| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Review
Volume 16, Number 6, June 2024, pages 273-283
Botulinum Toxin Type A and Hyaluronic Acid Dermal Fillers in Dentistry: A Systematic Review of Clinical Application and Indications
Marta Macia, c , Carlotta Fanellia, Mauro Lorussoa, Donatella Ferraraa, Marino Caropreseb, Michele Laurenzielloa, Michele Tepedinob, Domenico Ciavarellaa
aDepartment of Clinical and Experimental Medicine, School of Dentistry, University of Foggia, Foggia, Italy
bDepartment of Biotechnological and Applied Clinical Sciences, University of L’Aquila, L’Aquila, Italy
cCorresponding Author: Marta Maci, Department of Clinical and Experimental Medicine, School of Dentistry, University of Foggia, 71122 Foggia, Italy
Manuscript submitted May 13, 2024, accepted June 24, 2024, published online June 30, 2024
Short title: BoNT-A and HA Dermal Fillers in Dentistry
doi: https://doi.org/10.14740/jocmr5202
| Abstract | ▴Top |
Background: Botulinum toxin type A (BoNT-A) and hyaluronic acid (HA) dermal fillers are increasingly utilized in dentistry for therapeutic and aesthetic purposes. However, a comprehensive synthesis of their clinical applications and indications in dentistry is lacking. This systematic review aimed to analyze the clinical application and indications of BoNT-A and HA dermal fillers in dentistry, providing insights into their efficacy, safety profiles, and limitations.
Methods: A systematic search was conducted in PubMed/MEDLINE databases to identify relevant studies published between 2018 and 2024. Medical Subject Headings (MeSH) terms and keywords related to BoNT-A, HA dermal fillers, dentistry, clinical applications, and indications were used. Study selection criteria included randomized controlled trials (RCTs) and non-RCTs involving human participants of any age group. Data extraction and synthesis followed established guidelines, focusing on study characteristics, participant demographics, intervention details, outcome measures, and key findings related to BoNT-A and HA dermal fillers’ clinical application in dentistry.
Results: Systematic searches across electronic databases and grey literature identified 857 records, with an additional 73 from hand searches. After screening titles and abstracts, 542 records were excluded, leaving 374 full-text publications for evaluation. Ultimately, 12 RCTs and 13 non-RCTs were included. The systematic review encompassed diverse geographic locations: Brazil, Italy, Spain, Syria, India, Egypt, Korea, and the Netherlands, involving samples sizes ranging from 14 to 143 participants. The review synthesized findings on HA’s efficacy in various areas, including bone repair, gingivitis management, temporomandibular joint disorders, postoperative swelling reduction, periodontal defect treatment, chin and check projection and lips augmentation. BoNT-A exhibited promising efficacy in managing orofacial pain conditions, gummy smile treatment and neuromodulation of the lower third muscles. Safety profiles varied among studies, with some reporting minimal adverse effects while others noted dose-related concerns.
Conclusion: BoNT-A and HA dermal fillers offer a wide array of clinical applications in dentistry, ranging from therapeutic interventions to aesthetic enhancements. Despite promising efficacy, careful consideration and monitoring of safety outcomes are essential when integrating these interventions into clinical practice. Further research addressing methodological limitations and safety concerns is warranted to optimize their utilization and improve patient care in dentistry.
Keywords: Systematic review; Botulinum toxin type A; Hyaluronic acid; Dermal fillers; Dentistry; Clinical application; Indications; Efficacy
| Introduction | ▴Top |
Botulinum toxin type A (BoNT-A) and hyaluronic acid (HA) dermal fillers have emerged as valuable adjuncts in various medical specialties, including dentistry, owing to their diverse clinical applications and therapeutic benefits [1, 2]. While traditionally associated with cosmetic dermatology, these agents have garnered increasing attention for their potential roles in addressing a wide range of dental concerns, ranging from orofacial pain management to aesthetic enhancements [3, 4].
