| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 11, Number 3, March 2019, pages 213-218
Risk Factors of Hypoglycemic Encephalopathy and Prolonged Hypoglycemia in Patients With Severe Hypoglycemia
Rika Saikawaa, Hodaka Yamadaa, b, Daisuke Suzukia, Misato Amamotoa, Yuko Matsumotoa, Shunsuke Funazakia, Masashi Yoshidaa, Hideo Toyoshimaa, Kazuo Haraa
aDepartment of Medicine, Division of Endocrinology and Metabolism, Jichi Medical University, Saitama Medical Center, 1-847 Amanuma-cho, Omiya-ku, Saitama, Japan
bCorresponding Author: Hodaka Yamada, Department of Medicine, Division of Endocrinology and Metabolism, Jichi Medical University, Saitama Medical Center, 1-847 Amanuma-cho, Omiya-ku, Saitama 330-8503, Japan
Manuscript submitted December 17, 2018, accepted December 31, 2018
Short title: Risk Factors of Hypoglycemic Encephalopathy
doi: https://doi.org/10.14740/jocmr3728
| Abstract | ▴Top |
Background: The aim of the study was to investigate risk factors of hypoglycemic encephalopathy (HE) in patients with severe hypoglycemia.
Methods: We retrospectively enrolled patients with severe hypoglycemia who were transported to the emergency department in an ambulance. We defined severe hypoglycemia as plasma glucose level < 60 mg/dL (or capillary levels < 50 mg/dL). HE was defined as severe hypoglycemia with altered level of consciousness (Glasgow coma scale < 12) and prolonged HE as coma or stupor lasting > 24 h after glucose administration. We compared several parameters between patients with and without HE and between prolonged and recovered patients.
Results: Included were 173 patients with severe hypoglycemia; of them, 94 were diagnosed with HE, with 12 of them prolonged HE. Glucose level in HE patients was lower than that in those without HE (P < 0.001). Moreover, we noted a significant difference in glucose levels between the prolonged and recovered groups. Furthermore, body temperature was higher in prolonged versus recovered patients (P = 0.0017).
Conclusion: Blood glucose level may be correlated with severity of altered level of consciousness. In addition, body temperature may be related to coma or prolonged stupor.
Keywords: Hypoglycemic encephalopathy; Severe hypoglycemia; Body temperature
| Introduction | ▴Top |
Severe hypoglycemia is a common metabolic event in the emergency department (ED). It may cause irreversible altered levels of consciousness, cognitive decline, and death [1]. A case-control study examining patients’ social status and self-management revealed that a previous episode of hypoglycemia and inadequate blood glucose monitoring were risk factors for severe hypoglycemia [2]. In addition, hypoglycemic encephalopathy (HE) is a critical condition with poor clinical outcome [3]. Ikeda et al revealed that higher body temperature and low lactic acid levels may be factors in predicting poor prognosis in HE [4]. On magnetic resonance imaging, early changes in the brain due to hypoglycemia were seen in the internal capsule and extended to the hemispheric white matter [5]. Understanding the risk factors for HE is useful in treating hypoglycemia and preventing HE; however, few studies have examined the risk factors for progression to HE in severe hypoglycemic patients. Therefore, the aim of this study is to elucidate: 1) The risk of clinical background and metabolic parameters of HE; and 2) The prolonged aspect of HE.
| Materials and Methods | ▴Top |
Study participants
This was a retrospective, single-center, observational cohort study. We enrolled patients with severe hypoglycemia who were transferred by ambulance to the ED at Jichi Medical University, Saitama Medical Center between April 2009 and March 2018. The definition of severe hypoglycemia was an event requiring the assistance of another person and a plasma glucose level < 60 mg/dL (or capillary levels < 50 mg/dL) [6]. We included patients aged > 20 years who were transported to our ED with severe hypoglycemia, and we excluded patients with glucose levels ≥ 60 mg/dL or those with unknown glucose levels.
