| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 9, Number 7, July 2017, pages 560-566
Variables to Predict Nephrological Disease in General, and Glomerulonephritis in Particular, in Patients With Microhematuria
Carsten Paul Bramlagea, b, e, Manuel Wallbacha, David Ellenbergerc, Cornelia Deutschb, Joan Minguetb, d, Katherine Helen Smithd, Johanna Stocka, Alina Goninskia, Peter Bramlageb, Michael Kozioleka, Gerhard Anton Muellera
aDepartment of Nephrology and Rheumatology, Georg-August-University of Gottingen, Gottingen, Germany
bInstitute of Pharmacology and Preventive Medicine (IPPMED), Cloppenburg, Germany
cInstitute of Medical Statistics, Georg-August-University of Gottingen, Gottingen, Germany
dInstitute for Research and Medicine Advancement (IRM), Terrassa, Spain
eCorresponding Author: Carsten Paul Bramlage, Department of Nephrology and Rheumatology, Georg-August University of Gottingen, Robert-Koch-Str. 40, 37075 Gottingen, Germany
Manuscript accepted for publication March 17, 2017
Short title: Hematuria: Prediction of Glomerulonephritis
doi: https://doi.org/10.14740/jocmr2993w
| Abstract | ▴Top |
Background: Microhematuria (MH) is a symptom frequently leading to uncertainty as to when a nephrology referral is appropriate. Because MH may be indicative of severe kidney disorders, prompt diagnosis and potential treatment initiation can be important. We aimed to identify further variables that point at a nephrological cause, in particular of glomerulonephritis (GN), when MH is diagnosed.
Methods: A retrospective analysis of data acquired from patients attending a nephrology office due to MH was performed. Demographic information and diagnostic tests were evaluated in order to identify factors that were associated with a nephrological cause.
Results: Patients with MH (n = 805) as indicated by a urine stick analysis were included. Of these, MH was confirmed by urine sediment analysis in 543 patients (67.5%). Of those, 48.3% had a nephrological cause, including 12.4% with GN and 2.9% with rapid progressive GN (RPGN). A urine dipstick finding of ≥ 250 erythrocytes per microliter, microalbuminuria and elevated leukocytes increased the probability of having a GN to 62.4%. Furthermore, the presence of microalbuminuria, GFR < 60 mL/min, history of hypertension and diabetes mellitus increased the probability for all nephrological causes to 95.4%.
Conclusion: There are a number of factors available that help to assess the need for a nephrology referral in patients with microhematuria.
Keywords: Microhematuria; Kidney disease; Diagnostic; General practice; Urinalysis
| Introduction | ▴Top |
Microhematuria (MH) is commonly defined as the presence of at least three red blood cells per field of view under a high-power lens during analysis of a properly obtained urine specimen [1]. Usually asymptomatic, it is mostly an accidental finding that is considered to be of little importance unless patients are at an advanced age and are at risk of cancer [2]. Therefore, there is uncertainty as to the extent of workup needed [3-5].
The source of MH may be located anywhere along the urinary tract, with a wide variety of possible causes. These include benign reasons such as mild trauma or infections [6], but also malignancies [7]. Furthermore, a proportion of patients presenting with MH will have a nephrological condition that requires intervention in order to delay progression to end-stage renal disease (ESRD) and dialysis [1, 8]. The presence of dysmorphic red blood cells, proteinuria, cellular casts, and/or renal insufficiency indicates a need for further investigation to establish if there is a glomerular cause [1].
It is apparent from the literature that there is a high level of uncertainty surrounding the usefulness and accuracy of MH to predict serious nephrological disorders. This is particularly true among the majority of office-based physicians, which rarely have the means to perform urine sediment microscopy, and thus insufficient information to make a decision on the need for a nephrology referral [9, 10]. Clarification of this situation is essential for enabling accurate and rapid diagnosis.
We therefore carried out a retrospective evaluation of patients presenting with MH attending our nephrology office. The aim of the analysis was to identify factors that are routinely determined by the office-based physician and increase the likelihood of a nephrological cause of MH. Such information would improve the identification of patients that would benefit from early referral to a nephrologist, allowing for rapid diagnosis and initiation of appropriate treatment [8].
| Methods | ▴Top |
Study design
We carried out a retrospective analysis of data from patients who presented at our nephrology office between 1998 and 2014. All patients with MH in a prior urine test strip analysis were included. No further inclusion or exclusion criteria were applied.