BoNT-A, a neurotoxic protein derived from Clostridium botulinum, acts by inhibiting the release of acetylcholine at neuromuscular junctions, leading to temporary muscle paralysis. On the other hand, HA dermal fillers, composed of biocompatible HA, offer volumetric augmentation and tissue support, facilitating the correction of facial wrinkles, volume loss, and soft tissue defects [5, 6]. BoNT-A exerts its effects by inhibiting neurotransmitter release at the neuromuscular junction, thereby inducing temporary muscle paralysis, while HA functions as a volumizing agent, augmenting soft tissue contours and restoring facial harmony. These distinct mechanisms of action render BoNT-A and HA invaluable tools in addressing a myriad of aesthetic and functional concerns encountered in dental practice [3, 7, 8].
In recent years, the utilization of BoNT-A and HA dermal fillers in dentistry has expanded significantly, driven by advancements in treatment techniques, growing evidence supporting their efficacy and evolving patient preferences for minimally invasive procedures [8, 9].
BoNT-A effectively manages orofacial pain conditions and enhances functional outcomes, while HA fillers restore volume and improve soft tissue aesthetics [9, 10]. HA’s biocompatibility and reversibility make it ideal for addressing various dental concerns, including periodontal defects and temporomandibular joint (TMJ) disorders (TMDs), with minimal risk of adverse events. These treatments play crucial roles in enhancing patient satisfaction and optimizing dental care outcomes [11].
In an RCT by Briguglio et al, HA effectively treated two-wall infrabony periodontal defects, resulting in a mean clinical attachment level (CAL) gain of 1.9 ± 1.8 mm and a probing depth (PD) reduction of 1.6 ± 1.2 mm, surpassing gains from debridement. The study concludes that HA enhances CAL gain, PD reduction, and predictability of clinical outcomes in treating these defects. Another RCT comparing minimally invasive non-surgical therapy (MINST) with quadrant-wise subgingival instrumentation (Q-SI) in periodontitis patients found MINST significantly reduced PD, CAL, and bleeding scores versus Q-SI after 1 year. MINST was particularly effective in lowering C-reactive protein levels in patients with higher baseline values, suggesting potential cardiovascular benefits in periodontitis management.
The rationale for this systematic review lies in the increasing utilization of BoNT-A and HA dermal fillers in dental practice, despite a lack of comprehensive synthesis regarding their clinical applications and indications. BoNT-A and HA offer promising adjunctive treatments for various dental conditions, ranging from aesthetic enhancements to therapeutic interventions in TMDs and periodontal surgeries. Understanding their efficacy, safety profiles, and specific clinical indications is crucial for optimizing treatment outcomes and patient care. This review aimed to fill this gap by critically analyzing existing literature to provide evidence-based guidance on the use of BoNT-A and HA in dentistry.
| Methods | ▴Top |
Search strategy
A systematic search of electronic databases, including PubMed/MEDLINE, was conducted to identify relevant studies published between 2018 and 2024. Medical Subject Headings (MeSH) terms and keywords related to BoNT-A, HA dermal fillers, dentistry, clinical applications, and indications were utilized in the search strategy to ensure comprehensive coverage of the literature.
Study selection criteria
The inclusion and exclusion criteria for this study provided below.
Inclusion criteria were: 1) Studies investigating the clinical application and indications of BoNT-A and HA dermal fillers in dental practice; 2) RCTs for primary analysis to ensure rigorous scientific evaluation; 3) Non-randomized trials and other study designs to provide a comprehensive understanding of clinical applications, regardless of study design; 4) English language studies.
Specifically, RCTs were selected for the main analysis to ensure rigorous scientific evaluation. Additionally, non-RCTs were included to provide a comprehensive understanding of the clinical applications of HA and BoNT-A, regardless of study design. This inclusive approach allowed for a thorough exploration of the efficacy and clinical implications of these treatments in dental practice.
Exclusion criteria were: 1) Studies not relevant to the clinical application and indications of BoNT-A and HA dermal fillers in dentistry; 2) Animal studies lacking sufficient data or with unclear methodology; 3) Non-English language studies.
