| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Review
Volume 9, Number 4, April 2017, pages 266-272
Does Accidental Overcorrection of Symptomatic Hyponatremia in Chronic Heart Failure Require Specific Therapeutic Adjustments for Preventing Central Pontine Myelinolysis?
Renato De Vecchisa, f, Michel Noutsiasb, Carmelina Arianoa, c, Arturo Cesarod, Carmela Cioppaa, Anna Giasia, Nicola Maureae
aCardiology Unit, Presidio Sanitario Intermedio “Elena d’Aosta”, ASL Napoli 1 Centro, via Cagnazzi 29, 80137 Napoli, Italy
bDepartment of Internal Medicine I, Division of Cardiology, Pneumology, Angiology and Intensive Medical Care, University Hospital Jena, Friedrich-Schiller-University Jena, Erlanger Allee 101, D-07747 Jena, Germany
cDivision of Cardiology, Casa di Cura “Sollievo della Sofferenza”, viale Cappuccini 2, 71013 San Giovanni Rotondo, Italy
dDepartment of Cardiology, Second University of Napoli, Monaldi Hospital, via Leonardo Bianchi, 1, 80131 Napoli
eDivision of Cardiology, Istituto Nazionale per lo Studio e la Cura dei Tumori,“Fondazione Giovanni Pascale” IRCCS, via Mariano Semmola, 52, 80131 Napoli, Italy
fCorresponding Author: Renato De Vecchis, Via P.Gaurico 21, 80125 Napoli, Italy
Manuscript accepted for publication February 10, 2017
Short title: Hyponatremia Overcorrection in CHF
doi: https://doi.org/10.14740/jocmr2933w
- Abstract
- Introduction
- Epidemiological Aspects of Hyponatremia
- Prognostic Correlates of Hyponatremia
- Pathophysiology of Hyponatremia in the Course of CHF
- Symptomatic Hyponatremia: General Concepts
- Case Description
- Discussion
- Conclusions
- References
| Abstract | ▴Top |
This review aims at summarizing essential aspects of epidemiology and pathophysiology of hyponatremia in chronic heart failure (CHF), to set the ground for a practical as well as evidence-based approach to treatment. As a guide through the discussion of the available evidence, a clinical case of hyponatremia associated with CHF is presented. For this case, the severe neurological signs at presentation justified an emergency treatment with hypertonic saline plus furosemide, as indicated. Subsequently, as the neurological emergency began to subside, the reversion of the trend toward hyponatremia overcorrection was realized by continuous infusion of hypotonic solutions, and administration of desmopressin, so as to prevent the very feared risk of an osmotic demyelination syndrome. This very disabling complication of the hyponatremia correction is then briefly outlined. Moreover, the possible advantages related to systematic correction of the hyponatremia that occurs in the course of CHF are mentioned. Additionally, the case of tolvaptan, a vasopressin receptor antagonist, is concisely presented in order to underline the different views that have led to different norms in Europe with respect to the USA or Japan as regards the use of this drug as a therapeutic resource against the hyponatremia.
Keywords: Hyponatremia; Chronic heart failure; Therapy; Central pontine myelinolysis
| Introduction | ▴Top |
Mild to moderate hyponatremia (serum Na+ ranging from 120 to 135 mEq/L) in chronic heart failure (CHF) is associated with significantly reduced survival and can also be accompanied by various neurological symptoms, including postural instability, gait disorders, attention deficit and increased risk of falls and large bone fractures. Despite the ability to effectively normalize depressed serum sodium levels demonstrated for tolvaptan, i.e., the first-in-class drug among the vasopressin receptor antagonists, it did not prove to induce any significant increase in survival of CHF patients [1]. Thus, it was approved in European countries exclusively for euvolemic hyponatremia (essentially the syndrome of inappropriate secretion of antidiuretic hormone (SIADH), whether consequent to drugs (thiazide diuretics, selective serotonin reuptake inhibitors (SSRIs)), or arising from cerebral or pulmonary neoplasms or infections). Therefore, chronic mild-moderate hyponatremia in CHF patients, when associated with neurological symptoms, is currently treated in Europe with intravenous (IV) hypertonic saline solution (HSS), combined with IV furosemide.
