| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 16, Number 1, January 2024, pages 15-23
Intubation Time, Lung Mechanics and Outcome in COVID-19 Patients Suffering Acute Respiratory Distress Syndrome: A Single-Center Study
Diamanto Arethaa, c, Sotiria Kefalab, Alexandra Nikolopouloub, Vasilios Karamouzosb, Maria Valtab, Virginia Mplanib, Alexandra Georgakopouloub, Chrysavgi Papamichailb, Christina Sklavoub, Fotini Fligoub
aDepartment of Anesthesiology and Intensive Care Medicine, School of Medicine, University Hospital of Patras, Rion, 26504 Patras, Greece
bDepartment of Anesthesiology and Intensive Care Medicine, University Hospital of Patras, Rion, 26504 Patras, Greece
cCorresponding Author: Diamanto Aretha, Department of Anesthesiology and Intensive Care Medicine, School of Medicine, University Hospital of Patras, Rion, 26504 Patras, Greece
Manuscript submitted November 22, 2023, accepted January 17, 2024, published online January 31, 2024
Short title: Intubation Time and Lung Mechanics in COVID-19 Patients
doi: https://doi.org/10.14740/jocmr4984
| Abstract | ▴Top |
Background: We examined the effect of intubation time and the lung mechanics on clinical outcomes in coronavirus disease 2019 (COVID-19) patients.
Methods: Based on the patient’s hospital admission, intubation time was defined as early (≤ 2 days) or late (> 2 days). Patients were further divided into three groups; early (≤ 3 days), late (4 - 6 days), and very late (> 6 days) intubated.
Results: A total of 194 patients were included; 66.5% male, median age 65 years. Fifty-eight patients (29.9%) were intubated early and 136 (70.1%) late. Early intubated patients revealed lower mortality (44.8% vs. 72%, P < 0.001), were younger (60 vs. 67, P = 0.002), had lower sequential organ failure assessment (SOFA) scores (6 vs. 8, P = 0.002) and higher lung compliance on admission days 1, 6 and 12 (42 vs. 36, P = 0.006; 40 vs. 33, P < 0.001; and 37.5 vs. 32, P < 0.001, respectively). Older age (adjusted odds ratio (aOR) = 1.15, P < 0.001), intubation time (aOR = 1.15, P = 0.004), high SOFA scores (aOR = 1.81, P < 0.001), low partial pressure of oxygen (PaO2)/fractional inspired oxygen tension (FiO2) ratio (aOR = 0.96, P = 0.001), and low lung compliance on admission days 1 and 12 (aOR = 1.12, P = 0.012 and aOR = 1.14, P < 0.001, respectively) were associated with higher mortality. Very late and late intubated patients had higher mortality rates than patients intubated early (78.4% vs. 63.4% vs. 44.6%, respectively, P < 0.001).
Conclusions: Among COVID-19 intubated patients, age, late intubation, high SOFA scores, low PaO2/FiO2 ratio, and low lung compliance are associated with higher intensive care unit (ICU) mortality.
Keywords: COVID-19; Late intubation; ARDS; Mechanical ventilation
| Introduction | ▴Top |
Pneumonia associated with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (coronavirus disease 2019 (COVID-19)) is often associated with hypoxic respiratory failure, which is a key criterion for acute respiratory distress syndrome (ARDS). Delayed intubation and mechanical ventilation (MV), especially in patients with high respiratory drive, has been associated with patient self-inflicted lung injury (P-SILI) [1, 2], which is considered as an underlying mechanism and a potential prognosticator of a worsened patients’ clinical outcome. On the other hand, intubation and MV are associated with increased morbidity and mortality [3-5]. In four recent studies late intubation and MV are associated with increased mortality among COVID-19 patients [6-9]. In contrast, other studies, including a systematic review and meta-analysis of non-randomized studies, suggest that time to intubation and MV may have no impact on the morbidity and mortality of critically ill patients with COVID-19 [3, 10-15].