Study selection process and data extraction
Two independent reviewers screened the titles and abstracts of retrieved articles, followed by a full-text assessment of potentially relevant studies to determine eligibility for inclusion. Data extraction was conducted independently by two reviewers using a standardized data extraction form. Key data extracted from each included study included study characteristics (e.g., author, publication year, study design), participant demographics (e.g., sample size, age, gender distribution), intervention details (e.g., type and dosage of BoNT-A or HA dermal filler used), outcome measures assessed (e.g., clinical efficacy, adverse effects), and key findings related to the clinical application and indications of BoNT-A and HA dermal fillers in dentistry.
Data synthesis and analysis
Data synthesis involved a narrative summary of the findings based on the homogeneity of included studies. The systematic review adhered to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for reporting the findings. The PRISMA 27 checklist offers a comprehensive framework for systematic reviews. With main categories including “Title, Abstract, and Introduction”, “Methods”, “Results”, and “Discussion”, it guides in conducting and reporting reviews, ensuring transparency, reliability, and reproducibility, thus enhancing the integrity of the research process [12]. Methodological quality assessment of included studies was performed using appropriate tools, such as the Cochrane risk of bias tool for RCTs. Any discrepancies between reviewers were resolved through discussion or consultation with a third reviewer if necessary. Overall, the data analysis aimed to provide a comprehensive understanding of the existing evidence regarding the use of BoNT-A and HA dermal fillers in various dental applications, highlighting their efficacy, safety profile, and potential clinical implications.
Ethical considerations
Ethical approval was not required for this systematic review, as it involved the analysis of published literature and did not entail direct contact with human participants. All data were obtained from publicly available sources and were anonymized during analysis to ensure confidentiality and compliance with ethical standards.
| Results | ▴Top |
Systematic searches of electronic databases identified a total of 857 records and 73 from grey literature or hand searched. Of these, 542 were excluded during title and abstract screening, and 374 full-text publications were further evaluated for inclusion. After the full-text screening, 12 RCT and 13 non-RCT publications were included (Fig. 1).