| Epidemiological Aspects of Hyponatremia | ▴Top |
Classically hyponatremia is defined as a serum sodium concentration (serum Na+) < 135 mEq/L [2]: values of serum Na+ ranging from 135 to 130 mEq/L identify a condition of mild hyponatremia, values between 129 and 125 mEq/L are indicative of a moderate hyponatremia, while values of serum Na+ < 125 mEq/L identify a state of severe hyponatremia [3]. Furthermore, according to the common meaning, hyponatremia is defined as chronic when it has a duration > 48 h, e.g., the one commonly seen in the course of CHF. In general terms, hyponatremia appears to be the most common electrolyte disorder at the time of the first visit, irrespective of the clinical context (territorial, specialized ambulatory or hospital). For example, a study including more than 70,000 individuals, who had accessed the facilities of the emergency room, found that approximately 10% of these subjects had hyponatremia at admission [4]. However, a value of serum Na of 121 mEq/L, namely placed under the threshold, i.e., 125 mEq/L, beyond which the neurological symptoms caused by hyponatremia are relatively frequent, was seen only in 0.2% of these patients. In some diseases (cancer, heart failure, and cirrhosis of the liver in the stage of ascitic decompensation), hyponatremia is particularly prevalent [5]. For example, a recent study, based on laboratory data from about 4,700 hospitalizations concerning more than 3,000 cancer patients, reported a prevalence of hyponatremia at admission of 46% and highlighted an intra-hospital development of hyponatremia equal to 24% [6]. The majority of patients had mild or moderate hyponatremia, while severe hyponatremia accounted only for 1% of cases. Hyponatremia in cancer patients is mainly due to an SIADH. The tumors of the head and neck, breast and lung cancers are the most common malignancies in which a paraneoplastic SIADH coexists; in particular, in patients with small cell lung cancer, an SIADH is present in about 11-15% of cases.
In the vast majority of studies conducted in patients with heart failure, the prevalence of hyponatremia ranged from 20% to 25% [7-9]. Furthermore, even higher prevalence and incidence were observed in patients with clinical relapse of acute heart failure [10], in which hyponatremia was present at hospital admission in 38% of cases or occurred during hospital stay in 28% of patients.
| Prognostic Correlates of Hyponatremia | ▴Top |
Hyponatremia has a negative effect on prognosis, irrespective of the clinical context - hospital or outpatients’ clinic - in which it occurs. For example, in the NHANES study, subjects with apparently asymptomatic hyponatremia belonging to the general population exhibited a risk of all-cause death, after adjustment for age, sex and major comorbidities, which was approximately 2.4 times that of the normonatremic subjects [11]. This risk extended to subjects with mild hyponatremia in other general population studies [12]. Notably, in a retrospective study comprising over 20,000 patients referred to the emergency room, even hyponatremia associated with diuretic treatment appeared associated with a significantly increased risk of death after adjusting for age, gender and estimated glomerular filtration rate (eGFR) [13].
Even mild hyponatremia significantly increases the risk of death not only during hospitalization, but also at 1- and 5-year follow-ups [14]. Moreover, in the above-mentioned study, this risk was particularly high in patients with cardiovascular (CV) diseases, advanced malignant neoplasms and in those undergoing orthopedic surgery. By contrast, hyponatremia correction was accompanied by a lower risk of in-hospital death at a follow-up of 30 days [15, 16], as well as at 1 and 5 years [17], compared to patients with persistent hyponatremia in the course of hospital stay. Recently it was also shown that the development and progression of hyponatremia during hospital stay in patients hospitalized for acute exacerbation of CHF, but with normal serum Na+ at admission, were strongly correlated with an increase in both all-cause death and CV death.
Because most of the studies in the literature are observational, a cause-effect relationship between hyponatremia and mortality is still matter of debate [18]. In fact, a reduced serum Na+ may represent both a risk marker, as commonly associated with chronic conditions such as congestive heart failure, liver cirrhosis, malignant neoplasms, etc., as well as a causative factor, that would be implied in the genesis or aggravation of the clinical picture - directly, because of the serious damage caused to the central nervous system (CNS), when hyponatremia acutely occurs, or indirectly, when adverse effects on CNS arise as a consequence of a too rapid pharmacological correction of hyponatremia [19]. Finally, sufficient attention is not paid to mild to moderate chronic hyponatremia. In fact, this disorder is very often underestimated because it is apparently asymptomatic, but in fact the mild hyponatremia is associated with postural instability, attention deficit and increased risk of falls [20].