The definition of early and late intubation shows great variation, typically related to the intubation of patients within 24 h or after admission to the intensive care unit (ICU). However, various factors may influence the time of ICU admission: bed availability; age and illness duration, as younger and longer-surviving patients are usually transferred to the ICU more rapidly; initial clinical impression in terms of oxygenation and work of breathing; and the number of patients treated in the wards on oxygen support with high-flow nasal cannula (HFNC) or continuous positive airway pressure (CPAP).
Among patients with SARS-CoV-2 infection and respiratory insufficiency admitted to our hospital from whom treatment with HFNC or CPAP failed to cure severe hypoxemia and increased work of breathing, we hypothesized that late intubation and MV are associated with a worse outcome than early intubation. To investigate the effect of intubation time and lung mechanics, we analyzed prospectively collected data on COVID-19, mechanically ventilated patients hospitalized in our ICU.
| Materials and Methods | ▴Top |
This is a prospective observational cohort study that was carried out at the ICU of Patras General University Hospital, a tertiary, academic 750-bed hospital. All consecutive intubated and invasively mechanically ventilated patients over 18 years suffering severe COVID-19 pneumonia that were treated in the ICU, during the third pandemic wave (February 1, 2021, to February 28, 2022) were included in the study. Only patients with laboratory-confirmed severe ARDS coronavirus 2 (SARS-CoV-2) infection were included while patients without laboratory-confirmed COVID-19 were not included, even if they presented with a typical radiological pattern. The study protocol was approved by the Hospital Research Ethics Committee (PN: 10408), and the need for informed consent was waived. Our university hospital has 17 (both medical and surgical) ICU beds. During the COVID-19 pandemic, the ICU capacity of our hospital was expanded to 37 beds on an as-needed basis. However, there were insufficient ICU beds for patients with severe ARDS who required HFNC or CPAP. Consequently, after a given point (February 1, 2021), it was decided that only intubated cases should be treated in the ICU, while patients who required HFNC or CPAP continued to be treated in the wards under enhanced monitoring. COVID-19 was confirmed by a positive result on real-time reverse transcriptase-polymerase chain reaction of both nasal and pharyngeal swabs for SARS-CoV-2. The study was conducted in compliance with the ethical standards of our institution (General University Hospital of Patras) on human subjects as well as with the Helsinki Declaration.
In accordance with our hospital’s protocol, patients with initial hypoxemia and respiratory failure were first managed with a Venturi mask, HFNC and/or CPAP, and awake prone positioning when tolerated. Respiratory support methods were usually used in the order described, with a gradual increase in fractional inspired oxygen tension (FiO2) and positive-end-expiratory-pressure (PEEP) predicated on the assessment of the attending physicians. The decision for intubation and MV was also made by the attending physicians in the event of altered mentation, hemodynamic instability, and respiratory distress (evidenced by the usage of accessory respiratory muscles or inability to speak). Hypoxemia without dyspnea and respiratory distress (silent or “happy” hypoxemia, which is common in COVID-19 patients) [16] was not considered sufficient to warrant intubation. However, in critical disease including ARDS and severe hypoxemia or respiratory failure which was insisted or aggravated (PO2/FiO2 < 100) despite the application of HFNC (up to 60 L/min) or CPAP (up to 10 cm H2O PEEP), septic shock and/or multiple organ disfunction, the patients were intubated. A protective lung ventilation strategy was adopted [17-19], while using MV, which was initiated in pressure control ventilation mode, with a tidal volume of 6 - 8 mL/kg of ideal body weight, aiming at maintaining a driving pressure of < 15 cm H2O [20] and a plateau pressure (Ppl) of < 30 cm H2O. FiO2 was titrated to oxygen saturation measured via pulse oximetry of 92-94%, and PEEP was determined according to the best PEEP strategy [21]. The respiratory rate was titrated to maintain pH > 7.25, accepting mild hypercapnia (PCO2 < 52 mm Hg), unless contraindicated. Recruitment maneuvers were at the discretion of the attending physician and were not mandatory. In severe hypoxemia (partial pressure of oxygen (PaO2)/FiO2 < 150 mm Hg), a prone position was used for up to 24 h if there were no complications, and a neuromuscular blocker infusion was initiated. Following improvement in hypoxemia, protocolized spontaneous breathing trials were considered [22], while percutaneous tracheostomy was performed on patients undergoing prolonged MV. Post-extubation CPAP or HFNC was used when needed.