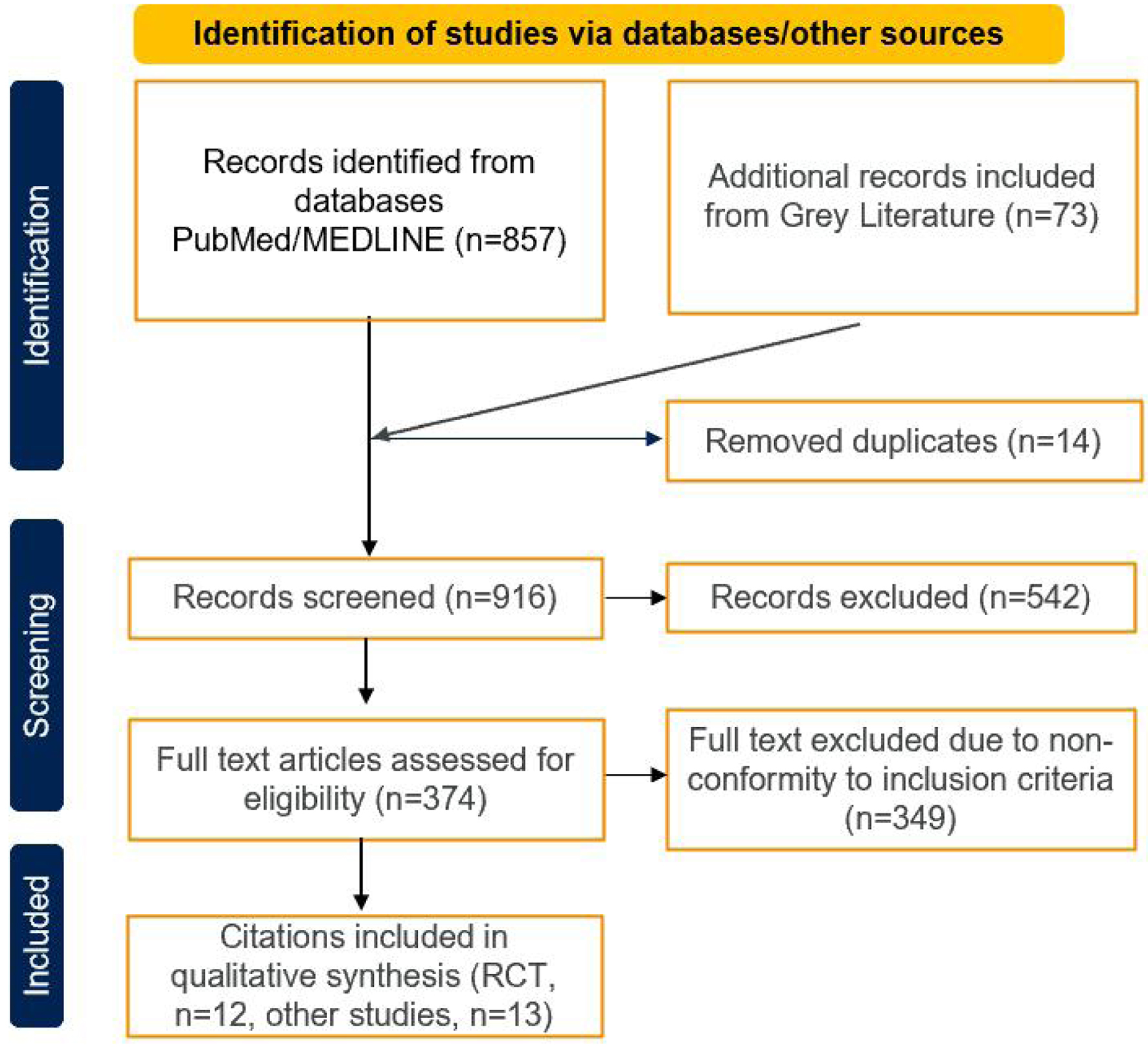 Click for large image | Figure 1. PRISMA flowchart. |
A PRISMA flowchart was created to summarize the selection process of the eligible studies. Potential content was extracted from selected studies [12].
Study characteristics
The systematic review included 12 clinical studies focusing on BoNT-A and HA dermal fillers in dentistry, with six studies dedicated to each [13-24]. Table 1 outlines the recommended treatment applications of BoNT-A and HA in various dental and aesthetic procedures. It details the specific areas of injection, the products used, and the typical duration of treatment effectiveness. These studies were conducted across diverse geographic locations: Brazil, Italy, Spain, Syria, India, Egypt, Korea, and the Netherlands. Sample sizes ranged from 14 to 143 participants, while participants’ mean age varied from 18.67 to 41 years [16-24]. Gender distribution varied across studies, with De la Torre Canales et al and Steenen et al having only female participants [17-19, 24]. This underscores the diverse demographic representation in the systematic review (Table 2 [13-24]). In addition to main RCTs, a total of 13 studies were included to provide the clinical application of HA and BoNT-A irrespective of study design.
 Click to view | Table 1. Treatment Application Guideline |
 Click to view | Table 2. Study Characteristics and Application in Dentistry |
Efficacy and advancements in dentistry with HA
The systematic review synthesized findings on the efficacy of HA in various dental applications (Supplementary Material 1, www.jocmr.org). One study demonstrated higher bone formation and fractal dimension values in treated sockets compared to controls post-operation [13]. Another study showed effective reduction of gingival inflammation with an HA and H2O2 mouth rinse [14]. In TMDs, HA improved maximum mouth opening, reduced pain, and enhanced oral health-related quality of life [15-20]. Additionally, HA reduced postoperative swelling and trismus compared to collagen alone after molar extraction. Improved clinical outcomes were reported with HA gel and debridement in periodontal defects [21]. Variations in the effectiveness of lip augmentation among different HA fillers were also noted, highlighting HA’s versatility in dental practice [24].