Study design
We defined HE as severe hypoglycemia with altered levels of consciousness and a score of < 12 on the Glasgow coma scale (GCS) (scores range from 3 to 15, with lower scores suggesting reduced levels of consciousness) [7]. In addition, we defined prolonged HE as comatose cases or those in a prolonged stupor for > 24 h after glucose administration. We divided the participants into two groups of those with and without HE (GCS ≥ 12). Moreover, HE patients were classified into the prolonged HE group and the recovered group. Cases with episodes of emergency transport for severe hypoglycemia or those in whom HE was suspected at least once were included in the HE group.
Data collection
We reviewed medical records and assessed the physical findings, blood tests, and clinical outcomes for all patients. Axial body temperatures were checked on arrival at the ED with an electronic thermometer. We obtained drug information and dosage for patients with diabetes mellitus.
We defined high-dose sulfonylurea as > 2 mg glimepiride, > 40 mg gliclazide, and > 1.25 mg glibenclamide.
Ethical approval
This study was approved by the Ethics Committee of the Jichi Medical University, Saitama Medical Center (No. S16-76) and conformed to the ethical guidelines of the Declaration of Helsinki. Formal consent is not required for this retrospective type of study.
Statistical analysis
Data were presented as mean ± standard deviation (SD), and skewed variables were described as median and interquartile range. We compared patient characteristics upon presentation to the ED between the two groups using the Student t-test or the Mann-Whitney U test. The Fisher’s exact test was used to compare categorical variables. All analyses were performed with EZR (Jichi Medical University, Saitama Medical Center), a graphical user interface for R (v. 2.13.0; The R Foundation for Statistical Computing, Vienna, Austria), and a modified version of the R commander (v. 1.6-3), which was designed to add statistical functions frequently used in biostatistics research [8]. P < 0.05 was considered statistically significant.
| Results | ▴Top |
Patient characteristics
Initially, we identified 182 eligible patients; however, nine were excluded because their glucose level was ≥ 60 mg/dL or unknown, for a total study enrollment of 173 patients (Fig. 1). Table 1 shows the patients’ basic characteristics. Severe hypoglycemia in other 168 patients was considerably caused by anti-hyperglycemic drugs. Figure 2a shows causative drugs for severe hypoglycemia in the overall patient population and Figure 2b shows those for type 2 diabetes. Insulin was the major causative agent in patients overall; however, sulfonylurea was the major agent in patients with type 2 diabetes. We itemized data for the other drugs in Supplementary Tables 1 and 2 (www.jocmr.org). None of the 173 patients were prescribed sodium-glucose cotransporter-2 inhibitors (SGLT2is). Three patients took glucagon-like peptide-1 receptor agonist (GLP-1RA), but one patient used GLP-1RA together with glinide and two patients used it with insulin. The sulfonylurea-induced hypoglycemic patients were significantly older than the insulin-induced hypoglycemic patients (P < 0.001). No significant difference in estimated glomerular filtration rates (eGFRs) was seen between those with insulin- and sulfonylurea-induced hypoglycemia (P = 0.083) (Table 2). Figure 3 shows the age and eGFR distribution in diabetic patients with sulfonylurea-, insulin-induced severe hypoglycemia and other hypoglycemic patients (n = 173). Patients taking sulfonylureas were older than those taking insulin; however, we could not identify a regular tendency of distribution.