| Pathophysiology of Hyponatremia in the Course of CHF | ▴Top |
In CHF, hyponatremia can typically occur in response to a state of reduced effective arterial blood volume, i.e., a condition of arterial underfilling [21]. Notably, in heart failure, arterial underfilling occurs in despite of a larger total blood volume that ensues from increased circulating volume in the venous system. At the level of the neuroendocrine cells in the supraoptic and paraventricular nuclei of the hypotalamus, the decreased effective arterial blood volume in CHF patients provokes non-osmotic release of arginine-vasopressin (AVP), that is added to the concomitant activation of sympathetic nervous system (SNS) and renin-angiotensin-aldosterone system (RAAS). AVP secretion in response to arterial underfilling is a mechanism that would intend to maintain volume homeostasis. In effect, by responding to changes in plasma tonicity as well as to the decrease in effective arterial circulation irrespective of plasma osmolality, AVP plays a major role in keeping volume homeostasis, through its interaction with receptors on cells in the renal colletting ducts, termed V2 receptors. In addition, AVP exerts its action also through non-osmotic receptors on vascular smooth muscle cells (V1a receptors) in order to keep the vascular tone. Hyponatremia, when it is present, arises from a hemodilution state consequent to the inhibition of free water clearance in the renal collecting ducts, triggered by increased serum AVP levels. In our opinion, the term “hypervolemic hyponatremia” applied to congestive CHF really is a rather misleading interpretation, since in this condition an increased blood volume is contained only in the venous system, thereby causing systemic venous hypertension that leads to chronic interstitial extravasation and related peripheral edema, while the effective arterial blood volume is really decreased owing to low cardiac output and a slowing down in circulation time [21]. In the pathogenesis of hyponatremia, a controversial role is played by diuretic and vasodilator treatments because these drugs can ameliorate cardiac performance and alleviate the symptoms of cardiac overload but they do not improve per se a preexisting condition of arterial underfilling [22]. Indeed, both diuretics and vasodilators have the potential for generating hypotension and relative arterial underfilling, thus inducing further AVP release. Particularly, hyponatremia is likely to be mostly propitiated by erroneous and/or overzealous diuretic therapy. Therefore, further impairment in effective arterial blood volume has frequently been blamed on excessive or inappropriate diuretic therapy, resulting in the worsening of renal flow and drop in GFR [23]. Both reduced GFR and stimulation of the thirst mechanism by angiotensin II may elicit the occurrence of hyponatremia. However, the pathogenesis of hyponatremia in edematous patients is still controversial and has not been completely clarified yet. Particularly, some authors argue in favor of a causative role of particular biohumoral patterns (poorly controlled RAAS overactivation, excess of BNP release [24], and relative adrenal insufficiency [25]) and disputable therapeutic approaches (intensive IV diuretic therapy, and thiazides [26]), concerning both the pathogenesis and persistence over time of this electrolyte trouble.
| Symptomatic Hyponatremia: General Concepts | ▴Top |
The symptoms associated with hyponatremia are the consequence of cerebral edema caused by the passage of water from the hypotonic extracellular fluid inside neuronal cells. Although most cases are asymptomatic, hyponatremia can cause neurologic symptoms that include headache, nausea, vomiting, muscle cramps, gait disturbances, dullness, disorientation, and lethargy. If the plasma sodium concentration is reduced rapidly or substantially, more severe manifestations may arise such as depression of reflexes, seizures, herniation of the brainstem, coma, and respiratory arrest (Fig. 1).
 Click for large image | Figure 1. Symptoms of hyponatremia depend on the extent of the electrolytic disorder, but also on the rapidity with which it occurs. |
| Case Description | ▴Top |
A patient presented to the emergency department with CHF and symptomatic hyponatremia. The serum Na+, measured on admission, was 98 mEq/L. The patient was staggering and was having difficulty with her motor function. She was unable to walk and her speech was difficult to understand.
Clinical history
The patient was a 74-year-old woman, smoker (20 cigarettes per day), suffering from chronic alcoholism, slightly obese (BMI = 31.5), with rheumatic mitral steno-insufficiency treated with biological prosthetic valve 5 years earlier, affected by chronic atrial fibrillation since about 2 years requiring warfarin therapy, with the dose adjusted according to INR measurement executed every 2 weeks, plus digoxin 0.125 mg once daily and enalapril 10 mg per day coupled with bisoprolol 5 mg twice daily. She reported repeated episodes of dyspnea on exertion, for which she received the diagnosis of CHF in NYHA class II. She also assumed fluoxetine 10 mg once daily because of depressive syndrome with phobic traits. Moreover, she was under treatment for new onset hypertension (association of hydrochlorothiazide 25 mg plus amiloride 2.5 mg per day, namely half tablet of Moduretic per day).
