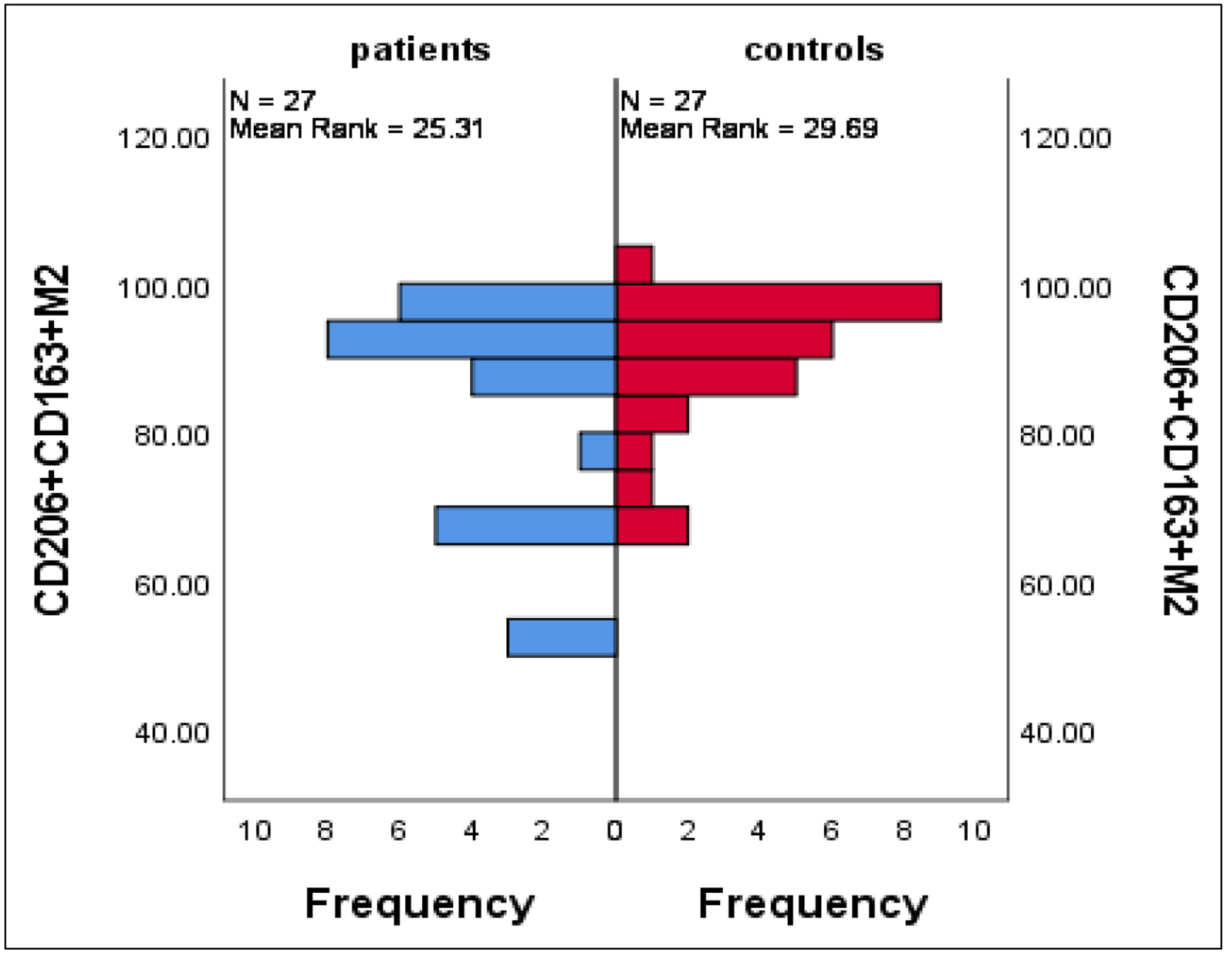 Click for large image | Figure 5. Differences in CD206+CD163+ M2 between patients and controls. Data are expressed as mean ± SE. Mann-Whitney U-test. SE: standard error of the mean. |
Correlations between inflammatory indices and macrophages
The results elicited significant positive correlations between the PC/MPV ratio and CD80+CD86+ M1 (r = 0.31, P = 0.04). At the same time, there was a significant negative correlation between the current ratio and CD80-CD206+ M2 (r = -0.31, P = 0.02) (Fig. 6).