Documentation
For all patients, demographic information was collected, along with the result of urine test strip analysis. Further data were recorded for those patients that tested positive for MH in the urine sediment analysis, being defined as ≥ 3 erythrocytes per visual field under a high power lens. A full medical history was taken, including the intake of relevant drugs (anticoagulation and NSAID), and additional diagnostic testing was documented. The estimated glomerular filtration rate (eGFR) was calculated using the CKD-EPI formula, and signs of a urinary tract infection (leukocyturia and nitrite) were recorded. Coagulation abnormalities (prothrombin time (PTT), Quick, international normalized ratio (INR), and thrombocytes) and systemic inflammation were evaluated.
The potential sources of the MH were taken from the patient files, which included the results of all diagnostic tests, including the kidney biopsy if one had been performed.
The study was conducted according to the principles of the Declaration of Helsinki.
Statistical analysis
Data were presented as means with standard deviations (SDs) and range or absolute values with percentages. Boosted regression was used to preselect variables affecting the likelihood of a nephrological cause (or glomerulonephritis (GN) specifically). The association analysis was based on patients with all necessary data available (n = 339 complete cases).
Logistic regression with Firth’s penalised likelihood approach was used in order to reduce potential bias caused by low event rates. Univariate and multivariate effect estimates (OR) along with 95% confidence intervals (CIs) were given. Furthermore effect measures on the log OR were dichotomised to create a risk score. This model was then recalibrated based on the total risk score sums to get appropriate values for the probability of an event, with the area under the curve (AUC) as a goodness-of-fit measure. A P-value of < 0.05 was considered to be statistically significant. All analyses were performed using the software Statistica 12 (Statsoft, Hamburg, Germany) and R (R Foundation, Vienna, Austria).
| Results | ▴Top |
Patient characteristics and MH
A total of 805 patients that presented at the nephrology office due to an office-based physician diagnosis of MH were included in the study. The mean age of this population was 56.3 ± 18.1 years (range: 6 - 91 years) (Table 1). At the time of the first nephrology visit, urine test strip analysis identified 90.3% of the 805 patients with ≥ 10 erythrocytes per microliter, and the remaining proportion with fewer than 10 erythrocytes per microliter. Macroscopic hematuria was documented for 4.6% of the 805 patients and chronic haematuria for 2.5% (Table 2).
 Click to view | Table 1. Patient Characteristics |
 Click to view | Table 2. Characteristics of Hematuria |
MH was confirmed by urine sediment analysis in 543 (67.5%) patients. Thus, a total of 262 patients (32.5%) were determined to have transient MH or false positive urine test strip analysis (Table 2).
For the patients with MH confirmed by the urine sediment analysis, 66.1% (359/543) of patients had a history of arterial hypertension, 32.8% (178/543) hyperlipidemia and 15.8% (86/543) diabetes mellitus (type 1 or 2).
The eGFR was found to be below the normal value of 90 mL/min in 74.1% of the patients with 47.1% (236/502) having an eGFR value below 60 mL/min (Table 1).
Underlying causes of MH
A potential nephrological cause of MH was identified for 48.3% (262/543) of patients (Table 3). The underlying disease was determined to be GN in 12.4% (68/543) of patients, with this diagnosed as RPGN in 2.9% (16/543). Interstitial nephritis, generally attributed to medication use, was found in 6.1% (33/543) of patients. High proportions of patients were found to have a first diagnosis of hypertensive (26.5%; 144/543) or diabetic (9.4%; 51/543) nephropathy.
 Click to view | Table 3. Potential Sources of MH Identified in the Nephrology Office (Multiple Selections Are Possible) |
Only 27.1% (147/543) of patients had a single potential cause, with multiple reasons particularly common for the patients with renal diseases that are not normally associated with MH, such as hypertensive and diabetic nephropathy.
The most frequent concomitant cause of MH was urological (20.8%; 113/543), with 54.0% also presenting with a nephrological disorder. Further concomitant reasons were systemic inflammation (22.8%; 124/543), urinary tract infections (10.1%; 55/543) and coagulation abnormality (11.8%; 64/541), with 8.1% (44/543) being treated with anticoagulants or anti-platelet agents (17.9%; 97/543) (Table 3).
Relationship between MH and a diagnosis of kidney disease
Kidney disease was diagnosed in 262 (48.3%) of the 543 patients that were found to have MH in the urine sediment analysis. In univariate analysis, a patient age of ≥ 40 years was associated with a nephrological disease, as was male gender and a BMI of > 30 kg/m2 (Table 4). In addition, a history of hypertension, blood pressure > 140/90 mmHg, eGFR impairment, diabetes mellitus (type 1 and 2), and elevated systemic inflammation were associated with kidney disease in our cohort.