Physical exam
Physical exam showed dyspnea on exertion (NYHA class II), PA 180/95 mm Hg, arrhythmic heart sounds from atrial fibrillation with average ventricular response of 100 beats/min, apical holosystolic murmur, jugular venous distention, bilateral pulmonary rales, and bilateral leg edema.
Her echocardiographic left ventricular ejection fraction was 48% and there was no echocardiographic evidence of prosthetic valve malfunction. The laboratory results revealed a substantially decreased serum Na+ (98 mEq/L). Contrary to the signs and cardiac symptoms dating back to a lot of time earlier, the recent onset of neurological symptoms such as the postural instability and disorders of speech prompted us to explore the possibility of a superimposed neurological degenerative disease or a new onset ischemic CV event with atypical clinical presentation. However, the absence of signs of pyramidal lesions and lack of signs or symptoms attributable to a degenerative disease of the basal ganglia (Parkinson’s), together with the non-significant findings inferred from the cerebral computed tomography (CT) scan, induced us to give value to the concomitant finding of a low level of serum sodium (98 mEq/L). Such a severe hyponatremia had never previously emerged on the basis of medical records concerning previous hospitalizations.
Management of hyponatremia
A significant increase in serum sodium was scheduled to be achieved during a presumptive period from 3 to 4 days. It was decided that the upper limit for the speed of correction of hyponatremia should not have exceeded 6 mEq/L per day. Therapy consisted of 100 mL of IV 3% hypertonic saline twice daily plus two small IV infusions of furosemide (40 mg in 100 mL of aqueous vehicle to be administered in 160 min: 15 mg/h) twice daily.
This relatively cautious approach was planned according to specific recommendations reported in the literature [3], aimed to prevent a too rapid correction of the chronic hyponatremia, that has potential for generating the so-called osmotic demyelination syndrome (ODS) or central pontine myelinolysis (CPM) [27, 28], that is the most important type of demyelination syndrome associated with too rapid correction of a state of hyponatremia. CPM is heterogeneous and depends on the regions of the brain involved. A succinct synopsis of its symptoms is reported in Table 1.
 Click to view | Table 1. Osmotic Demyelination Syndrome (Otherwise Termed “Central Pontine Myelinolysis”): Main Prodromes and Symptoms |
During the first 48 h of hospitalization, the patient gradually retrieved normal neurological conditions, and restarted to eat and intake liquids, although not regularly; thus, considering also the good clinical result, the infusion of hypertonic saline (NaCl 2 M, i.e., 11.6% NaCl; 10 mL vials; 5 vials in electric syringe, 7.5 mL/h plus furosemide given as slow IV boluses) was continued. The upper limit for the speed of correction of hyponatremia was established not to be superior to 6 mEq/24 h.
Hyponatremia was in this case considered chronic, with possible overlap of an acute episode in a patient at higher risk of osmotic myelinolysis for the presence of chronic alcoholic abuse and advanced age. As apparent from the graph (Fig. 2), however, the slope of the straight line (dashed green line) that identifies the daily rate of effective correction of serum sodium (points in blue) appears much steeper compared to the slope of the straight line (dashed blue line) that identifies instead the ideal correction speed, as programmed in view of the high risk of myelinolysis postulated for this patient.