Data collection
Patients’ basic clinical and demographic data were retrospectively collected from the electronic clinical records on the day of intubation and ICU admission. From that moment onwards, the data of interest were collected prospectively, and survival was assessed at ICU discharge. Driving pressures were calculated. Radiological and laboratory data were collected from the central computerized recording system of the hospital.
Outcomes
The time from hospital admission to intubation and MV was defined as intubation time. The primary outcome was the impact of the time to intubation on ICU survival. Based on the patient’s hospital admission, intubation time was defined as: early (≤ 2 days) or late (> 2 days). Secondary outcomes included MV duration, lung mechanics and ICU length of stay (LOS). The impact of time to intubation on MV duration and ICU LOS were also studied. In addition, depending on the time to intubation from the time of hospital admission, a secondary analysis was performed, with patients further divided into three groups: early intubation (≤ 3 days), late intubation (4 - 6 days), and very late intubation (> 6 days), and the differences between groups were studied.
Statistical analysis
Normality of data was tested using the Shapiro-Wilk test and the Kolmogorov Smirnov test, and all the parameters tested exhibited a non-normal distribution. Proportional and categorical data were compared with the Chi-square test or Fisher’s exact test, while the Mann-Whitney U-test was used for continuous data analysis. Accordingly, the descriptive statistics are presented as medians (interquartile range 25 - 75), or percentages (%).
According to a predefined analysis plan, that was considered before data collection, three different analyses were performed. The first analysis was aimed at determining the factors that differ between early and late intubated patients. The second analysis was aimed at detecting predictors of ICU mortality in patients who were intubated upon ICU admission. The third, a secondary analysis, was aimed at determining the impact of the time of intubation (early, late, or very late) on patients’ survival (secondary analysis).
Predictors of ICU mortality were identified by using univariable and multivariable logistic regression models (backward stepwise). Variables with P values ≤ 0.01 in the univariate regression were included in the multivariable model, while the choice of variables was also based on considered potential collinearity and scientific knowledge. The possibility of ICU survival was assessed via survival analysis using Kaplan-Meier curves. The predictive value of the multivariable regression model was estimated using the receiver operating characteristic (ROC) and the area under the ROC curve (AUC).
The data were analyzed using the SPSS statistical package for Windows (version 27.0; IBM, Armonk, NY, USA) and GraphPad Prism Version 9.3.1. A two-tailed P value of < 0.05 was considered statistically significant.
| Results | ▴Top |
Early intubated patients compared to late intubated patients
We included 194 consecutive intubated patients in this study; 66.5% were male, and the median age was 65 years old. Of the 194 study participants, 136 patients (70.1%) were intubated late (> 2 days) and 58 (29.9%) were intubated early (≤ 2 days). Total ICU mortality was 64%, and mortality among early intubated patients was 44.8% compared to 72% among late intubated patients ((P < 0.001) (Fig. 1a); (P < 0.016) (Fig. 1c)). Among the patients who survived, the time to intubation was significantly shorter (3.5 vs. 7 days, P < 0.001) than among non-survivors (Fig. 1b). Early intubated patients and late intubated patients had similar body mass index (30 vs. 28, P = 0.554), admission PaO2/FiO2 ratio (120 vs. 110, P = 0.295), admission plateau pressure (26 vs. 26, P = 0.101), median driving pressure (13.5 vs. 14; P = 0.081), MV days (12 vs. 12; P = 0.902), ICU LOS (14.5 vs. 12; P = 0.344) and comorbidity number (2 vs. 2, P = 0.340). However, early intubated patients were younger (60 vs. 67, P = 0.002), and had lower sequential organ failure assessment (SOFA) scores (6 vs. 8, P = 0.002) than late intubated patients. Furthermore, early intubated patients had higher static compliance of the respiratory system than late intubated patients on admission day 1 (42 vs. 36, P = 0.006), day 6 (40 vs. 33, P < 0.001) and day 12 (37.5 vs. 32, P < 0.001). Before intubation, CPAP or/and HFNC was administered to 164 (84.5%) patients. There was a statistically significant difference in CPAP or HFNC use between early intubated patients (42 of 58, i.e., 72.4%) and late intubated patients (122 of 136, i.e., 89.7%; P = 0.004). Furthermore, the median days under CPAP/HFNC administration were significantly less among the early intubated group than among the late intubation group (1 vs. 4.5, P < 0.001). The differences between early and late intubation patients are presented in Table 1.