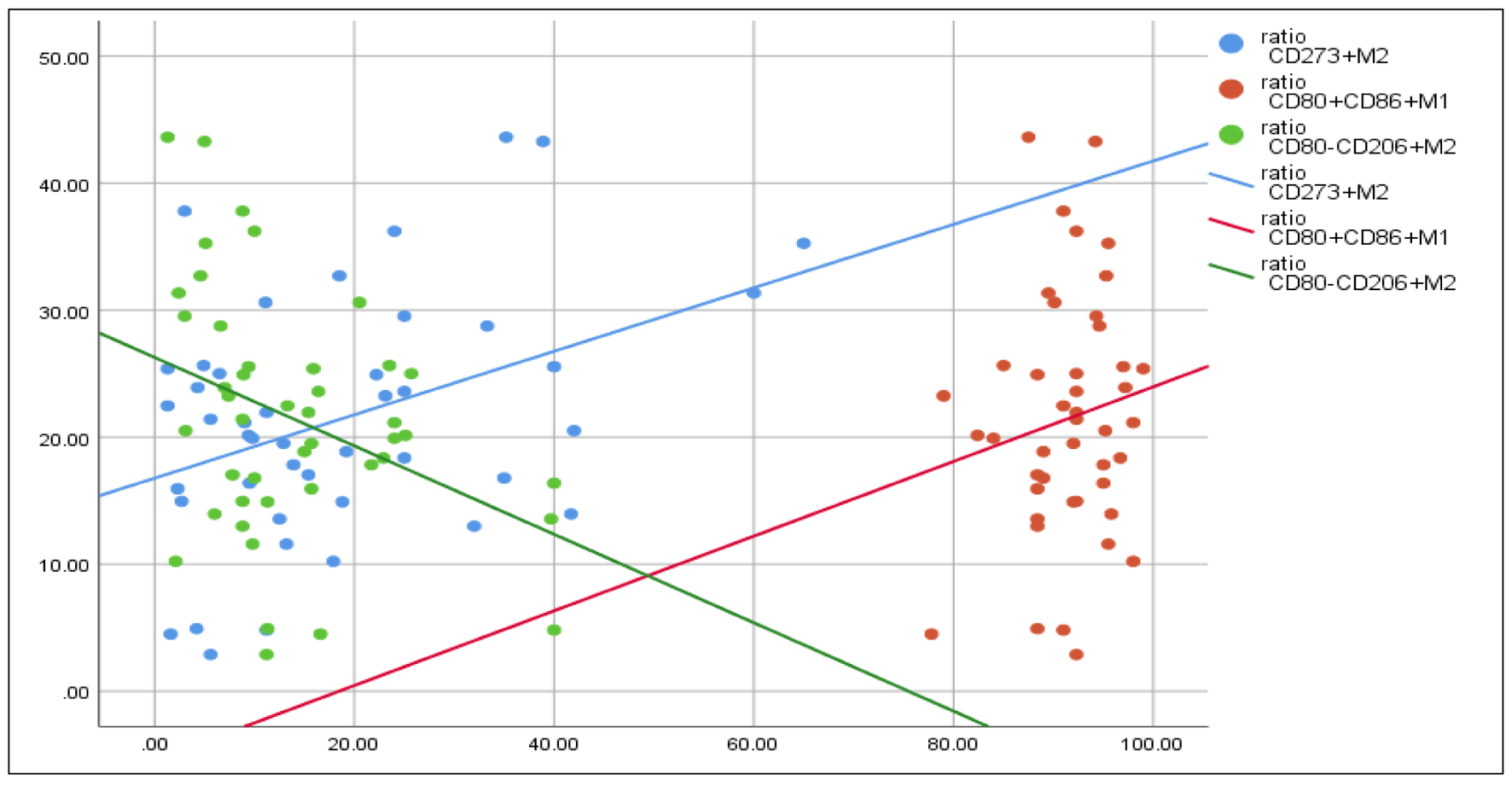 Click for large image | Figure 6. Correlations between PC/MPV ratio and, CD80+CD86+ M1, CD80-CD206+ M2. Spearman rho test is used for correlation. PC: platelet count; MPV: mean platelet volume. |
| Discussion | ▴Top |
Our findings indicate a higher proportion of macrophages with the specific phenotype (CD68+CD11b+) in patients compared to individuals in good health. This observation can potentially be attributed to the differentiation of bone marrow and blood monocytes into various effector cells with unique antimicrobial functions when a mammalian host encounters a virulent pathogen [22]. The elevation in circulating monocytes in response to infection or non-infectious inflammation is regulated by molecules such as CCL2 and CCL7 [23]. These results were in harmony with those of Florentin et al, who documented that inflammatory monocytes were increased in the blood of hypoxic mice and human pulmonary arterial hypertensive patients [24].
