| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 14, Number 10, October 2022, pages 409-415
Clinical Utility of the Geriatric Nutritional Risk Index Before Surgical Intervention for Epithelial Ovarian Cancer Patients: A Retrospective Study
Shinji Oguraa, Kohei Seoa, b, Mitsuyoshi Ichiharaa, Kiyotake Ichizukaa, Masaaki Nagatsukaa
aDepartment of Obstetrics and Gynecology, Showa University Northern Yokohama Hospital, Kanagawa, Japan
bCorresponding Author: Kohei Seo, Department of Obstetrics and Gynecology, Showa University Northern Yokohama Hospital, Tsuzuki- ku, Yokohama-shi, Kanagawa 224-8503, Japan
Manuscript submitted August 27, 2022, accepted October 6, 2022, published online October 28, 2022
Short title: GNRI for Epithelial Ovarian Cancer
doi: https://doi.org/10.14740/jocmr4816
| Abstract | ▴Top |
Background: The aim of the study is to analyze the impact of the geriatric nutritional risk index (a patient nutritional assessment item) on the prognoses of epithelial ovarian cancer patients.
Methods: In this retrospective study conducted at a single hospital, we retrospectively analyzed 75 epithelial ovarian cancer patients who underwent surgical treatment at our hospital from 2010 to 2015. The geriatric nutritional risk index cut-off value was calculated using the receiver operating characteristic curve. Patients were divided into two groups on the basis of the calculated value. Kaplan-Meier curves were prepared for each group, and the difference in survival rates was calculated using the log-rank test. Cox proportional hazards regression analysis was used to compare other factors that affect prognosis.
Results: The geriatric nutritional risk index was calculated to be 97.3. The survival rate was 61.9% for the group of patients with an index value > 97.3, and 39.4% for patients with an index value < 97.3 at 48 months (P < 0.001). A univariate analysis was performed with the following variables: age > 60 years, albumin level < 3.5 g/dL, body mass index < 22, presence of ascites, cancer antigen 125 level > 35 U/mL, type of tumor tissue, residual lesion, and geriatric nutritional risk index < 97.3. Albumin level, residual lesion, and geriatric nutritional risk index showed significant differences. A multivariate analysis was also performed, and only the geriatric nutritional risk index showed a significant difference (P = 0.0481).
Conclusions: The geriatric nutritional risk index may have a strong influence on the prognoses of epithelial ovarian cancer patients. We recommend utilizing these findings in daily clinical practice and incorporating them into treatment strategies for epithelial ovarian cancer.
Keywords: Carcinoma; Ovarian epithelial; Geriatric assessment; Nutrition assessment; Nutritional status
| Introduction | ▴Top |
According to the statistical report of the National Cancer Centre in Japan, the total number of cancer-related deaths in 2017 was 373,334, of which 86.4% involved individuals aged > 65 years. In addition, 72.1% of all cancer patients were aged > 65 years. This increasing association between old age and cancer is becoming more prominent with time. Men usually show prostate cancer, gastric cancer, and colon cancer, whereas women usually show breast cancer, colon cancer, and lung cancer. Breast, uterine, and ovarian cancer patients tend to be relatively younger; however, the number of cases and age of the patients are increasing in Japan, where the population is rapidly aging. The annual incidence of ovarian cancer has exceeded 10,000, indicating the need to consider treatment options for ovarian cancer among the elderly.