 Click for large image | Figure 1. Mortality differences in early vs. late intubation group of patients (a), differences in intubation timing (days) in survivors vs. non-survivors (b) and probability of survival between the two groups of patients (c). |
 Click to view | Table 1. Differences Between Early and Late Intubated Patients |
Age (P < 0.001), high SOFA score at ICU admission (P < 0.001), time to intubation (P = 0.004), low PaO2/FiO2 ratio after intubation (P = 0.001), low static compliance of the respiratory system on admission day 1 and day 12 (P = 0.012 and P < 0.001, respectively), and a high white blood cell (WBC) number at ICU admission (P = 0.001) were independently associated with mortality. Predictors of ICU mortality in patients who were intubated upon ICU admission are presented in Table 2. The predictive value of the multivariable regression model is rather high (AUC = 0.967, P < 0.001) (Supplementary Material 1, www.jocmr.org). The variance inflation factor (VIF) was used to test multicollinearity issues. VIF was lower than 7, therefore our analysis does not present any significant multicollinearity issues.
 Click to view | Table 2. Predictors of Intensive Care Unit Mortality Based on Uni- and Multivariable Logistic Regression Model |
Secondary analysis: early intubated patients compared to late and very late intubated patients
Sixty-five patients (33.5%) were intubated early (≤ 3 days), 41 patients (21.1%) were intubated late (4 - 6 days), and 88 patients (45.4%) were intubated very late (> 6 days) after hospital admission. There was a statistically significant difference in mortality between the early intubated patients and the late intubated patients (P = 0.045), and between the early intubated patients and the very late intubated patients (P < 0.001). However, there was no statistically significant difference in mortality between the late intubated patients and the very late intubated patients (P = 0.58) (Fig. 2a). Among the early intubated patients, mortality was 44.6%, compared to 63.4% among the late intubated and 78.4% among the very late intubated patients (P < 0.001) (Fig. 2b). The statistically significant differences between these three groups of patients are presented here (Supplementary Material 2, www.jocmr.org).
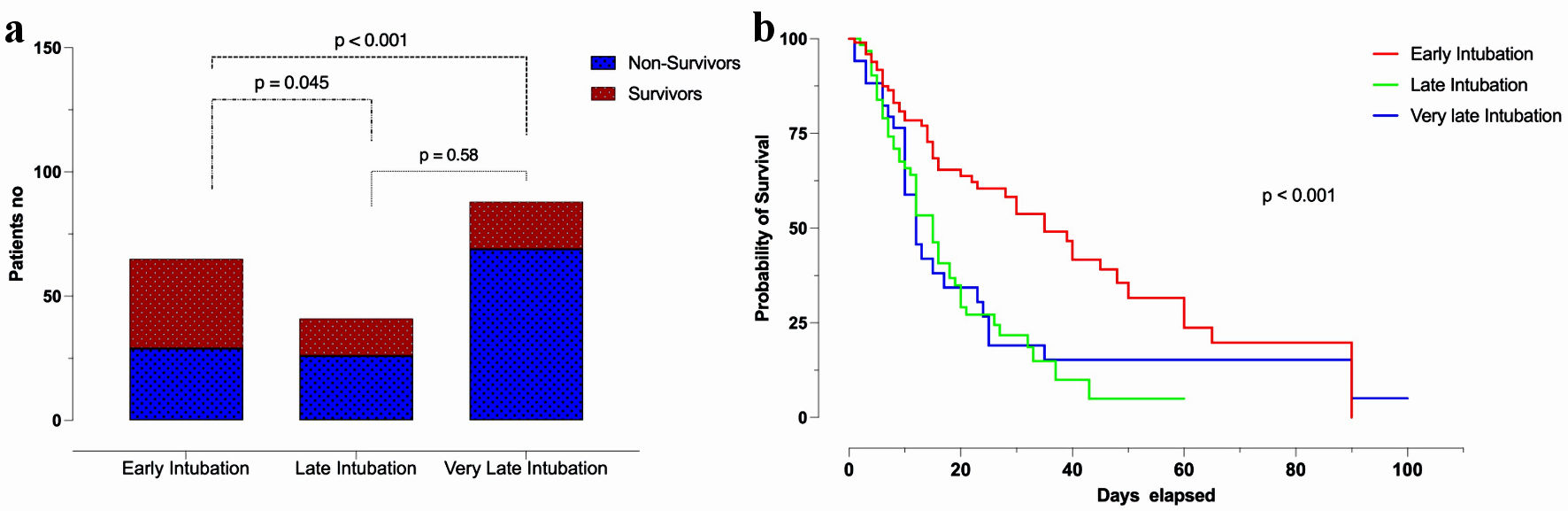 Click for large image | Figure 2. Patients’ mortality and time to intubation (a) and Kaplan-Meier curve for survival in early, late, and very late intubated patients (b). |
| Discussion | ▴Top |