Alternatively, Italiani et al presented a different perspective, suggesting that the accumulation of inflammatory monocytes in inflamed tissues results primarily from their migration from the bloodstream rather than from their capacity to proliferate. It is worth noting that inflammatory signals originating from microbial sources typically hinder their proliferation [25]. The research by Davies et al (2013) revealed that inflammatory monocyte-derived macrophages can indeed undergo proliferation during specific phases in the resolution of zymosan-induced peritonitis [26]. Regarding the inclination of macrophage polarization during viral infections, it is important to understand that macrophage polarization involves various activation pathways that enable these cells to execute their defensive roles. This versatility allows macrophages to adapt and perform specific functions in diverse contexts, emphasizing the significant functional diversity that characterizes these cells [27, 28]. In this context, our results confirm that there is an increase in the M2 in patients rather than the control and vice versa for M1. These findings could be interpreted and explained in light of the individual viruses that are causing the infection. Burdo et al have documented that certain viruses have the ability to drive macrophage polarization towards the M1 phenotype, while others facilitate M2 polarization.
Additionally, various viruses can induce intricate patterns of macrophage polarization, which may vary based on factors such as viral strains, the stage of infection, and the gender of the host. Typically, virus-infected macrophages tend to adopt pro-inflammatory M1 phenotypes in the early stages of infection, transitioning to anti-inflammatory M2 phenotypes in the later stages of infection [29]. Another theory that could explain the variation in the macrophage polarization according to the individual virus infection could be supported by the report of Ferrer et al, who stated that, in most cases, virulent virus strains tend to suppress the antiviral reactions of M1-polarized macrophages and push the polarization of macrophages towards the M2 phenotype. Conversely, weakened or attenuated virus strains typically promote the M2 phenotype [30]. In this context, our results could support the attenuated pathogenic nature of the virus infection of the studied group.
Regarding the pattern of expression of immune checkpoints on macrophages and monocytes, our results confirm that there is increased expression of CD274 (PD-L1) on both types of M1 and M2 macrophages compared to the control group. These findings partially align with the observations made by Lai et al. In their study, they observed an elevation in the expression of programmed cell death protein 1, also known as PD-1 (CD279), and its ligand PD-L1 (CD274), particularly in differentiated (CD68+CD11b+) macrophages [24]. However, in contrast to our results, they highlighted varying expressions of inducible PD-L1 (CD274) and PD-1 (CD279) among distinct specialized macrophage populations. Furthermore, their research revealed a decrease in the levels of these checkpoints in THP-1-differentiated M2 macrophages [14]. In another investigation conducted by Xiong et al, it was proposed that PD-L1 might have an association with M1/M2 polarization, resulting in modifications in cytokine release and the expression of surface markers in macrophages [31]. One potential rationale for this observation is that classically activated M1 macrophages tend to exhibit higher levels of PD-L1 (CD274) expression, possibly due to the influence of Th1 cells and a combination of LPS/IFN-γ [32].