 Click to view | Table 4. Relationship Between MH and Renal Disease |
Factors associated with a patient that had a nephrological cause of MH were determined by selecting variables found to be statistically significant in multivariable logistic regression, while also being available in the daily physician office. The variables identified were a history of hypertension (OR: 3.98; 95% CI: 2.16 - 7.77; P < 0.001), eGFR < 60 mL/min (OR: 5.17; 95% CI: 3.05 - 9.21; P < 0.001), the presence of microalbuminuria (OR: 3.76; 95% CI: 2.20 - 6.75; P < 0.001), and diabetes mellitus (type 1 or 2; OR: 2.84; 95% CI: 1.29 - 7.24; P = 0.013). The number of these indicators that are present can be regarded as a risk score for having a nephrological disease (AUC = 0.850), with an estimated probability of 95.4% whenever all four indicators are present (Fig. 1).
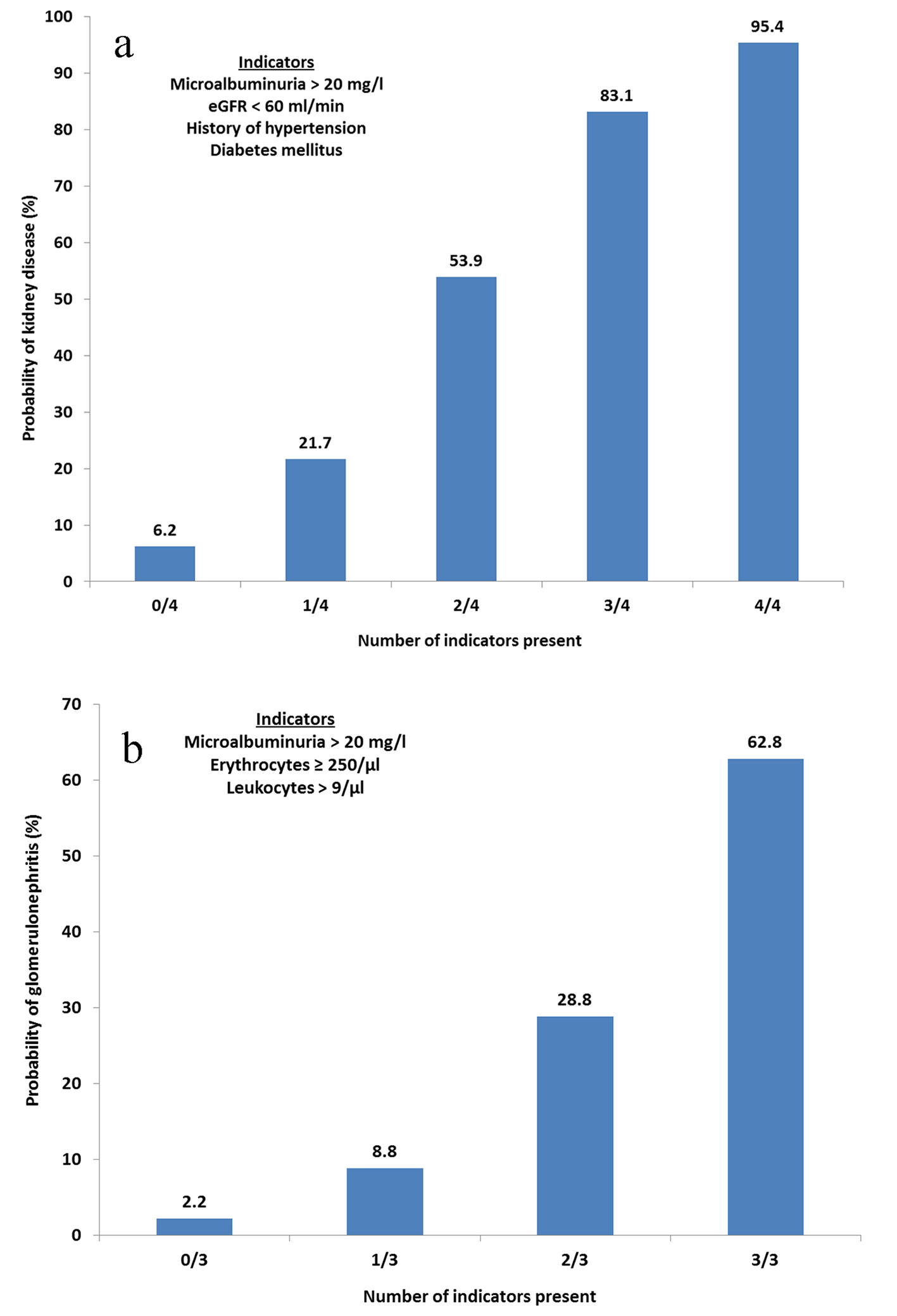 Click for large image | Figure 1. (a) Probability of kidney disease based on clinical findings. Calculated probabilities of kidney disease based on factors found to be significant in the multivariate logistic regression. Any combination of indicators can be present. eGFR, estimated glomerular filtration rate. (b) Probability of glomerulonephritis based on clinical findings. Calculated probabilities of glomerulonephritis based on factors found to be significant in the multivariate logistic regression. Any combination of indicators can be present. Erythrocyte concentration determined by urine stick analysis. |
In the univariate analysis (328 complete cases), the likelihood of gomerulonephritis specifically was found to be higher for patients with a urine test strip results of ≥ 250 erythrocytes/µL (OR: 5.09; 95% CI: 2.44 - 7.01; P < 0.001), those with microalbuminuria (OR: 6.79; 95% CI: 2.93 - 21.8; P < 0.001), and those with elevated leukocytes (≥ 10/µL; OR: 2.31; 95% CI: 1.28 - 4.02; P = 0.004). The cut-off of ≥ 10 leukocytes/µL was chosen from the 80th percentile of the data as a cut-off value of ≥ 11/µL yielded too few elevated cases (Table 4). The quantity of these three indicators that are present can be regarded as a risk score for having GN (AUC = 0.773), with an estimated probability of 62.8% in the case of all three indicators being present (Fig. 1).
| Discussion | ▴Top |
In order to identify factors that may indicate the need for a referral to a nephrologist for an individual presenting with MH, we retrospectively analyzed data from patients attending our nephrology office. Certain factors were found to be associated with a greater likelihood of the presence of an underlying nephrological cause of the patient’s MH. From the collated data, we calculated probabilities of nephrological disease in general and GN specifically, which could be used by an office-based physician when determining the necessity for a nephrology referral.