The main finding of our study is that, among intubated patients with severe respiratory failure and COVID-19, intubation after 2 days of hospital admission was associated with increased mortality. Furthermore, patients who were intubated 6 days after hospital admission had a much higher mortality rate than those patients who were intubated early (i.e., within 3 days of admission). In general, survivors had a shorter time to intubation than non-survivors. In addition, age, a high SOFA score, a high WBC number at ICU admission, a low PaO2/FiO2 ratio after intubation, and low static compliance of the respiratory system were also associated with increased mortality.
A valid reason for this clinical course could be the spontaneous, prolonged ventilatory efforts before intubation, inducing the progression of patients’ lung damage, also known as P-SILI [1, 2, 23]. Both disease progression and superimposed P-SILI could result in failure of the CPAP and/or HFNC support therapies, and a need for intubation. Noninvasive respiratory support has been considered a very effective therapy for overcoming gas exchange impairment and potentially averting the need for intubation in ARDS patients [24]. However, patients failing noninvasive ventilation have been shown to have a particularly poor prognosis [25]. By decreasing inspiratory effort and tidal volumes, intubation and MV yield protective effects thus limiting the extent of P-SILI. In order to prevent lung injury there was an early hypothesis-driven advisory that COVID-19 patients should be intubated and mechanically ventilated early in the disease progression [26]. However, other studies on COVID-19 respiratory management and outcomes have challenged the above theory and have called this paradigm into question [27, 28]. As such, early management of COVID-19-induced hypoxemia employs noninvasive forms of oxygenation to forestall the need for intubation and MV.
In our study, reduction in respiratory system compliance, especially in late and very late intubated patients, is probably associated with a significant increase in non-aerated lung tissue caused by alveolar and interstitial edema, consolidation and/or fibrosis. We do not know the impact of late intubation and prolonged HFNC and CPAP administration on the development of these lung lesions, but our findings are similar to those of other clinical trials [29-31].
In COVID-19 patients, the time to intubation is still the subject of intense debate [32]. Our findings are similar to those of other studies, suggesting that delayed intubation in patients with severe hypoxemia worsens their prognosis, especially after a prolonged CPAP trial [6-9]. However, many observational studies [10-13] and one meta-analysis of non-randomized cohort studies, spanning approximately 9,000 patients [14] report non-statistically significant differences in mortality between patients intubated early or late during the course of the disease.