As regards the differential expression of CD273 (PD-L2) on different subtypes of macrophages, our results reveal no significant difference in the expression between patients and the control group. This could be supported by the findings of Loke and Allison, who reported that PD-L2 is absent in inflammatory macrophages but can be prompted to appear through alternative activation triggered by IL-4. While PD-L1 can be readily induced on various antigen-presenting cell lines and resident macrophages, it is noteworthy that PD-L2 shows its most significant inducibility, specifically in the context of inflammatory macrophages [32]. Studying the correlation of different macrophage subtypes M1 and M2 revealed a significant positive correlation between inflammatory indices (PC/MPV, NLR) and M1. At the same time, these inflammatory indices and M2 had a significant negative correlation. These observations are consistent with the general characteristics of M1 macrophages, which, when activated classically by IFN-γ, tend to secrete increased levels of pro-inflammatory cytokines and chemokines. This secretion promotes the activation of Th1 responses, facilitates complement-mediated phagocytosis, and contributes to type I inflammation [11]. In various infections, the polarization of macrophages towards the M1 phenotype can actually enhance the establishment of viruses. For instance, human immunodeficiency virus (HIV)-1 induces acute inflammation and promotes the recruitment of monocytes and T cells to the site of viral infection [12].
On the contrary, M2-like macrophages play a role in regulating Th2 responses by producing anti-inflammatory mediators. This, in turn, leads to the recruitment of neutrophils, monocytes, and T lymphocytes. M2-like macrophages are often characterized as highly endocytic and partially phagocytic [11]. Another study by Feng et al [13] concluded that M1 activation promotes inflammation in symptomatic juvenile idiopathic arthritis. Conversely, M2a macrophages exhibit a rapid response in suppressing inflammation in this context. In cases of inactive systemic juvenile idiopathic arthritis, M2b and M2c macrophages play a predominant role in curbing inflammation.
Limitations
The current study had some limitations. First, the effector function of the macrophages was not assessed by the different cytokines milieu to prove the subtype polarization effector function. Furthermore, type 2 macrophages have different subtypes, M2a, M2b, and M2c, which are not investigated in this work. Numerous questions still need to be answered and represent potential avenues for future research. How might these findings influence the course of disease and the development of therapeutic approaches? Moreover, the customized response of the immune system according to the type of infectious agent could not be assessed in this work. Most of the available resources and experimental models included the antitumor effect of macrophages, so we recommend further examination of the macrophages in the context of the viral infection perse. Checkpoint modulation presents a highly promising strategy. Nevertheless, there is a significant demand for more in-depth investigations, particularly regarding various checkpoint molecules and their cell-specific distribution patterns, which can have an impact on underlying pathologies.
Conclusion
The examination of clinical specimens obtained from patients with signs of acute respiratory viral infection showed the role of the macrophage in the immune response. Dysfunction in macrophages results in heightened immune activity and inflammation, which plays a role in the progression of viral diseases and the emergence of accompanying health issues. This malfunction in macrophages is a common characteristic seen in various viruses, making it a promising focus for antiviral therapies with broad applicability. The immune checkpoint could be a target for immune modulation in patients with severe symptoms.
| Supplementary Material | ▴Top |
Suppl 1. Case 1. A 30-year-old male patient with anterior segments of the bilateral lower lung lobes showed consolidation with mild interlobar thickening and GGO, suggesting bronchopneumonia. Case 2. A 42-year-old male patient with bilateral lower lung lobes showed GGO with tree-in bud consolidation suggesting bronchopneumonia (viral pneumonia) COVID-19, CORADS 3, mild severity score. Case 3. A 20-year-old female patient with bilateral GGO exhibited mild inhomogeneous consolidation involving both lower lung lobes, suggesting bronchopneumonia.
Acknowledgments
None to declare.
Financial Disclosure
No external funding was received for this study.
Conflict of Interest
The authors disclose no conflict of interest.
Informed Consent
All subjects participating in the study provided informed consent.
Author Contributions
AMZ, HAMH, AAT, TAM, MEA, and AAW contributed to the study’s design, patient follow-up, and data analysis. AMZ, AS, AAE, NGS, WAA, and AR conducted all investigations for the study. AMZ, MMA, WAA, and KS prepared the initial manuscript draft. All authors participated in the critical review of the final manuscript version. All authors approved the manuscript in its submitted form and agreed to take responsibility for all aspects of the work.