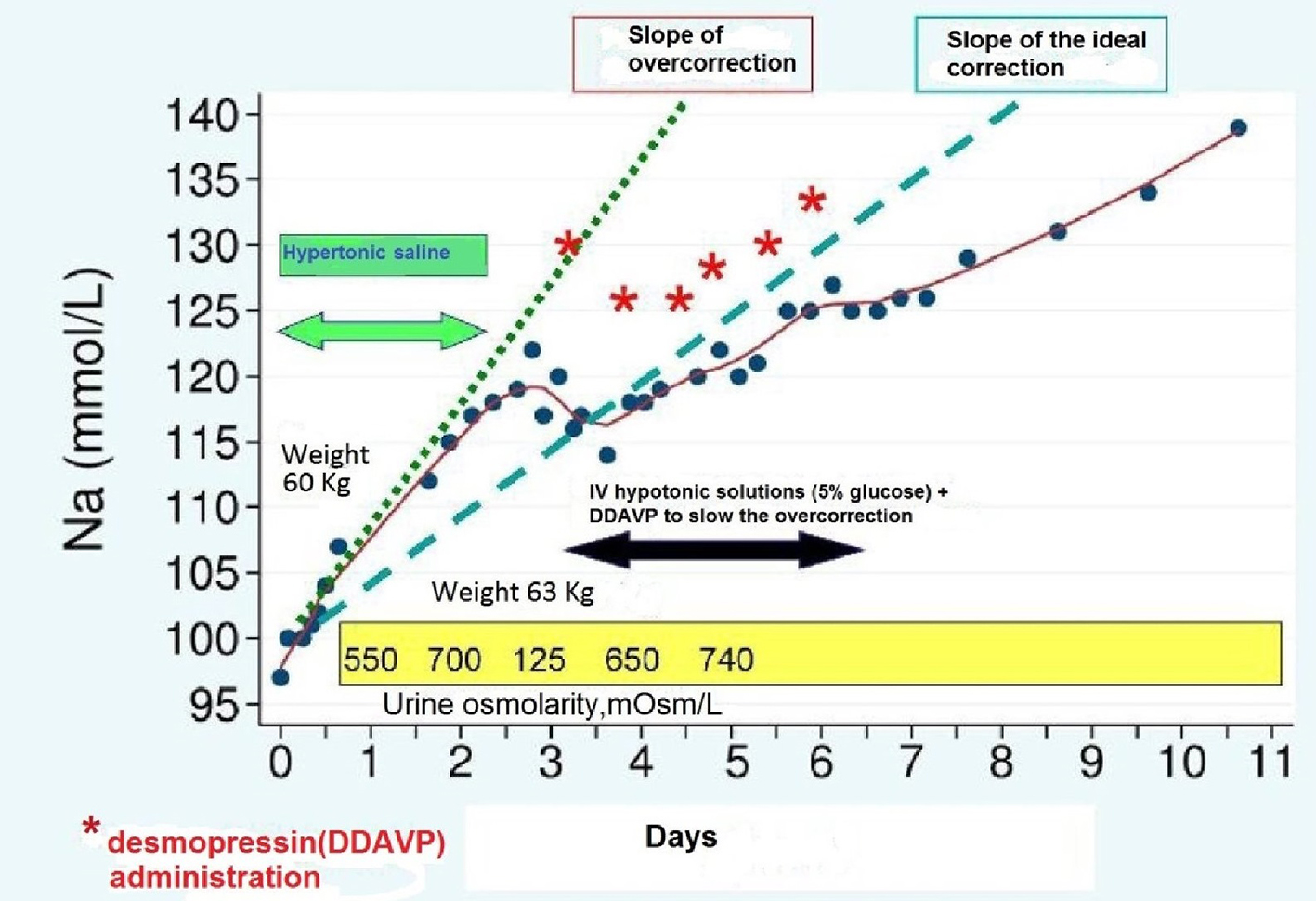 Click for large image | Figure 2. Correction speed of the serum Na+ in the various phases of therapy. |
Consequently, 48 h after admission, the patient serum sodium concentration became equal to 122 mEq/L (12 mEq/L higher than the expected target). This was attributed to the combination of hypertonic saline infusion and rapid recovery, albeit not maximal, in the capacity of urine dilution (judging by the relatively rapid transition from 700 to 125 mOsm/L), after rapid cancellation of the effect on ADH exerted by the SSRI drug (fluoxetine) and owing to the interruption of thiazide assumption. Moreover, a partial recovery of effective circulating blood volume cannot be excluded, due to increased spontaneous introduction of liquids and foods by the patient. For this reason, it was decided to reduce the speed of correction of serum sodium using hypotonic fluids (NaCl 0.45% solution) and desmopressin, the latter being given at doses of 2 μg per every subcutaneous injection (asterisks in red). This entailed the realignment of serum sodium on the straight line which expresses a speed of more appropriate correction for the patient. In relation to the recovery of urinary dilution capacity following the volume expansion and the suspension of pharmacological interference by SSRIs and thiazide, the previous clinical picture of SIADH was regarded as a transient phenomenon, and a treatment with tolvaptan was not indicated accordingly. After 10 days in hospital, the patient was discharged after retrieving of her normal serum sodium level.