The majority of these studies are retrospective, with a small number of patients treated with MV. In a study by Hernandez-Romieu et al, of the 231 patients admitted to the ICU, only 97 (47.2%) were eventually intubated, while the remaining were treated with HFNC [11]. The short median period between hospital and ICU admission (1 day) may have limited the appearance of different phenotypes of lung damage and disease progression [33, 34]. Concerning the meta-analysis [14], there was a significant variability in the definition of early and late intubation, which was a major limitation of the study.
Patients who were intubated late were older, had higher SOFA scores, decreased lung compliance and higher WBC counts. None of these factors would make an impact based on the decision to intubation criteria but, on the other hand, suggest a progressive course of COVID-19 infection pneumonia. Therefore, in our opinion, the decision on intubation should also include the progression of the disease and the possible deterioration of the inflammation markers. According to our study, older age, higher SOFA scores, higher WBC counts and worsening pulmonary lesions, which are revealed by computed tomography, should also be considered in the decision to intubation [35, 36].
Our study has some critical limitations. First, it is a single-center, observational, cohort study, and our results do not necessarily reflect the reality of other hospitals, even in our own country. In our study, however, ICU data were prospectively collected, and we included a considerable number of intubated patients. Second, we included only intubated patients, most of whom had failed the HFNC/CPAP treatment, while there were many patients with severe hypoxemia in our hospital treated with noninvasive ventilation who survived without intubation. Therefore, determining the optimal time for intubation remains a challenge.
In conclusion, our study findings indicate that among critically ill intubated COVID-19 patients, late intubation is associated with poor outcomes. During patients’ hospitalization, additional risk factors such as age, a high SOFA score and a high WBC number may increase the mortality risk associated with late intubation. A lower PaO2/FiO2 ratio following intubation and low static compliance of the respiratory system are also significant risk factors. Further prospective studies are required to establish the best time for intubation in COVID-19 patients suffering from severe ARDS.
| Supplementary Material | ▴Top |
Suppl 1. ROC curve (predictive value of the multivariable regression model).
Suppl 2. Differences between early, late, and very late intubated patients.
Acknowledgments
None to declare.
Financial Disclosure
Support was provided solely from institutional and/or departmental sources.
Conflict of Interest
Part of the study’s data were presented as abstract in the annual congress of the European Society of Intensive Care Medicine (ESICM), Paris, October 23 - 25, 2022. All the authors declare no competing interest.
Informed Consent
The study protocol was approved by the Hospital Research Ethics Committee (PN: 10408), and the need for informed consent was waived.
Author Contributions
Diamanto Aretha is the guarantor of the content of the manuscript including data and analysis. Study conception and design was performed by DA, and FF. Statistical analysis was performed by DA, who also wrote the manuscript and prepared the tables and the figures of the manuscript. Data collection was performed by SK, VK, AN, VM, AG, MV, CP and CS. The first draft of the manuscript was written by DA, while all the authors commented on previous versions of the manuscript. FF critically revised the final version of the manuscript. All authors have approved the final version and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Data Availability
The datasets generated and/or analyzed during the current study are not publicly available due to our hospital’s strategy and its scientific committee for sensitive personal data but are available from the corresponding author on reasonable request.
Abbreviations
ARDS: acute respiratory distress syndrome; CPAP: continuous positive airway pressure; HFNC: high flow nasal cannula; ICU: intensive care unit; IQR: interquartile range; LOS: length of stay; MV: mechanical ventilation; P-SILI: patient self-inflicted lung injury; SOFA score: sequential organ failure assessment score; FiO2: fractional inspired oxygen tension; PEEP: positive end expiratory pressure; Ppl: plateau pressure; WBC: white blood cell
| References | ▴Top |
- Brochard L, Slutsky A, Pesenti A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am J Respir Crit Care Med. 2017;195(4):438-442.
doi pubmed - Battaglini D, Robba C, Ball L, Silva PL, Cruz FF, Pelosi P, Rocco PRM. Noninvasive respiratory support and patient self-inflicted lung injury in COVID-19: a narrative review. Br J Anaesth. 2021;127(3):353-364.
doi pubmed pmc - Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G, Bonanomi E, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in lombardy, Italy. JAMA Intern Med. 2020;180(10):1345-1355.
doi pubmed pmc - Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475-481.
doi pubmed pmc - Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, Greninger AL, et al. COVID-19 in critically ill patients in the Seattle region - case series. N Engl J Med. 2020;382(21):2012-2022.
doi pubmed pmc - Vera M, Kattan E, Born P, Rivas E, Amthauer M, Nesvadba A, Lara B, et al. Since January 2020 Elsevier has created a COVID-19 resource centre with free information in English and Mandarin on the novel coronavirus COVID- 19. The COVID-19 resource centre is hosted on Elsevier Connect, the company’s public news and information. 2020.