Although physicians carefully consider whether treatment is indicated for elderly cancer patients, the implications of the word “treatment indication” are quite often unclear. Usually, the attending physician consults with the patient and their family to decide on the best treatment strategy. Since the elderly are often worried about therapeutic intervention, it may be easier to refer to informed consent if there are objective numbers and data when considering therapeutic intervention. Regarding ovarian cancer, the concept of a “comprehensive geriatric assessment (CGA)” has become popular in recent years as an index that reflects the patient’s general condition, not in relation with the tumor stage or histological type, but as a factor that determines prognosis. Thus, the relationship between CGA and ovarian cancer is now being analyzed. CGA is a concept and tool that facilitates the provision of comprehensive medical care by allowing evaluation of the physical, mental, and social functions of patients with respect to multiple perspectives. The term “geriatric assessment” (GA) is used when only a comprehensive health assessment is performed for the purpose of determining the appropriate interventions [1]. In 2018, the American Society of Clinical Oncology published the guidelines, “Practical evaluation methods and interventions for vulnerable elderly patients who start chemotherapy” [2]. The guidelines state that “GA should be used to identify vulnerabilities that are not routinely detected in patients older than 65 years who start chemotherapy.” A number of GA evaluation items have been created and advocated; one of them is the Barthel index [3], which focuses on physical activities of daily living, the controlling nutritional status score, the prognostic nutritional index (PNI), and the geriatric nutritional risk index (GNRI). Several studies have retrospectively analyzed the prognostic influence of using each item in the management of various carcinomas in recent years [4, 5]. In addition to chemotherapy, surgical treatment, dialysis, and the relationship between heart failure and GA are being evaluated [6]. Regarding ovarian cancer, the influence of PNI and the association between the preoperative C-reactive protein/albumin ratio and the prognoses of epithelial ovarian tumor patients treated with chemotherapy has been reported [7, 8]. However, research on the prognostic relationship between GNRI and ovarian cancer is lacking. In clinical practice, surgical interventions are often difficult due to the poor general condition of the patient even if their performance status (PS) is 0 - 1. The general impression is that emaciated patients have poor prognoses. Therefore, we aimed to analyze the influence of nutrition on the prognoses of ovarian cancer patients by assessing the impact of GNRI (a patient nutritional assessment item) on the prognoses of epithelial ovarian cancer patients. We chose GNRI, since it considers the patient’s body mass index (BMI) in its formula. To the best of our knowledge, this is the first study to report the analysis of the impact of GNRI on the prognosis of epithelial ovarian cancer.
| Materials and Methods | ▴Top |
This study was approved by the Institutional Review Board of Showa University Northern Yokohama Hospital (approval number: 20H021). We retrospectively selected and analyzed patients who were diagnosed with epithelial ovarian cancer (based on histopathological investigations) and underwent surgical interventions in our hospital from 2010 to 2015. The total number of patients included was 75. GNRI was calculated using the data collated immediately before the operation. The cut-off value of the GNRI was calculated using the receiver operating characteristic (ROC) curve. GNRI was calculated as follows: (1.489 × serum albumin value (g/L)) + 41.7 × %ideal body weight (IBW). Patients were divided into two independent groups based on the calculated cut-off value. Kaplan-Meier curves were created for each group, and the difference between the survival rates of both groups at 48 months was calculated using the log-rank test. Cox proportional hazards regression analysis was used to compare other factors that affect prognosis. This analysis was retrospective and there were variations between the background data of the patients within the two groups. Therefore, we analyzed whether there were variations in the backgrounds of seven items in the patients’ background data. The items included age, BMI, albumin level, cancer stage (International Federation of Gynecology and Obstetrics (FIGO) classification), presence or absence of ascites, residual lesion, cancer antigen (CA)-125 levels, and histopathological cancer type. The Mann-Whitney U test was used to analyze continuous variables, whereas Fisher’s exact test and Pearson’s chi-square test were used for nominal variables. Results with significant differences were evaluated using multivariate analysis. We considered a P value of < 0.05 to be statistically significant. All analyses were performed using the R software program (version 1.26, Saitama Medical Center, Jichi Medical University, Saitama, Japan) [9].
The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
| Results | ▴Top |
Based on the ROC curve, the cut-off value of the GNRI was calculated to be 97.3 (area under the curve, 0.644); the study population was divided into two groups based on this cut-off value. The survival rate at 48 months was 61.9% in the group of patients with GNRI values higher than 97.3, and 39.4% for the group of patients with indices lower than 97.3. The P value was < 0.001 and the difference was statistically significant (Fig. 1). Table 1 shows the differences in the patients’ background factors. BMI, albumin levels, surgical completion rate, and fibrinogen levels were found to be significantly different between the two groups. The analyses results showed that age > 60 years, albumin level < 3.5 g/dL, BMI < 20 kg/m2, ascites, CA-125 level > 35 U/mL, type of tumor tissue, postoperative residual tumor, and GNRI < 97.3 were poor prognostic factors. Multivariate analysis was used to assess albumin level < 3.5 g/dL, postoperative residual tumor, cancer stage, and GNRI < 97.3; only GNRI showed a significant influence on prognosis (P = 0.0481) (Table 2).