These changes in electrolyte profile were achieved without adverse repercussions on systemic hemodynamics. In fact, the IV furosemide at small doses was discontinued simultaneously with the suspension of infusions of hypertonic saline. The clinical picture remained similar to that prior to admission, i.e., patient in NYHA class II with dyspnea on effort, namely dyspnea was evoked by the usual efforts of the relation life. However, there was a partial regression of the bilateral leg edema and a slight weight loss (-2 kg in comparison with admission).
| Discussion | ▴Top |
Hyponatremia (serum Na+ < 135 mEq/L) is an electrolyte disorder that often testifies the existence of a chronic medical condition, e.g., cancer in advanced stage in the case of euvolemic hyponatremia due to ectopic or inappropriate secretion of antidiuretic hormone (as per SIADH syndromes), or even liver cirrhosis or CHF in the case of the so-called hypervolemic hyponatremia, namely, that is accompanied by clinical signs and symptoms of hydrosaline congestion. Hypervolemic hyponatremia, that is associated with manifestations of hydrosaline retention, is regarded by the majority of scholars as an epiphenomenon and marker, rather than as a causative factor of the disease (CHF or cirrhosis of the liver). However, one can assume for hypervolemic hyponatremia a role as an aggravating factor of the disease, because the neurological symptoms of this electrolyte disorder, although sometimes clinically inconspicuous, may contribute to worsen the patient’s state of discomfort and disability. In particular, cognitive disorders and motor coordination deficiencies, associated with even initial stages of hyponatremia, can be disabling, as frequently described for the decline in mental concentration and reduction in level of attention, and also for postural instability and gait disturbances, that are major causes of clinical troubles in these patients.
In the case of hyponatremia that occurs in the course of heart failure, non-osmotic stimulation of the supraoptic and paraventricular nuclei of the hypothalamus, which accurately sense any reduction in arterial effective circulating volume, is regarded as the primum movens able to elicit an increase in the secretion of ADH from the posterior pituitary gland. The latter then acting at the level of the distal nephron inhibits the clearance of the free water thereby promoting a state of hemodilutional hyponatremia within a clinical picture with widespread edema from systemic venous hypertension in the case of congestive CHF or characterized by ascites from portal venous hypertension in the case of liver cirrhosis.
In the genesis of hypervolemic hyponatremia, i.e., the one that typically occurs during CHF or liver cirrhosis, a frequent overlap of iatrogenic causes has been demonstrated, primarily consisting of overzealous or excessive use of drastic diuretic therapies, capable of worsening the state of reduced arterial effective circulating volume so as to elicit further secretion of ADH. The generation of a state of relative intravascular volume depletion may be prevented using albumin [29] or hypertonic saline solution [30], capable of preserving an adequate vascular refilling rate, subsequently to the depletion of the circulating volume arising from the adoption of high doses of IV loop diuretics.
In the daily medical practice, hyponatremia whose grade is rated mild (134 - 130 mEq/L) or moderate (129 - 125 mEq/L) is not corrected because the patient is usually free from neurological complaints. Instead, in the case of serum sodium less than 125 - 120 mEq/L, in the CHF or liver cirrhosis scenarios, the current recommended approach in Europe provides for a limitation of dietary water intake - a measure poorly tolerated by the vast majority of patients - or alternatively, requires use of 3% hypertonic saline, given intravenously, the latter mandatorily complemented by the administration of IV furosemide as a slow infusion or by means of repeated IV boluses.
In other countries (Japan and USA), the incidence of iatrogenic hyponatremia caused by incorrect administration of high doses of loop diuretics is now minimized by the combined use of tolvaptan, an ADH receptor antagonist. This association allows to obtain an aqueous diuresis with low sodium excretion, due to specific inhibition of free water reabsorption, that tolvaptan typically achieves by acting on the distal part of the nephron, i.e., the seat of the ADH action. Therefore, it is possible to effectively counter the hemodynamic congestion without paying the price of an excessive loss of sodium through the urine. However, the use of aquaretic drugs like tolvaptan for correction of non-symptomatic hyponatremia in CHF has been not approved by the European Regulatory Authority for Medicines. Indeed in Europe the use of tolvaptan is allowed only for cases of severe and symptomatic hyponatremia, which typically are found associated with paraneoplastic SIADH or even in SIADH of patients treated by psychotropic medications, in particular, SSRI; indeed the hyponatremia of heart failure and cirrhosis seldom attains levels below 125 - 120 mEq/L.