Data Availability
The data supporting the findings of this study are accessible from the corresponding author KS upon reasonable request.
Abbreviations
CBC: complete blood count; CCL2: chemokine ligand 2; CD: cluster of differentiation; COVID: coronavirus disease; CS & T beads: Cytometer Setup and Tracking beads; CXCL9: C-X-C motif chemokine ligand 9; IFN-γ: interferon-gamma; IL-12: interleukin-12; LPS: lipopolysaccharide; LMR: lymphocyte-to-monocyte ratio; MCP-1: monocyte chemoattractant protein-1; MPV/PC: mean platelet volume/platelet count ratio; MPV: mean platelet volume; NLR: neutrophil/lymphocyte ratio; NO: nitric oxide; PD-1: programmed cell death protein 1; PD-L1: programmed cell death protein 1 ligand; PMT: photomultiplier tube; RBCs: red blood cells; SE: standard error of the mean; TGF-β: transforming growth factor-beta; Th1: T-helper 1; TNF-α: tumor necrosis factor-alpha; VCAM-1: vascular cell adhesion molecule-1
| References | ▴Top |
- El-Badawy O, Elsherbiny NM, Abdeltawab D, Magdy DM, Bakkar LM, Hassan SA, Hassan EA, et al. COVID-19 Infection in Patients with Comorbidities: Clinical and Immunological Insight. Clin Appl Thromb Hemost. 2022;28:10760296221107889.
doi pubmed pmc - Shapouri-Moghaddam A, Mohammadian S, Vazini H, Taghadosi M, Esmaeili SA, Mardani F, Seifi B, et al. Macrophage plasticity, polarization, and function in health and disease. J Cell Physiol. 2018;233(9):6425-6440.
doi pubmed - Anderson NR, Minutolo NG, Gill S, Klichinsky M. Macrophage-based approaches for cancer immunotherapy. Cancer Res. 2021;81(5):1201-1208.
doi pubmed - Reece MD, Taylor RR, Song C, Gavegnano C. Targeting macrophage dysregulation for viral infections: novel targets for immunomodulators. Front Immunol. 2021;12:768695.
doi pubmed pmc - Boeuf P, Drummer HE, Richards JS, Scoullar MJ, Beeson JG. The global threat of Zika virus to pregnancy: epidemiology, clinical perspectives, mechanisms, and impact. BMC Med. 2016;14(1):112.
doi pubmed pmc - Abbas W, Tariq M, Iqbal M, Kumar A, Herbein G. Eradication of HIV-1 from the macrophage reservoir: an uncertain goal? Viruses. 2015;7(4):1578-1598.
doi pubmed pmc - Yan Z, Gibson SA, Buckley JA, Qin H, Benveniste EN. Role of the JAK/STAT signaling pathway in regulation of innate immunity in neuroinflammatory diseases. Clin Immunol. 2018;189:4-13.
doi pubmed pmc - Liu Y, Holdbrooks AT, Meares GP, Buckley JA, Benveniste EN, Qin H. Preferential recruitment of neutrophils into the cerebellum and brainstem contributes to the atypical experimental autoimmune encephalomyelitis phenotype. J Immunol. 2015;195(3):841-852.
doi pubmed pmc - Liew PX, Kubes P. The Neutrophil's role during health and disease. Physiol Rev. 2019;99(2):1223-1248.
doi pubmed - Prame Kumar K, Nicholls AJ, Wong CHY. Partners in crime: neutrophils and monocytes/macrophages in inflammation and disease. Cell Tissue Res. 2018;371(3):551-565.
doi pubmed pmc - Atri C, Guerfali FZ, Laouini D. Role of human macrophage polarization in inflammation during infectious diseases. Int J Mol Sci. 2018;19(6):1801.
doi pubmed pmc - Herbein G, Varin A. The macrophage in HIV-1 infection: from activation to deactivation? Retrovirology. 2010;7:33.