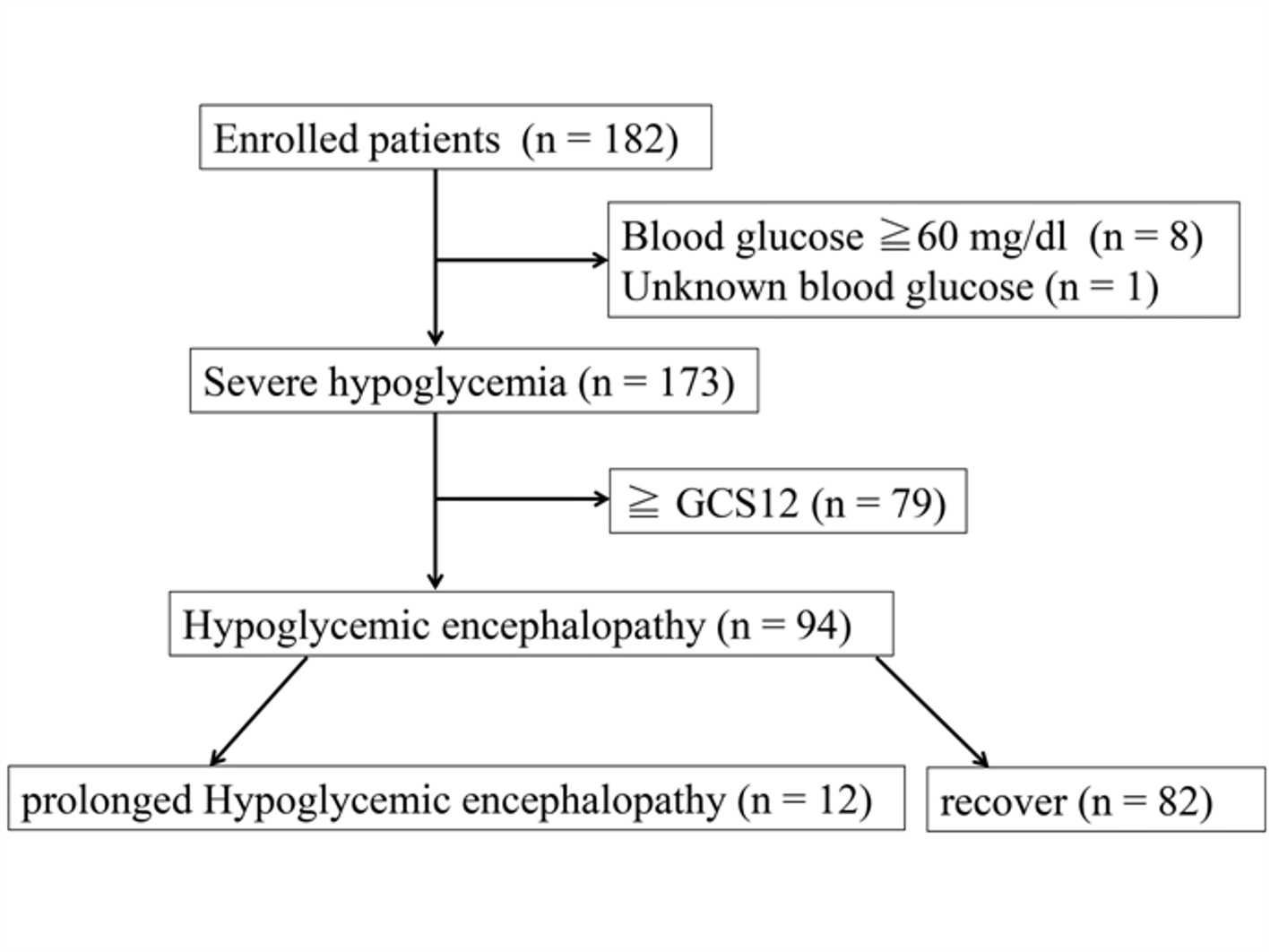 Click for large image | Figure 1. Flow chart of patient enrollment throughout the study. |
 Click to view | Table 1. Baseline Demographic and Clinical Parameters of Participants (n = 173) |
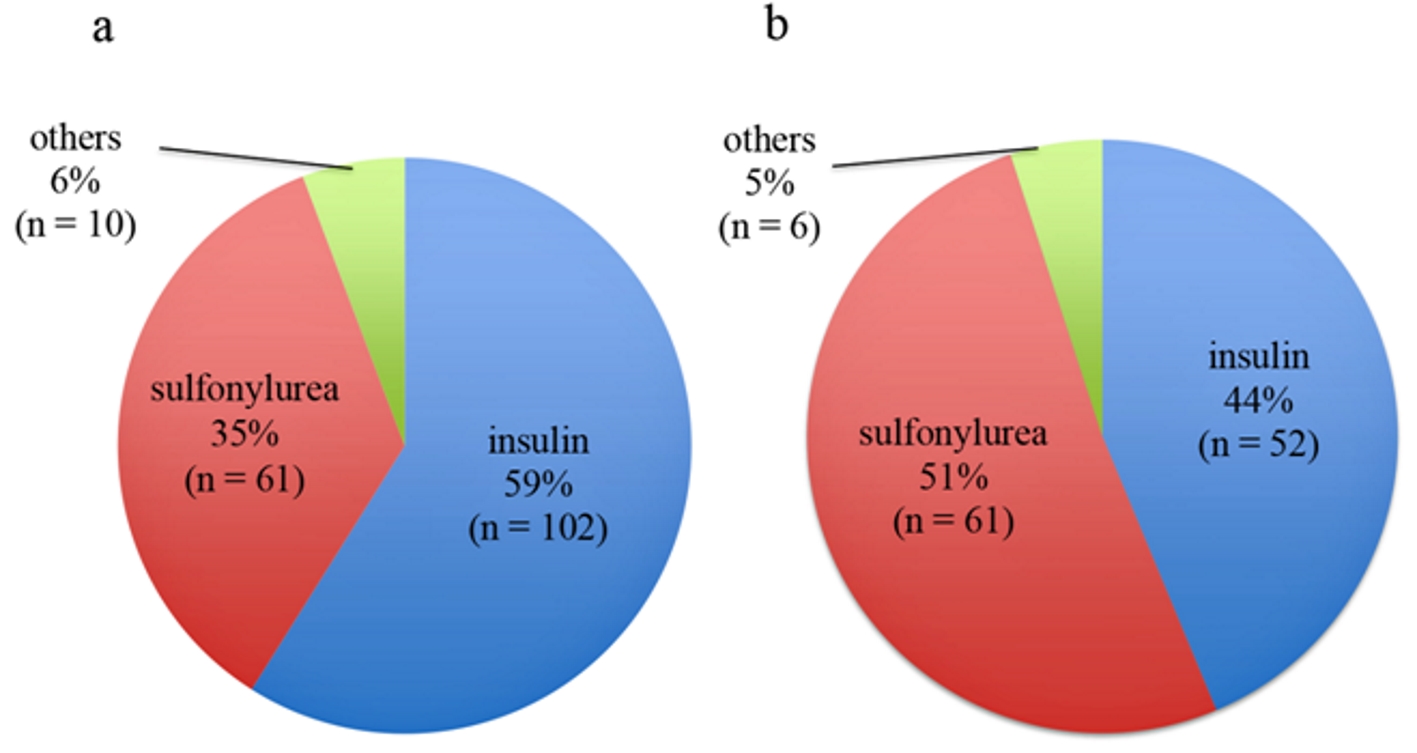 Click for large image | Figure 2. Major causative anti-hyperglycemic agents of severe hypoglycemia in the overall patient population (n = 173) (a) and those with type 2 diabetes (n = 119) (b). |
 Click to view | Table 2. Comparison of Age and Renal Function Between Insulin and Sulfonylurea Users With Severe Hypoglycemia |
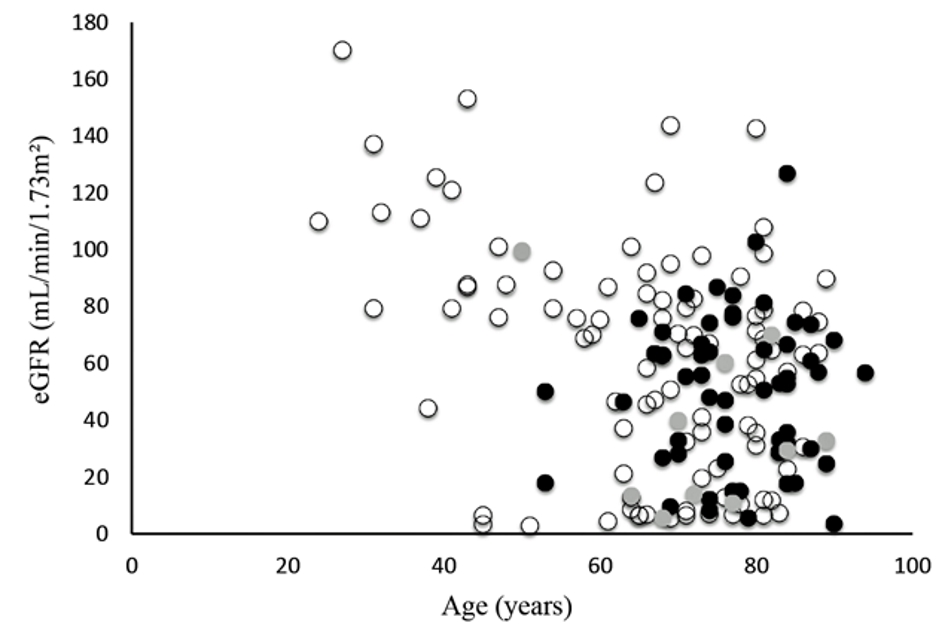 Click for large image | Figure 3. Distribution of age and eGFR in sulfonylurea- or insulin-induced severe hypoglycemia. Black circles indicate sulfonylurea-induced hypoglycemia, white circles indicate insulin-induced hypoglycemia, and gray circles indicate other severe hypoglycemic patients. |
Comparison of clinical parameters between patients with and without HE
We examined several clinical parameters between HE patients and those with severe hypoglycemia (GCS ≥ 12) (Table 3). HE patients had significantly lower blood glucose levels before glucose administration compared with non-HE patients. No significant difference was noted between causative drugs in those with or without HE (sulfonylurea, insulin, and other anti-hyperglycemic drugs) (P = 0.767). We reviewed the dosage of sulfonylureas (high or low dose) in patients with sulfonylurea-induced severe hypoglycemia, and the differences between high and low dosage of sulfonylurea were not significant with glimepiride (P = 0.554) or glibenclamide (P = 1.000). Only two patents used gliclazide and both patients had HE.
 Click to view | Table 3. Difference Between the Hypoglycemic Encephalopathy Group and the Severe Hypoglycemic Group |