| Conclusions | ▴Top |
Considering that even mild or moderate grades of hyponatremia can cause symptoms (dullness, reduced level of attention, postural instability with increased risk of falls, etc.) hyponatremia occurring in the course of CHF or liver cirrhosis should be treated with appropriate measures (hypertonic saline plus loop diuretic, or tolvaptan). However, there are established medical cultural prejudices that hinder the interventional approach and instead favor the maintenance over time and progressive deterioration of this electrolytic disorder. In the present case report, it is then evident that chronic hyponatremia required a well-tailored and cautious correction because an approach that instead adopts a relatively high speed of sodium correction could be burdened by the risk of osmotic demyelination syndrome. Even for the availability of new drugs active especially in chronic hyponatremia (tolvaptan, conivaptan and other ADH receptor antagonists), the current trend is shifting toward a less conservative approach and a more liberal use of aquaretic drugs, particularly of tolvaptan. However, some concerns still remain to be solved, related to the high cost of these drugs as well as to the feared risk of side effects (in particular, liver toxicity reported for the prolonged use of tolvaptan).
| References | ▴Top |
- Konstam MA, Gheorghiade M, Burnett JC, Jr., Grinfeld L, Maggioni AP, Swedberg K, Udelson JE, et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: the EVEREST Outcome Trial. JAMA. 2007;297(12):1319-1331.
doi pubmed - Lippi G, Aloe R. Hyponatremia and pseudohyponatremia: first, do no harm. Am J Med. 2010;123(9):e17.
doi pubmed - Spasovski G, Vanholder R, Allolio B, Annane D, Ball S, Bichet D, Decaux G, et al. Clinical practice guideline on diagnosis and treatment of hyponatraemia. Nephrol Dial Transplant. 2014;29 (Suppl 2):i1-i39.
doi pubmed - Arampatzis S, Frauchiger B, Fiedler GM, Leichtle AB, Buhl D, Schwarz C, Funk GC, et al. Characteristics, symptoms, and outcome of severe dysnatremias present on hospital admission. Am J Med. 2012;125(11):1125 e1121-1125 e1127.
- Schrier RW, Sharma S, Shchekochikhin D. Hyponatraemia: more than just a marker of disease severity? Nat Rev Nephrol. 2013;9(1):37-50.
doi pubmed - Doshi SM, Shah P, Lei X, Lahoti A, Salahudeen AK. Hyponatremia in hospitalized cancer patients and its impact on clinical outcomes. Am J Kidney Dis. 2012;59(2):222-228.
doi pubmed - De Luca L, Klein L, Udelson JE, Orlandi C, Sardella G, Fedele F, Gheorghiade M. Hyponatremia in patients with heart failure. Am J Cardiol. 2005;96(12A):19L-23L.
doi pubmed - Klein L, O'Connor CM, Leimberger JD, Gattis-Stough W, Pina IL, Felker GM, Adams KF, Jr., et al. Lower serum sodium is associated with increased short-term mortality in hospitalized patients with worsening heart failure: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) study. Circulation. 2005;111(19):2454-2460.
doi pubmed - Gheorghiade M, Abraham WT, Albert NM, Gattis Stough W, Greenberg BH, O'Connor CM, She L, et al. Relationship between admission serum sodium concentration and clinical outcomes in patients hospitalized for heart failure: an analysis from the OPTIMIZE-HF registry. Eur Heart J. 2007;28(8):980-988.
doi pubmed - Wald R, Jaber BL, Price LL, Upadhyay A, Madias NE. Impact of hospital-associated hyponatremia on selected outcomes. Arch Intern Med. 2010;170(3):294-302.
doi pubmed - Mohan S, Gu S, Parikh A, Radhakrishnan J. Prevalence of hyponatremia and association with mortality: results from NHANES. Am J Med. 2013;126(12):1127-1137 e1121.