- Pandya A, Kaur NA, Sacher D, O'Corragain O, Salerno D, Desai P, Sehgal S, et al. Ventilatory mechanics in early vs late intubation in a cohort of coronavirus disease 2019 patients with ARDS: a single center's experience. Chest. 2021;159(2):653-656.
doi pubmed pmc - Camous L, Pommier JD, Martino F, Tressieres B, Demoule A, Valette M. Very late intubation in COVID-19 patients: a forgotten prognosis factor? Crit Care. 2022;26(1):89.
doi pubmed pmc - Ball L, Robba C, Herrmann J, Gerard SE, Xin Y, Pigati M, Berardino A, et al. Early versus late intubation in COVID-19 patients failing helmet CPAP: A quantitative computed tomography study. Respir Physiol Neurobiol. 2022;301:103889.
doi pubmed pmc - Lee YH, Choi KJ, Choi SH, Lee SY, Kim KC, Kim EJ, Lee J. Clinical significance of timing of intubation in critically ill patients with COVID-19: a multi-center retrospective study. J Clin Med. 2020;9(9):1-12.
doi pubmed pmc - Hernandez-Romieu AC, Adelman MW, Hockstein MA, Robichaux CJ, Edwards JA, Fazio JC, Blum JM, et al. Timing of intubation and mortality among critically ill coronavirus disease 2019 patients: a single-center cohort study. Crit Care Med. 2020;48(11):e1045-e1053.
doi pubmed pmc - McKay B, Meyers M, Rivard L, Stankewicz H, Stoltzfus JC, Rammohan G. Comparison of early and late intubation in COVID-19 and its effect on mortality. Int J Environ Res Public Health. 2022;19(5):3075.
doi pubmed pmc - Siempos, II, Xourgia E, Ntaidou TK, Zervakis D, Magira EE, Kotanidou A, Routsi C, et al. Effect of early vs. delayed or no intubation on clinical outcomes of patients with COVID-19: an observational study. Front Med (Lausanne). 2020;7:614152.
doi pubmed pmc - Papoutsi E, Giannakoulis VG, Xourgia E, Routsi C, Kotanidou A, Siempos II. Effect of timing of intubation on clinical outcomes of critically ill patients with COVID-19: a systematic review and meta-analysis of non-randomized cohort studies. Crit Care. 2021;25(1):121.
doi pubmed pmc - Network C-IGobotR, the C-ICUI. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: a prospective cohort study. Intensive Care Med. 2021;47(1):60-73.
doi pubmed pmc - Dhont S, Derom E, Van Braeckel E, Depuydt P, Lambrecht BN. Conceptions of the pathophysiology of happy hypoxemia in COVID-19. Respir Res. 2021;22(1):12.
doi pubmed pmc - Putensen C, Theuerkauf N, Zinserling J, Wrigge H, Pelosi P. Meta-analysis: ventilation strategies and outcomes of the acute respiratory distress syndrome and acute lung injury. Ann Intern Med. 2009;151(8):566-576.
doi pubmed - Thompson BT, Chambers RC, Liu KD. Acute respiratory distress syndrome. N Engl J Med. 2017;377(6):562-572.
doi pubmed - Fan E, Brodie D, Slutsky AS. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319(7):698-710.
doi pubmed - Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, Stewart TE, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372(8):747-755.
doi pubmed - Gattinoni L, Collino F, Maiolo G, Rapetti F, Romitti F, Tonetti T, Vasques F, et al. Positive end-expiratory pressure: how to set it at the individual level. Ann Transl Med. 2017;5(14):288.