GN was a potential source of MH for 12.4% of patients, with 2.9% of these cases determined to be rapid progressive GN, a condition that can result in ESRD and a need for dialysis within a few weeks of its development. It is for these patients that a prompt consultation with a nephrologist is the most important. A urine test strip finding of ≥ 250 erythrocytes/µL, microalbuminuria, and elevated leukocyte count were all associated with GN, with a calculated probability of this condition being present of 62.8% if all three indicators were found. These could therefore be important parameters to indicate the presence of a serious nephrological disorder to the office-based physician, possibly even before a decrease in kidney function/eGFR becomes apparent. All of these variables are readily available to the office-based physician.
Interestingly, a high proportion of patients with MH were diagnosed as having hypertensive or diabetic nephropathy. These glomerulopathies are not normally associated with MH, thus, it is likely that these patients had an additional source. Indeed, approximately 40% of the patients included in our analysis had their MH attributed to more than one potential cause. This includes the use of anticoagulants or anti-platelet agents, infections and urological diseases. Therefore, although hypertensive or diabetic nephropathy is unlikely to be the sole cause of the MH, the presence of hypertension or diabetes should indicate to the office-based physicians that a nephrology referral may be warranted.
It should be noted that hypertensive nephropathy is over-represented in our cohort. While few of these patients had a kidney biopsy, Perkowska-Ptasinska et al. showed a low prevalence of hypertension-related lesions in the biopsies they performed on their nephrology patients, a high proportion of whom had hypertension [11]. Therefore, in patients presenting with MH and elevated blood pressure, other renal diseases have to be ruled out
The presence of dysmorphic erythrocytes or cellular casts is a reliable indicator of a glomerular cause of the MH [12-14]. However, many nephrological conditions present with erythrocytes of unaltered morphology [15]. In addition, office-based physicians often do not have the facilities to perform urine sediment microscopy to make a decision regarding the need for a nephrology referral [10]. Thus, we decided to include all patients with a renal cause of MH, independent of its characteristics, in the statistical analysis and development of the probability scores. In order to aid the physician in their decision-making process, we statistically determined the individual risks associated with parameters that are routinely available in general clinical practice. Microalbuminuria, an eGFR below 60 mL/min, a history of hypertension, and the presence of diabetes mellitus type 1 or 2 were found to be highly associated with kidney disease. The probability of a nephrological disorder increased with the number of these indicators that were present, reaching approximately 95% when all four were found. Whilst we do not go so far as to recommend a cut-off point for a nephrology referral, this system provides useful guideline for the office-based physician. Validation of the model in a large cohort of patients displaying MH in a clinical practice setting may provide an estimation of an appropriate risk level at which a patient should be referred to a nephrologist.