Comparison of clinical parameters between prolonged and recovered HE
We examined the parameters between prolonged and recovered HE patients (Table 4), and found that blood glucose levels were significantly lower and body temperatures higher in prolonged HE patients with severe hypoglycemia.
 Click to view | Table 4. Difference in Clinical Parameters Between the Prolonged and Recovered Groups of Patients With Hypoglycemic Encephalopathy |
| Discussion | ▴Top |
In the present study, we showed that low blood glucose levels and higher body temperatures were predictors for HE in patients with severe hypoglycemia. In addition, almost all episodes of hypoglycemia were due to anti-hyperglycemic agents, and sulfonylureas in particular were a major causative factor in older patients with type 2 diabetes. Ikeda et al analyzed 165 HE patients and evaluated their clinical outcome, demonstrating that prolonged hypoglycemia, higher body temperature, and low levels of lactic acid were prognostic indicators of HE. In particular, hypothermia (< 35°) was observed more frequently in the patients with good outcomes compared to those with poor clinical outcomes [4]. Our results revealed that blood glucose levels and body temperature can affect HE outcome. Hypoglycemia is reported to induce neuronal death via mitochondrial dysfunction and oxidative stress [9, 10]. Ultimately, we considered hypoglycemia severity as a risk factor for HE and its progression. A retrospective study showed that hypothermia was observed in severe hypoglycemic patients and associated with lower body temperature and good GCS [11]. Hypothermia in hypoglycemia was considered to decrease energy demand [11]. Alternatively, Barbara et al reported that body temperature