doi pubmed pmc - Feng D, Huang WY, Niu XL, Hao S, Zhang LN, Hu YJ. Significance of macrophage subtypes in the peripheral blood of children with systemic juvenile idiopathic arthritis. Rheumatol Ther. 2021;8(4):1859-1870.
doi pubmed pmc - Lai CY, Tseng PC, Chen CL, Satria RD, Wang YT, Lin CF. Different induction of PD-L1 (CD274) and PD-1 (CD279) expression in THP-1-differentiated types 1 and 2 macrophages. J Inflamm Res. 2021;14:5241-5249.
doi pubmed pmc - Stone ND, Ashraf MS, Calder J, Crnich CJ, Crossley K, Drinka PJ, Gould CV, et al. Surveillance definitions of infections in long-term care facilities: revisiting the McGeer criteria. Infect Control Hosp Epidemiol. 2012;33(10):965-977.
doi pubmed pmc - Chang A, Masante C, Buchholz UJ, Dutch RE. Human metapneumovirus (HMPV) binding and infection are mediated by interactions between the HMPV fusion protein and heparan sulfate. J Virol. 2012;86(6):3230-3243.
doi pubmed pmc - Franquet T. Imaging of pulmonary viral pneumonia. Radiology. 2011;260(1):18-39.
doi pubmed - Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14(10):986-995.
doi pubmed pmc - Gordon S, Pluddemann A, Martinez Estrada F. Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunol Rev. 2014;262(1):36-55.
doi pubmed pmc - Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13.
doi pubmed pmc - Shibru B, Fey K, Fricke S, Blaudszun AR, Furst F, Weise M, Seiffert S, et al. Detection of immune checkpoint receptors - a current challenge in clinical flow cytometry. Front Immunol. 2021;12:694055.
doi pubmed pmc - Serbina NV, Jia T, Hohl TM, Pamer EG. Monocyte-mediated defense against microbial pathogens. Annu Rev Immunol. 2008;26:421-452.
doi pubmed pmc - Tsou CL, Peters W, Si Y, Slaymaker S, Aslanian AM, Weisberg SP, Mack M, et al. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Invest. 2007;117(4):902-909.
doi pubmed pmc - Florentin J, Coppin E, Vasamsetti SB, Zhao J, Tai YY, Tang Y, Zhang Y, et al. Inflammatory macrophage expansion in pulmonary hypertension depends upon mobilization of blood-borne monocytes. J Immunol. 2018;200(10):3612-3625.
doi pubmed pmc - Italiani P, Boraschi D. From monocytes to M1/M2 macrophages: phenotypical vs. functional differentiation. Front Immunol. 2014;5:514.
doi pubmed pmc - Davies LC, Rosas M, Jenkins SJ, Liao CT, Scurr MJ, Brombacher F, Fraser DJ, et al. Distinct bone marrow-derived and tissue-resident macrophage lineages proliferate at key stages during inflammation. Nat Commun. 2013;4:1886.
doi pubmed pmc - Mills CD. M1 and M2 macrophages: oracles of health and disease. Crit Rev Immunol. 2012;32(6):463-488.
doi pubmed - Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol. 2000;164(12):6166-6173.
doi pubmed - Burdo TH, Walker J, Williams KC. Macrophage polarization in AIDS: dynamic interface between anti-viral and anti-inflammatory macrophages during acute and chronic infection. J Clin Cell Immunol. 2015;6(3):333.
pubmed pmc - Ferrer MF, Thomas P, Lopez Ortiz AO, Errasti AE, Charo N, Romanowski V, Gorgojo J, et al. Junin virus triggers macrophage activation and modulates polarization according to viral strain pathogenicity. Front Immunol. 2019;10:2499.
doi pubmed pmc - Xiong H, Mittman S, Rodriguez R, Moskalenko M, Pacheco-Sanchez P, Yang Y, Nickles D, et al. Anti-PD-L1 treatment results in functional remodeling of the macrophage compartment. Cancer Res. 2019;79(7):1493-1506.
doi pubmed - Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci U S A. 2003;100(9):5336-5341.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