Efficacy and advancements in dentistry with BoNT-A
The review also examined the efficacy of BoNT-A in dental applications across six studies [16-19, 22, 23] (Supplementary Material 1, www.jocmr.org). One study demonstrated reduced exposed gingiva and muscle activity with BoNT-A injections for gummy smile treatment [16]. Another study showed significant pain reduction and increased pressure pain threshold with BoNT-A in persistent myofascial pain (MFP) [17]. Improved mouth opening and reduced muscle pain were highlighted with BoNT-A compared to controls at 180 days in a different study [18]. Sustained pain reduction and increased pain threshold up to 6 years post-treatment were revealed in another investigation [19]. Combined BoNT-A and oral appliance were suggested for effective lower face contouring. Additionally, reduced gingival display with zinc supplementation + BoNT-A was demonstrated, indicating its prolonged efficacy in maintaining decreased gingival display [22, 23]. These findings underscore BoNT-A’s diverse therapeutic potential in enhancing oral health and esthetics, ranging from gummy smile treatment and MFP management to lower face contouring in dental practice.
Safety profile of HA and BoNT-A
The safety profile of the interventions across the studies varied, with some reporting minimal adverse effects, while others noted dose-related concerns (Supplementary Material 1, www.jocmr.org). Overall, while some interventions demonstrated favorable safety profiles with minimal or no reported adverse events, others indicated potential concerns, emphasizing the importance of careful consideration and monitoring of safety outcomes in dental treatments. However, De la Torre Canales et al highlighted dose-related adverse effects of BoNT-A [17].
BoNT-A application in dentistry and clinical indication
The review examined six RCTs on BoNT-A’s applications in dentistry [16-19, 22, 23]. BoNT-A injections addressed gummy smiles, MFP, and mandibular motion issues [16-19]. They also reduced gingival display and enhanced lower facial contours [22, 23] (Table 2, Supplementary Material 1, www.jocmr.org).
Additionally, a total of six studies were included to elucidate the clinical applications of BoNT-A, irrespective of study design. These studies collectively highlight the diverse applications of BoNT-A in addressing various dental conditions, emphasizing its potential as an adjunctive therapy for enhancing dental aesthetics and managing orofacial pain.
Masseter for bruxism and masseteric hypertrophy
BoNT-A injections in the masseter muscle provide a non-invasive solution for bruxism and masseteric hypertrophy. By selectively targeting the masseter muscle, BoNT-A can reduce muscle activity, alleviating symptoms such as teeth grinding and jaw clenching. Studies have demonstrated the efficacy of BoNT-A in managing bruxism-related symptoms and reducing masseteric hypertrophy, thus improving patient comfort and preventing dental complications [25, 26].
Gummy smile
BoNT-A injections into the orbicularis muscle offer an effective treatment for excessive gingival display, commonly referred to as a gummy smile. By inhibiting the hyperactivity of the upper lip elevator muscles, BoNT-A can achieve a balanced smile by reducing the visibility of the gums. This non-surgical approach provides patients with a more aesthetically pleasing smile while preserving facial harmony [27].
Orbicularis and mentalis muscles
BoNT-A injections into the orbicularis and mentalis muscles are valuable in addressing hyper functional lines and wrinkles around the mouth area. By temporarily paralyzing these muscles, BoNT-A can smooth out facial lines and enhance overall facial aesthetics [28, 29].
Facial pain and TMD
BoNT-A injections are effective in managing facial pain and TMD by reducing muscle hyperactivity and alleviating associated symptoms. By targeting specific muscle groups involved in jaw movement, BoNT-A can relieve pain and improve jaw function, thus enhancing the quality of life for patients suffering from TMD. Research supports the efficacy of BoNT-A therapy in providing long-lasting relief from facial pain and improving overall oral health [30].
These applications demonstrate the effectiveness of BoNT-A in dentistry, offering minimally invasive solutions for a wide range of concerns related to muscular activity, facial aesthetics, and TMDs.