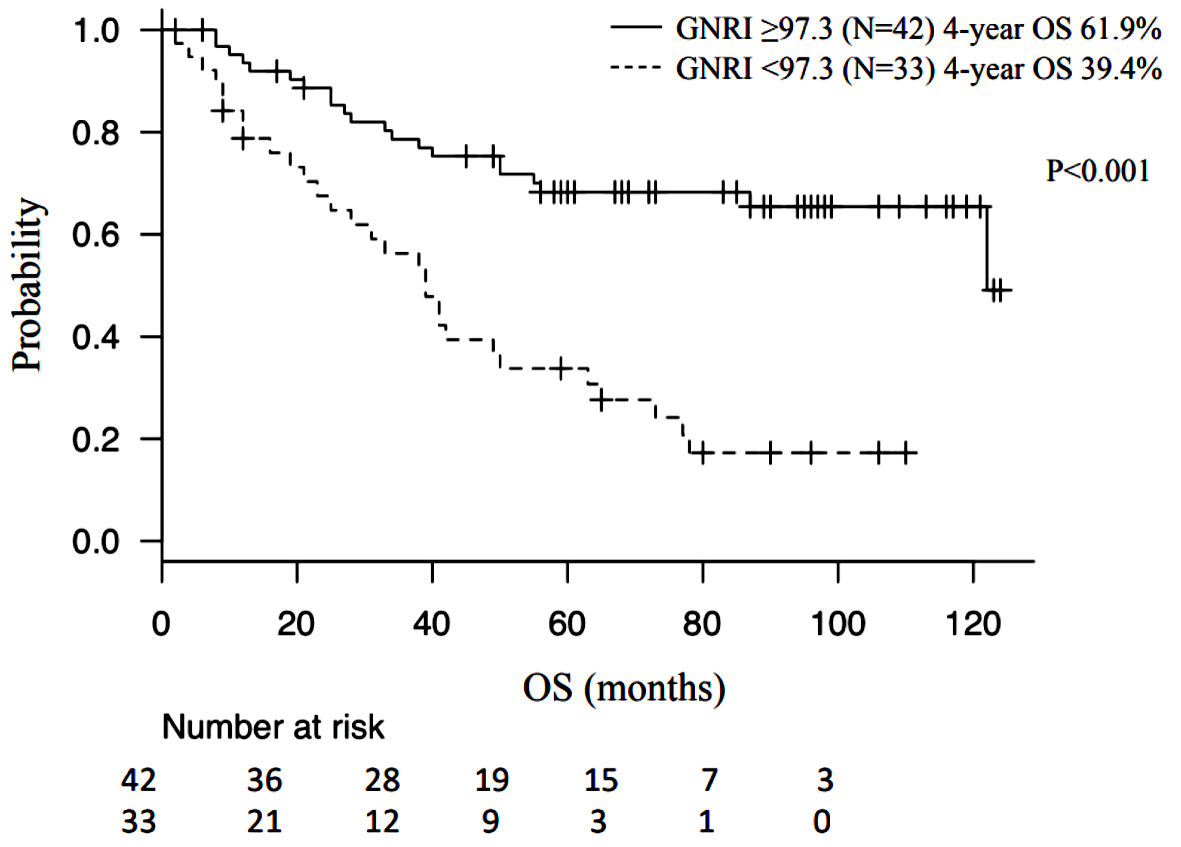 Click for large image | Figure 1. Kaplan-Meier curve and log-rank test. GNRI: geriatric nutritional risk index; OS: overall survival. |
 Click to view | Table 1. Patient Characteristics Categorized According to GNRI |
 Click to view | Table 2. Univariate and Multivariate Analysis of Risk Factors Associated With Overall Survival of Epithelial Ovarian Cancers |
| Discussion | ▴Top |
In this study, we analyzed the influence of GNRI on the prognoses of epithelial ovarian cancer patients. The study results show that albumin levels and amount of residual tumor postoperatively have a major influence on the prognosis of epithelial ovarian cancer patients, but the findings also suggest that GNRI may be an even more important prognostic factor. The study results also indicate that the median age of epithelial ovarian cancer patients who undergo surgical intervention is steadily increasing every year (Fig. 2).