was not related to neurological outcome in hypoglycemic patients in an intensive care unit (ICU) [3]. These differences were due to patient background, disease severity, percentage of diabetes, and the department (ICU or ED). In addition, missing data might affect the results of retrospective studies; hence, further investigation is necessary to determine whether temperature significantly affects hypoglycemia or whether hypothermia may be a therapeutic option for HE patients.
In our causative drug analysis in patients with diabetes, use of sulfonylureas was a major risk factor for severe hypoglycemia, especially in older patients; however, there was no correlation with progression to HE. The patients with sulfonylurea-induced severe hypoglycemia were older compared to those taking insulin, both in the overall population of severe hypoglycemic patients and the type 2 diabetes patients. Sulfonylurea dosage was not significantly associated with renal function (eGFR), suggesting that hypoglycemia severity can be associated with HE regardless of sulfonylurea dosage. Namba et al reported that in patients with type 2 diabetes, the sulfonylurea-induced group was older and their eGFRs were lower than in the insulin-induced group (Japan Diabetes Society Scientific Survey/Research Ethics Committee) [6]. This discrepancy can be attributed to the presence of dialysis patients. Our study included 12 dialysis patients and nine were insulin users. Therefore, this could contribute to the comparatively low eGFR in the insulin-induced group. In our study, non-sulfonylurea agents (glinide, dipeptidyl peptidase-4 inhibitor, biguanide) were low risk for causing severe hypoglycemia (Supplementary Tables 1 and 2) (www.jocmr.org), consistent with a previous multicenter survey [6].