doi pubmed pmc - Subira C, Hernandez G, Vazquez A, Rodriguez-Garcia R, Gonzalez-Castro A, Garcia C, Rubio O, et al. Effect of pressure support vs T-piece ventilation strategies during spontaneous breathing trials on successful extubation among patients receiving mechanical ventilation: a randomized clinical trial. JAMA. 2019;321(22):2175-2182.
doi pubmed pmc - Cruces P, Retamal J, Hurtado DE, Erranz B, Iturrieta P, Gonzalez C, Diaz F. A physiological approach to understand the role of respiratory effort in the progression of lung injury in SARS-CoV-2 infection. Crit Care. 2020;24(1):494.
doi pubmed pmc - Alhazzani W, Moller MH, Arabi YM, Loeb M, Gong MN, Fan E, Oczkowski S, et al. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19). Intensive Care Med. 2020;46(5):854-887.
doi pubmed pmc - Brochard L, Lefebvre JC, Cordioli RL, Akoumianaki E, Richard JC. Noninvasive ventilation for patients with hypoxemic acute respiratory failure. Semin Respir Crit Care Med. 2014;35(4):492-500.
doi pubmed - Matta A, Chaudhary S, Bryan Lo K, DeJoy R, 3rd, Gul F, Torres R, Chaisson N, et al. Timing of intubation and its implications on outcomes in critically ill patients with coronavirus disease 2019 infection. Crit Care Explor. 2020;2(10):e0262.
doi pubmed pmc - Marini JJ, Gattinoni L. Management of COVID-19 Respiratory Distress. JAMA. 2020;323(22):2329-2330.
doi pubmed - Rola P, Farkas J, Spiegel R, Kyle-Sidell C, Weingart S, Duggan L, Garrone M, et al. Rethinking the early intubation paradigm of COVID-19: time to change gears? Clin Exp Emerg Med. 2020;7(2):78-80.
doi pubmed pmc - Schenck EJ, Hoffman K, Goyal P, Choi J, Torres L, Rajwani K, Tam CW, et al. Respiratory mechanics and gas exchange in COVID-19-associated respiratory failure. Ann Am Thorac Soc. 2020;17(9):1158-1161.
doi pubmed pmc - Ziehr DR, Alladina J, Petri CR, Maley JH, Moskowitz A, Medoff BD, Hibbert KA, et al. Respiratory pathophysiology of mechanically ventilated patients with COVID-19: a cohort study. Am J Respir Crit Care Med. 2020;201(12):1560-1564.
doi pubmed pmc - Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 does not lead to a "Typical" acute respiratory distress syndrome. Am J Respir Crit Care Med. 2020;201(10):1299-1300.
doi pubmed pmc - Cabrini L, Ghislanzoni L, Severgnini P, Landoni G, Baiardo Redaelli M, Franchi F, Romagnoli S. Early versus late tracheal intubation in COVID-19 patients: a "pros/cons" debate also considering heart-lung interactions. Minerva Cardiol Angiol. 2021;69(5):596-605.
doi pubmed - Wang X, Jehi L, Ji X, Mazzone PJ. Phenotypes and subphenotypes of patients with COVID-19: a latent class modeling analysis. Chest. 2021;159(6):2191-2204.
doi pubmed pmc - Gattinoni L, Chiumello D, Caironi P, Busana M, Romitti F, Brazzi L, Camporota L. COVID-19 pneumonia: different respiratory treatments for different phenotypes? Intensive Care Med. 2020;46(6):1099-1102.
doi pubmed pmc - Bavishi AA, Mylvaganam RJ, Agarwal R, Avery RJ, Cuttica MJ. Timing of intubation in coronavirus disease 2019: a study of ventilator mechanics, imaging, findings, and outcomes. Crit Care Explor. 2021;3(5):e0415.
doi pubmed pmc - Francone M, Iafrate F, Masci GM, Coco S, Cilia F, Manganaro L, Panebianco V, et al. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur Radiol. 2020;30(12):6808-6817.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