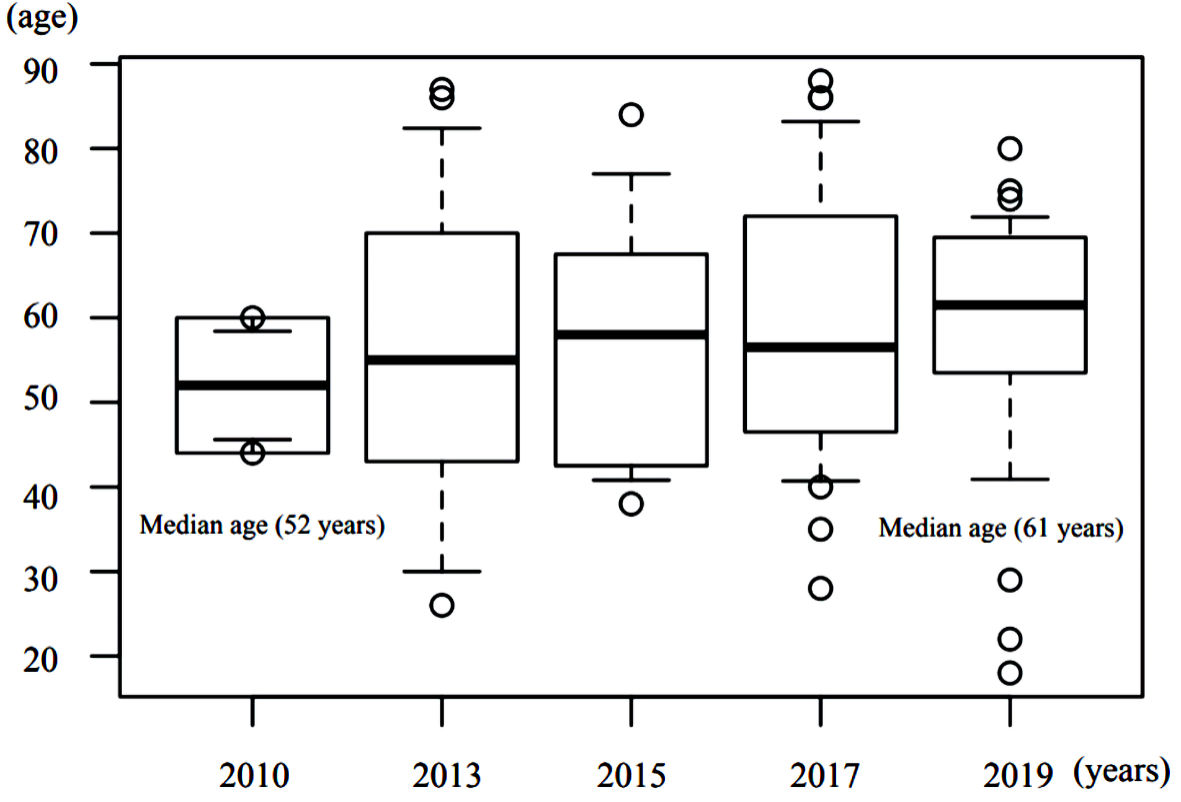 Click for large image | Figure 2. Yearly transition of median ages of epithelial ovarian cancer patients who underwent surgical intervention. |
On the basis of these results, we strongly believe that in addition to accurate evaluation of the impact of tumor-related factors, such as the stage of disease and the amount of residual tumor, on prognosis, evaluation of the impact of the patient’s general health on the prognosis is also essential. GNRI is a nutritional assessment method based on %IBW and serum albumin levels [10]. GNRI is a modification of the nutritional risk index (NRI), which was used as an index for estimating the occurrence of postoperative complications related to nutritional disorders in the surgical field; however, both indices have two distinctly different characteristics [11, 12]. First, the NRI uses the usual body weight, which is the ratio of the current weight to the normal weight, whereas GNRI uses %IBW, which is a comparison of the current weight to the ideal weight. The %IBW is a more appropriate parameter for these evaluations because the normal weight of a patient is often uncertain with increasing age. Second, GNRI uses the estimated height from knee-height to calculate %IBW, assuming that it is difficult to maintain a standing position due to the sequelae of cerebrovascular accident or physical fragility. In advanced countries including Japan, the median age of epithelial ovarian cancer patients is expected to increase further in the future. In that scenario, the GNRI will be a more effective method for evaluating the patient’s general condition.