HA application in dentistry and clinical indication
The systematic review identified six RCTs exploring the clinical applications of HA in dentistry [13-15, 20, 21, 24]. HA was effective in bone repair post mandibular premolar extraction and improving gingivitis when used in a mouth rinse [13, 14]. It served as an adjunct in temporomandibular joint arthroscopy and managed postoperative trismus and swelling [15, 20]. HA gel aided in treating chronic periodontitis, especially intra-bony defects, while HA dermal fillers were effective in lip augmentation [21, 24] (Table 2, Supplementary Material 1, www.jocmr.org).
In addition to the RCT, a total of six additional studies were included to elucidate the clinical applications of HA, irrespective of study design, to provide a comprehensive understanding regarding HA application in dentistry. HA has gained significant recognition in dentistry for its versatile applications, particularly in addressing aesthetic concerns and promoting tissue regeneration. HA not only offers functional therapeutic approaches in dentistry but also serves as a valuable tool for enhancing facial aesthetics. Its intraoral use presents opportunities for both functional and aesthetic improvements, catering to a wide range of patient needs and preferences.
Gummy smile treatment
Myo modulation with HA offers several advantages. It is a minimally invasive procedure performed in-office, often requiring only topical anesthesia. It presents a promising option for the treatment of gummy smiles, offering patients a safe, effective, and customizable solution with minimal downtime and natural-looking results [31].
Periodontal and implant surgeries
HA fillers play a crucial role in periodontal and implant surgeries by promoting tissue regeneration and enhancing wound healing. Studies have demonstrated the effectiveness of HA-based materials in augmenting soft tissue volume and improving the stability of dental implants. By providing structural support and promoting angiogenesis, HA fillers contribute to the successful outcome of periodontal and implant procedures [32].
Enhancement of lip and perioral volume
HA fillers offer a non-invasive solution for enhancing lip and perioral volume, addressing aesthetic concerns related to facial symmetry and harmony. In orthodontics, HA fillers can be strategically injected into the lips and perioral tissues to compensate for skeletal discrepancies. For instance, upper lip fillers can be used to compensate for class III skeletal orthodontics, while chin fillers can help balance class II skeletal orthodontics, resulting in improved facial aesthetics and patient satisfaction [33, 34].
Chin projection and jaw contouring
HA fillers offer a non-surgical approach to increasing chin projection and contouring the jawline, particularly in cases of class II orthodontic profiles. By augmenting the chin and reshaping the jawline with HA injections, clinicians can achieve improved facial harmony and balance. This aesthetic enhancement not only complements orthodontic treatment outcomes but also enhances the overall facial appearance, leading to enhanced patient confidence and satisfaction [35].
Gum regeneration
HA has shown promise in promoting gum regeneration and tissue repair in cases of periodontal disease and gingival recession. By delivering HA-based materials directly into the periodontal tissues, clinicians can stimulate collagen production, enhance fibroblast activity, and promote tissue regeneration. Research studies have demonstrated the efficacy of HA fillers in improving periodontal health and restoring gum tissue integrity, thereby preserving tooth structure and supporting long-term dental function [36].
Table 1 outlines the treatment goals, areas of injection, products used, and treatment duration for various dental and facial conditions. It includes guidelines for using BoNT-A and HA in treating conditions such as bruxism, facial pain, TMD, gummy smile, and others. These clinical indications highlight the diverse applications of HA in dentistry and orthodontics, ranging from aesthetic enhancements to tissue regeneration, and underscore its significance in achieving optimal oral health and patient satisfaction.
Risk of bias assessment
The majority of reviewed studies exhibited a low risk of bias across all assessed domains (D1-D5), indicating a generally satisfactory level of methodological rigor. However, two studies, Castano-Joaqui et al, 2021, regarding D4 (measurement of outcome), and Steenen et al, 2023, raised some concerns in D2 (bias deviation due to intended intervention) and D5 (reporting of results) domains [15, 24]. It is important to consider these concerns when interpreting the findings of these studies. Overall, the included studies offer valuable insights into the subject matter despite these isolated issues (Fig. 2).