- Gankam-Kengne F, Ayers C, Khera A, de Lemos J, Maalouf NM. Mild hyponatremia is associated with an increased risk of death in an ambulatory setting. Kidney Int. 2013;83(4):700-706.
doi pubmed - Arampatzis S, Funk GC, Leichtle AB, Fiedler GM, Schwarz C, Zimmermann H, Exadaktylos AK, et al. Impact of diuretic therapy-associated electrolyte disorders present on admission to the emergency department: a cross-sectional analysis. BMC Med. 2013;11:83.
doi pubmed - Waikar SS, Mount DB, Curhan GC. Mortality after hospitalization with mild, moderate, and severe hyponatremia. Am J Med. 2009;122(9):857-865.
doi pubmed - Donze JD, Beeler PE, Bates DW. Impact of Hyponatremia Correction on the Risk for 30-Day Readmission and Death in Patients with Congestive Heart Failure. Am J Med. 2016;129(8):836-842.
doi pubmed - De Vecchis R, Di Maio M, Di Biase G, Ariano C. Effects of Hyponatremia Normalization on the Short-Term Mortality and Rehospitalizations in Patients with Recent Acute Decompensated Heart Failure: A Retrospective Study. J Clin Med. 2016;5(10).
doi - Konishi M, Haraguchi G, Ohigashi H, Sasaoka T, Yoshikawa S, Inagaki H, Ashikaga T, et al. Progression of hyponatremia is associated with increased cardiac mortality in patients hospitalized for acute decompensated heart failure. J Card Fail. 2012;18(8):620-625.
doi pubmed - Chawla A, Sterns RH, Nigwekar SU, Cappuccio JD. Mortality and serum sodium: do patients die from or with hyponatremia? Clin J Am Soc Nephrol. 2011;6(5):960-965.
doi pubmed - Hoorn EJ, Zietse R. Hyponatremia and mortality: moving beyond associations. Am J Kidney Dis. 2013;62(1):139-149.
doi pubmed - Renneboog B, Musch W, Vandemergel X, Manto MU, Decaux G. Mild chronic hyponatremia is associated with falls, unsteadiness, and attention deficits. Am J Med. 2006;119(1):71 e71-78.
- Schrier RW. Decreased effective blood volume in edematous disorders: what does this mean? J Am Soc Nephrol. 2007;18(7):2028-2031.
doi pubmed - MacFadyen RJ, Ng Kam Chuen MJ, Davis RC. Loop diuretic therapy in left ventricular systolic dysfunction: has familiarity bred contempt for a critical but potentially nephrotoxic cardio renal therapy? Eur J Heart Fail. 2010;12(7):649-652.
doi pubmed - Brandimarte F, Mureddu GF, Boccanelli A, Cacciatore G, Brandimarte C, Fedele F, Gheorghiade M. Diuretic therapy in heart failure: current controversies and new approaches for fluid removal. J Cardiovasc Med (Hagerstown). 2010;11(8):563-570.
doi pubmed - Hansen O, Sorensen P, Hansen KH. The occurrence of hyponatremia in SCLC and the influence on prognosis: a retrospective study of 453 patients treated in a single institution in a 10-year period. Lung Cancer. 2010;68(1):111-114.
doi pubmed - Lovelock JD, Coslet S, Johnson M, Rich S, Gomberg-Maitland M. Relative adrenal insufficiency in severe congestive heart failure with preserved systolic function: a case report. J Cardiovasc Med (Hagerstown). 2007;8(9):754-757.
doi pubmed - Friedman E, Shadel M, Halkin H, Farfel Z. Thiazide-induced hyponatremia. Reproducibility by single dose rechallenge and an analysis of pathogenesis. Ann Intern Med. 1989;110(1):24-30.
doi pubmed - Lampl C, Yazdi K. Central pontine myelinolysis. Eur Neurol. 2002;47(1):3-10.
doi pubmed - Sterns RH, Cappuccio JD, Silver SM, Cohen EP. Neurologic sequelae after treatment of severe hyponatremia: a multicenter perspective. J Am Soc Nephrol. 1994;4(8):1522-1530.
pubmed - Filippatos GS, Desai RV, Ahmed MI, Fonarow GC, Love TE, Aban IB, Iskandrian AE, et al. Hypoalbuminaemia and incident heart failure in older adults. Eur J Heart Fail. 2011;13(10):1078-1086.
doi pubmed - Paterna S, Di Gaudio F, La Rocca V, Balistreri F, Greco M, Torres D, Lupo U, et al. Hypertonic Saline in Conjunction with High-Dose Furosemide Improves Dose-Response Curves in Worsening Refractory Congestive Heart Failure. Adv Ther. 2015;32(10):971-982.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