Previous reports have suggested that the factors that worsen the prognosis of ovarian cancer are the stage of the disease (FIGO classification) [13], PS, age, surgical completion rate, tumor grade, and presence of ascites. In the multivariate analysis, and even in the univariate analysis, there was no significant difference in factors that were previously considered to have a large impact on prognosis. As shown in the Figure 2, Japan is aging very rapidly, especially in areas around our hospital. The current median age differs from that at the times when prognostic factors were calculated. Therefore, it is thought that items that reflect the patient’s degree of health now have more influence, while other tumor-related factors have less influence. As Japan’s aging population is expected to further accelerate in the future, these influences are expected to become stronger. Ovarian cancer shows few early symptoms and is often detected in its advanced stages; thus, regular examinations are important for early detection. Regarding the type of tumor tissue, various studies have assessed clinical frequency and progression tendency, chemotherapy reactivity, TP53/KRAS/BRAF mutations, estrogen receptor status, and the effects of hormone therapy; however, no study has directly compared the prognosis of the disease in relation to all of these factors [14]. In the present study, we categorized tumors as serous adenocarcinoma, mucinous adenocarcinoma, endometrioid adenocarcinoma, and clear cell adenocarcinoma to assess the effect of the histological type of cancer on the prognosis of ovarian cancer. However, the results of the analysis showed that histological type does not have a relatively large effect on prognosis. The survival curve of epithelial ovarian cancer at our hospital is shown in Figure 3; the prognosis did not differ notably among histological types. The degree of surgical completion has also been reported to have significant effects on prognosis, and the smaller the residual tumor diameter, the better the prognosis [15-18]. The results of the present study showed that residual tumor volume had the second highest hazard ratio next to GNRI, and it is thought to have a major influence on prognosis.
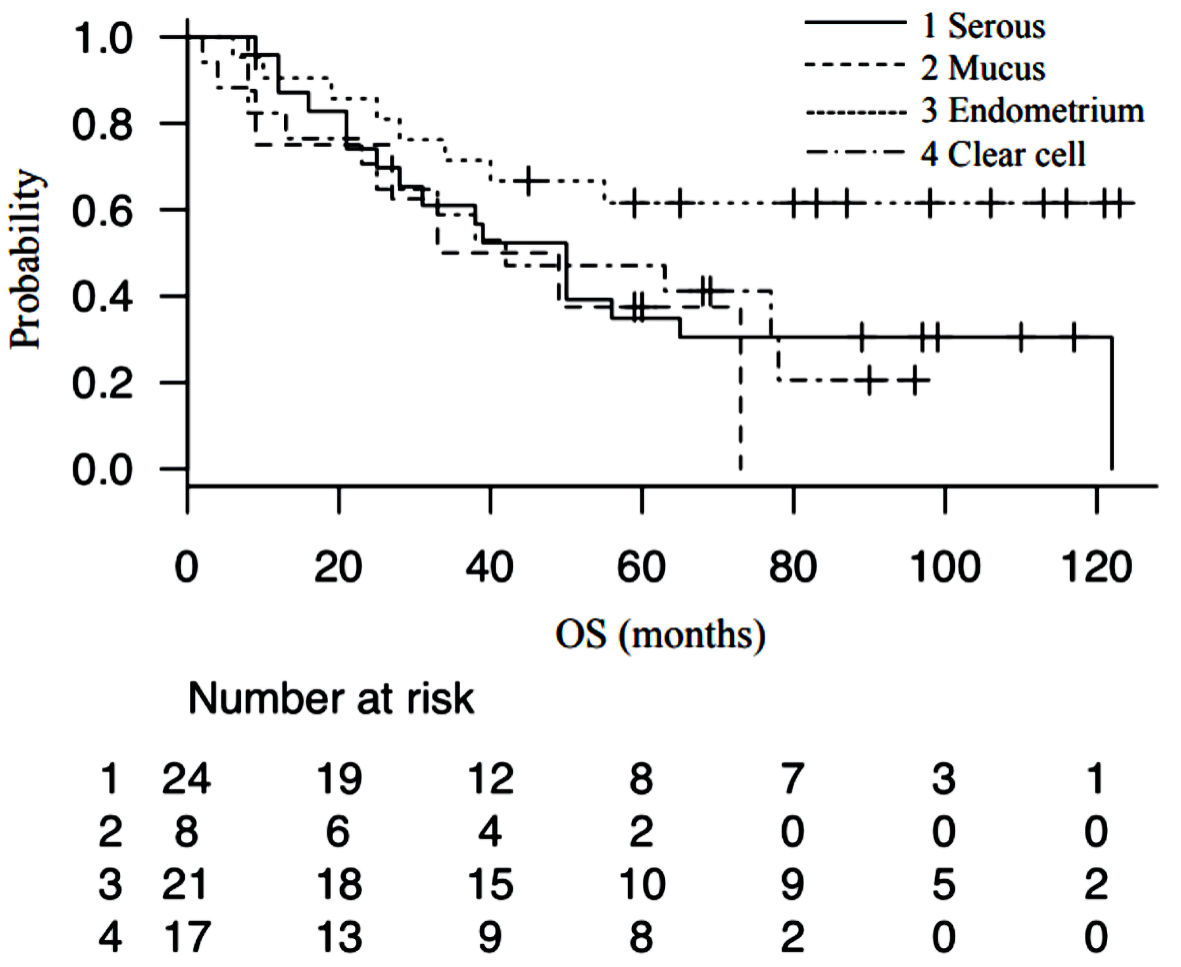 Click for large image | Figure 3. Survival curve of epithelial ovarian cancer patients stratified by histological type of cancer. OS: overall survival. |
There are currently various cancer staging classifications such as the FIGO [19] and the Gynaecologic Oncology Group classification; however, there is no classification method that is widely accepted worldwide. In the World Health Organization classification, cancer is classified into grades 1 - 3 based on cell atypia and construction. However, the definition of grades is not clear, and the evaluation varies slightly depending on each pathologist. We determined that incorporating uncertain definitions in our multivariate analyses will reduce the accuracy of the analyses. In future research, we would like to work with a pathologist to determine the definition of grade, perform objective evaluations, and incorporate the data into the analysis.