This study has several limitations that should be addressed. First, this was a single-center, retrospective cohort study; therefore, a larger number of participants are necessary. Second, we retrospectively collected data of hypoglycemic patients who were transferred to the ED; therefore, there were some missing values. In the statistical analysis, these missing data affected the result in this small number study. Finally, we only checked the axillary body temperature, and we could not investigate the air temperature at the time of arrival at the ED. The timing of transfer, whether during the day or night, and the seasons in Japan may affect the axillary body temperature.
Conclusions
Blood glucose levels before administration can be a clinical predictor of severity of altered levels of consciousness, and body temperature can be a risk factor for prolonged HE.
Conflict of Interest
The authors declare no conflict of interest.
Grant Support
This work was supported by a Grant-in-Aid for Research Activity start-up (17H07056) to H.Y. and Scientific Research (C) (18K08525) from the Japan Society for the Promotion of Science to M.Y.
| References | ▴Top |
- Ren S, Chen Z, Liu M, Wang Z. The radiological findings of hypoglycemic encephalopathy: A case report with high b value DWI analysis. Medicine (Baltimore). 2017;96(43):e8425.
doi pubmed - Jeon JY, Kim SR, Kim HJ, Kim DJ, Lee KW, Lee JD, Han SJ. Risk factors of severe hypoglycemia requiring medical assistance and neurological sequelae in patients with diabetes: A case-control study. Medicine (Baltimore). 2016;95(47):e5365.
doi pubmed - Barbara G, Megarbane B, Argaud L, Louis G, Lerolle N, Schneider F, Gaudry S, et al. Functional outcome of patients with prolonged hypoglycemic encephalopathy. Ann Intensive Care. 2017;7(1):54.
doi pubmed - Ikeda T, Takahashi T, Sato A, Tanaka H, Igarashi S, Fujita N, Kuwabara T, et al. Predictors of outcome in hypoglycemic encephalopathy. Diabetes Res Clin Pract. 2013;101(2):159-163.
doi pubmed - Johkura K, Nakae Y, Kudo Y, Yoshida TN, Kuroiwa Y. Early diffusion MR imaging findings and short-term outcome in comatose patients with hypoglycemia. AJNR Am J Neuroradiol. 2012;33(5):904-909.
doi pubmed - Namba M, Iwakura T, Nishimura R, Akazawa K, Matsuhisa M, Atsumi Y, Satoh J, et al. The current status of treatment-related severe hypoglycemia in Japanese patients with diabetes mellitus: a report from the committee on a survey of severe hypoglycemia in the Japan Diabetes Society. Diabetol Int. 2018;9(2):84-99.
doi pubmed - Koh E, Tsai LK, Hong CT. Serum calcium concentration affects signal changes on diffusion-weighted imaging in hypoglycemic encephalopathy. AJNR Am J Neuroradiol. 2012;33(2):297-300.
doi pubmed - Kanda Y. Investigation of the freely available easy-to-use software 'EZR' for medical statistics. Bone Marrow Transplant. 2013;48(3):452-458.
doi pubmed - Suh SW, Gum ET, Hamby AM, Chan PH, Swanson RA. Hypoglycemic neuronal death is triggered by glucose reperfusion and activation of neuronal NADPH oxidase. J Clin Invest. 2007;117(4):910-918.
doi pubmed - Auer RN. Progress review: hypoglycemic brain damage. Stroke. 1986;17(4):699-708.
doi pubmed - Tran C, Gatiani K, Herrmann R, et al. Hypoglycemia is a frequent sign of hypoglycemia in patients with diabetes. Diabetes Metab. 2012;38(4):370-372.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