Limitations
One limitation to the present analysis is its retrospective nature. In addition, only patients with MH were included and there was no comparison to patients without MH. This analysis was based on the data of a single office rather than being a multi-center study. Furthermore, there are no data available from office-based physicians that may have seen these patients prior to the nephrology visit. Such information would have allowed for analysis of the appropriateness of current referral practices and adherence to expert guidelines.
Conclusions
In a cohort of patients attending a nephrology clinic due to a finding of MH, a number of factors were found to be associated with the presence of the condition, including hypertension, diabetes mellitus, microalbuminuria, and a reduced eGFR. Furthermore, a urine dipstick finding of ≥ 250 erythrocytes per microliter, microalbuminuria, and elevated leukocytes were independently associated with the presence of GN specifically. In order to aid office-based physicians to determine when a nephrology referral is necessary for a patient with MH, we calculated probabilities of a nephrological disease being present, based on variables routinely available in clinical practice.
Acknowledgments
We thank Katrin Bramlage and Laura Brockmann for their help in data sampling.
Conflicts of Interest
None.
| References | ▴Top |
- Davis R, Jones JS, Barocas DA, Castle EP, Lang EK, Leveillee RJ, Messing EM, et al. Diagnosis, evaluation and follow-up of asymptomatic microhematuria (AMH) in adults: AUA guideline. J Urol. 2012;188(6 Suppl):2473-2481.
doi pubmed - Elias K, Svatek RS, Gupta S, Ho R, Lotan Y. High-risk patients with hematuria are not evaluated according to guideline recommendations. Cancer. 2010;116(12):2954-2959.
doi pubmed - Jung H, Gleason JM, Loo RK, Patel HS, Slezak JM, Jacobsen SJ. Association of hematuria on microscopic urinalysis and risk of urinary tract cancer. J Urol. 2011;185(5):1698-1703.
doi pubmed - Madeb R, Golijanin D, Knopf J, Davis M, Feng C, Fender A, Stephenson L, et al. Long-term outcome of patients with a negative work-up for asymptomatic microhematuria. Urology. 2010;75(1):20-25.
doi pubmed - Ogasawara M, Aoki K, Matsuura E, Kunimatsu M, Ohkubo I, Galli M, Sasaki M, et al. Anticardiolipin antibodies in patients with pregnancy loss induce factor Xa production in the presence of beta 2-glycoprotein I. Am J Reprod Immunol. 1995;34(5):269-273.
doi pubmed - Jaffe JS, Ginsberg PC, Gill R, Harkaway RC. A new diagnostic algorithm for the evaluation of microscopic hematuria. Urology. 2001;57(5):889-894.
doi - Kaufman DS, Shipley WU, Feldman AS. Bladder cancer. Lancet. 2009;374(9685):239-249.
doi - Smart NA, Dieberg G, Ladhani M, Titus T. Early referral to specialist nephrology services for preventing the progression to end-stage kidney disease. Cochrane Database Syst Rev. 2014;(6):CD007333.
doi - De Coster C, McLaughlin K, Noseworthy TW. Criteria for referring patients with renal disease for nephrology consultation: a review of the literature. J Nephrol. 2010;23(4):399-407.
pubmed - Kelly JD, Fawcett DP, Goldberg LC. Assessment and management of non-visible haematuria in primary care. BMJ. 2009;338:a3021.
doi pubmed - Perkowska-Ptasinska A, Deborska-Materkowska D, Bartczak A, Stompor T, Liberek T, Bullo-Piontecka B, Wasinska A, et al. Kidney disease in the elderly: biopsy based data from 14 renal centers in Poland. BMC Nephrol. 2016;17(1):194.
doi pubmed - Crop MJ, de Rijke YB, Verhagen PC, Cransberg K, Zietse R. Diagnostic value of urinary dysmorphic erythrocytes in clinical practice. Nephron Clin Pract. 2010;115(3):c203-212.
doi pubmed - Wollin T, Laroche B, Psooy K. Canadian guidelines for the management of asymptomatic microscopic hematuria in adults. Can Urol Assoc J. 2009;3(1):77-80.
doi pubmed - Kohler H, Wandel E, Brunck B. Acanthocyturia - a characteristic marker for glomerular bleeding. Kidney Int. 1991;40(1):115-120.
doi pubmed - Heine GH, Sester U, Girndt M, Kohler H. Acanthocytes in the urine: useful tool to differentiate diabetic nephropathy from glomerulonephritis? Diabetes Care. 2004;27(1):190-194.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