Regarding residual tumor volume, operator subjectivity is an important factor in the evaluation of residual lesions; thus, the accuracy of an analysis based on this factor will reduce accordingly. Moreover, internal debulking surgery (IDS) has to be considered after several cycles of neoadjuvant chemotherapy (NAC), and the prognoses of primary debulking surgery (PDS) and IDS will differ even if the surgical results are the same. Notably, the usefulness of NAC has also been verified previously [20]. In our hospital, NAC was only performed in cases when it is clinically judged to be appropriate. IDS cases were excluded from our analysis because the NAC enforcement criteria remain unclear. In future studies, we will establish NAC enforcement standards and evaluate the influence of GNRI on PDS and IDS. Regarding ascites, the presence or absence of ascites was evaluated in the present study using preoperative computed tomography (CT) and intraoperative findings. However, ascites is often found in small amounts even in stage I, and consequently, ascites was present in almost all cases in the present study. In future studies, the definition of ascites should be limited to extrapelvic ascites, preset estimated accumulated ascites volume in CT images, and cytology-positive cases. In the present study, we evaluated cases in which the patient underwent surgical intervention; patients who had PS 3 - 4 were excluded from the study as a result.
Thrombosis is an additional factor to be considered in regarding the prognosis of ovarian cancer. Ovarian cancer is reported to present a higher risk of thrombosis than other cancers, and its perioperative management has been studied [21]. Several reports have indicated that the Wells score and D-dimer values are useful for prevention of thrombosis [22-24]. In addition, the relationship between hyperfibrinogenemia and thrombosis in ovarian cancer patients and its influence on prognosis has been reported; similar results have been reported for other malignant tumors as well [25, 26]. Our analysis showed that the fibrinogen level was significantly higher in the low GNRI group. Due to the lack of data, we could not study the relationship between frailty and fibrinogen levels and the effect of fibrinogen levels on prognosis; therefore, we avoided adding data on fibrinogen levels to prevent inaccuracies in the analysis. In the future, we plan to measure and analyze not only fibrinogen but also plasminogen, protein S, protein C, antithrombin III (AT-III), etc. in all blood test items at the time of registration.
The median age for ovarian cancer patients from 2010 to 2015 was 58 years, which is lower than that of the patients in the present GA study. However, the analysis of this relatively young age group also showed that GNRI has a high hazard ratio. Figure 2 shows that the median age of ovarian cancer patients has increased by 9 years over the last 9 years. On the basis of this trend, we believe that GA will become more important in the field of ovarian cancer. The purpose of GA is to classify elderly patients into groups in which normal treatment can be administered (fit), treatment attenuation is recommended (vulnerable), or treatment is not recommended (frail). These classifications can be used as reference data for implementation of treatment policies. We believe that frail patients should not be treated with robust therapy that exceeds the limits of their general condition since this may cause adverse effects and yield poorer prognoses. However, obtaining informed consent, which deals with treatment decisions in the actual clinical setting, can be very difficult. Even if the potency of the treatment exceeds the limit of the patient’s physical strength, the course of positive treatment until death may be important and necessary for patients and their families.
This study had some limitations. This was a single-center retrospective study with differences in the patients’ background data. In future prospective trials, background factors should have no significant differences and should not affect analysis; the background factors should also be aligned so as not to affect the analysis. We are proceeding with a prospective study in which the background factors of both groups will be matched as much as possible. Another limitation is that the effects of chemotherapy should have been evaluated. All the patients in this current study had good PS and were able to complete standard treatment, and there were no cases of IDS. We were unable to evaluate differences in standard chemotherapy, maintenance therapy, and BRCA gene mutations. Regarding tumor grade, we could not perform grade analysis in this study. In some additional analysis, there was no significant difference in prognosis between the no residual and residual disease groups; however, there seemed to be a trend of influence, Similar results were obtained in the I - II group. We will increase the overall sample size and improve the accuracy of analysis with a prospective and background-matched study design.
In conclusion, this study shows the future possibilities of the clinical utility of GNRI in the creation of treatment strategies for epithelial ovarian cancer in super-aged societies in developed countries. We hope that the results of the present study do not diminish the hopes of elderly cancer patients but rather are used as a resource to make their lives more meaningful.
Acknowledgments
We would like to thank ROTSHAK for English language editing.
Financial Disclosure
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of Interest
None to declare.
Informed Consent
This study was a retrospective study. We asked on the message board if we could use their data anonymously in our hospital. We have obtained all the informed consents for publication.
Author Contributions
Shinji ogura: all processes including data collection, analysis, paper writing and so on. Seo kohei and Mitsuyoshi Ichihara were responsible for literacy search. Kiyotake Ichizuka and Masaaki Nagatsuka: final proofreading of this thesis.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
CGA: comprehensive geriatric assessment; GNRI: geriatric nutritional risk index; IDS: internal debulking surgery; NAC: neoadjuvant chemotherapy; PDS: primary debulking surgery; PS: performance status
| References | ▴Top |
- Wildiers H, Heeren P, Puts M, Topinkova E, Janssen-Heijnen ML, Extermann M, Falandry C, et al. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595-2603.
doi pubmed - Mohile SG, Dale W, Somerfield MR, Schonberg MA, Boyd CM, Burhenn PS, Canin B, et al. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology. J Clin Oncol. 2018;36(22):2326-2347.
doi pubmed - Mahoney FI, Barthel DW. Functional Evaluation: The Barthel Index. Md State Med J. 1965;14:61-65.
doi - Kamiya T, Ito C, Fujita Y, Ogura S, Mizuno K, Sakurai A, Aisa Y, et al. The prognostic value of the controlling nutritional status score in patients with multiple myeloma. Leuk Lymphoma. 2020;61(8):1894-1900.
doi pubmed - Ogura S, Nakazato T. Prognostic significance of the modified Barthel index in elderly patients with diffuse large B-cell lymphoma. Eur J Intern Med. 2020;75:110-111.
doi pubmed - Kinugasa Y, Kato M, Sugihara S, Hirai M, Yamada K, Yanagihara K, Yamamoto K. Geriatric nutritional risk index predicts functional dependency and mortality in patients with heart failure with preserved ejection fraction. Circ J. 2013;77(3):705-711.
doi pubmed - Liu Y, Chen S, Zheng C, Ding M, Zhang L, Wang L, Xie M, et al. The prognostic value of the preoperative c-reactive protein/albumin ratio in ovarian cancer. BMC Cancer. 2017;17(1):285.
doi pubmed - Miao Y, Li S, Yan Q, Li B, Feng Y. Prognostic significance of preoperative prognostic nutritional index in epithelial ovarian cancer patients treated with platinum-based chemotherapy. Oncol Res Treat. 2016;39(11):712-719.
doi pubmed - Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452-458.
doi pubmed - Bouillanne O, Morineau G, Dupont C, Coulombel I, Vincent JP, Nicolis I, Benazeth S, et al. Geriatric Nutritional Risk Index: a new index for evaluating at-risk elderly medical patients. Am J Clin Nutr. 2005;82(4):777-783.
doi pubmed - Buzby GP, Knox LS, Crosby LO, Eisenberg JM, Haakenson CM, McNeal GE, Page CP, et al. Study protocol: a randomized clinical trial of total parenteral nutrition in malnourished surgical patients. Am J Clin Nutr. 1988;47(2 Suppl):366-381.
doi pubmed - Buzby GP, Williford WO, Peterson OL, Crosby LO, Page CP, Reinhardt GF, Mullen JL. A randomized clinical trial of total parenteral nutrition in malnourished surgical patients: the rationale and impact of previous clinical trials and pilot study on protocol design. Am J Clin Nutr. 1988;47(2 Suppl):357-365.
doi pubmed - Heintz AP, Odicino F, Maisonneuve P, Quinn MA, Benedet JL, Creasman WT, Ngan HY, et al. Carcinoma of the ovary. FIGO 26th Annual Report on the Results of Treatment in Gynecological Cancer. Int J Gynaecol Obstet. 2006;95(Suppl 1):S161-192.
doi - Gurung A, Hung T, Morin J, Gilks CB. Molecular abnormalities in ovarian carcinoma: clinical, morphological and therapeutic correlates. Histopathology. 2013;62(1):59-70.
doi pubmed - Chi DS, Eisenhauer EL, Lang J, Huh J, Haddad L, Abu-Rustum NR, Sonoda Y, et al. What is the optimal goal of primary cytoreductive surgery for bulky stage IIIC epithelial ovarian carcinoma (EOC)? Gynecol Oncol. 2006;103(2):559-564.
doi pubmed - Eisenhauer EL, Abu-Rustum NR, Sonoda Y, Aghajanian C, Barakat RR, Chi DS. The effect of maximal surgical cytoreduction on sensitivity to platinum-taxane chemotherapy and subsequent survival in patients with advanced ovarian cancer. Gynecol Oncol. 2008;108(2):276-281.
doi pubmed - Winter WE, 3rd, Maxwell GL, Tian C, Carlson JW, Ozols RF, Rose PG, Markman M, et al. Prognostic factors for stage III epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2007;25(24):3621-3627.
doi pubmed - Winter WE, 3rd, Maxwell GL, Tian C, Sundborg MJ, Rose GS, Rose PG, Rubin SC, et al. Tumor residual after surgical cytoreduction in prediction of clinical outcome in stage IV epithelial ovarian cancer: a Gynecologic Oncology Group Study. J Clin Oncol. 2008;26(1):83-89.
doi pubmed - Manka I. [Classification and stages of malignant tumors in minor pelvis (report from the Cancer Committee of the International Federation of Gynecology and Obstetrics)]. Cesk Gynekol. 1971;36(8):502-504.
- Vergote I, Trope CG, Amant F, Kristensen GB, Ehlen T, Johnson N, Verheijen RH, et al. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363(10):943-953.
doi pubmed - Iodice S, Gandini S, Lohr M, Lowenfels AB, Maisonneuve P. Venous thromboembolic events and organ-specific occult cancers: a review and meta-analysis. J Thromb Haemost. 2008;6(5):781-788.
doi pubmed - Kawaguchi R, Furukawa N, Kobayashi H. Cut-off value of D-dimer for prediction of deep venous thrombosis before treatment in ovarian cancer. J Gynecol Oncol. 2012;23(2):98-102.
doi pubmed - Ljungqvist M, Soderberg M, Moritz P, Ahlgren A, Larfars G. Evaluation of Wells score and repeated D-dimer in diagnosing venous thromboembolism. Eur J Intern Med. 2008;19(4):285-288.
doi pubmed - Satoh T, Oki A, Uno K, Sakurai M, Ochi H, Okada S, Minami R, et al. High incidence of silent venous thromboembolism before treatment in ovarian cancer. Br J Cancer. 2007;97(8):1053-1057.
doi pubmed - Qiu J, Yu Y, Fu Y, Ye F, Xie X, Lu W. Preoperative plasma fibrinogen, platelet count and prognosis in epithelial ovarian cancer. J Obstet Gynaecol Res. 2012;38(4):651-657.
doi pubmed - Ogura S, Yonei S, Tanigawa T, Akimoto M, Sakurai A, Fujita Y, Ito C, et al. Prognostic significance of hyperfibrinogenemia in patients with lower-risk myelodysplastic syndromes. Ann Hematol. 2020;99(1):189-191.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