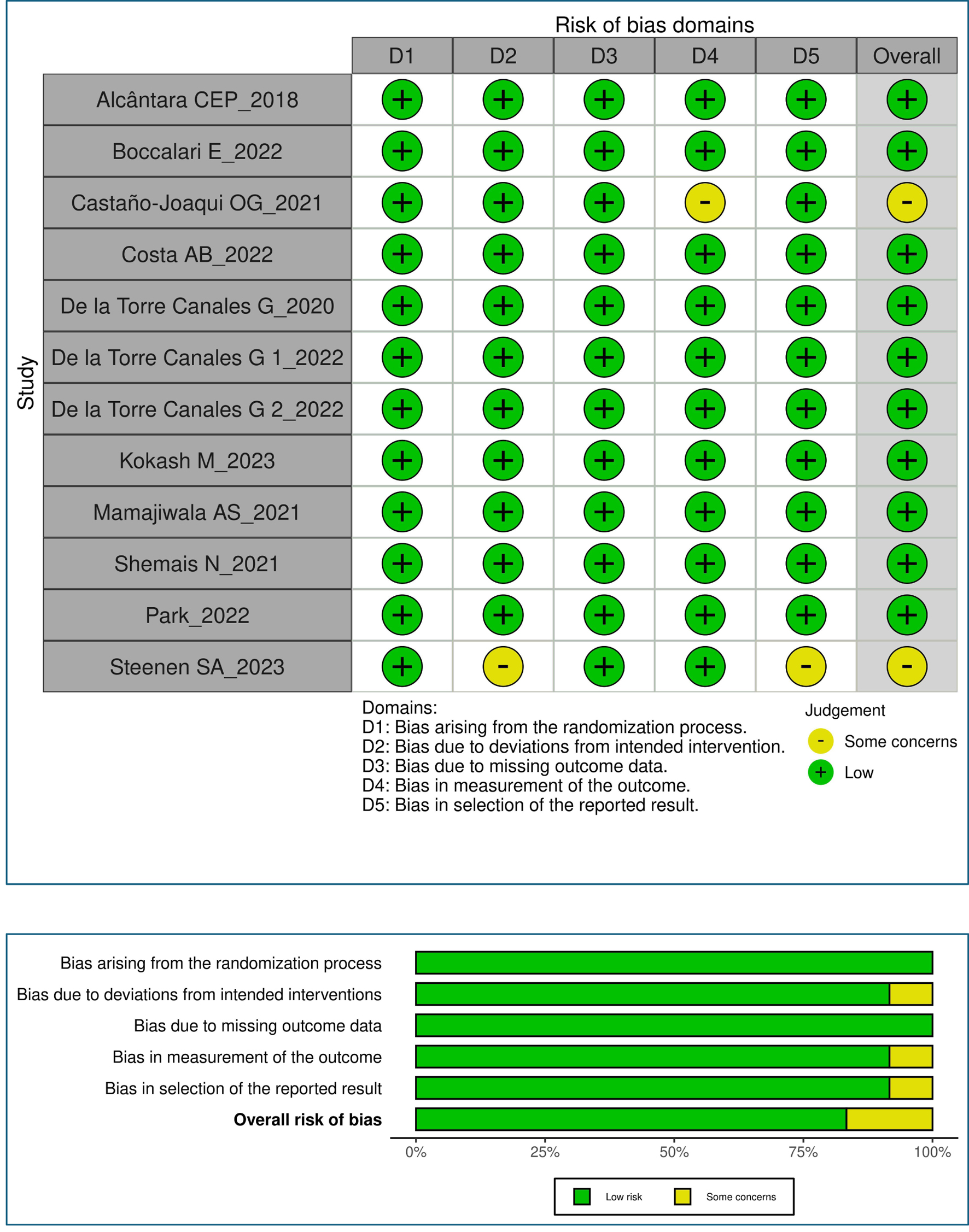 Click for large image | Figure 2. Risk of bias assessment. |
| Discussion | ▴Top |
BoNT-A and HA dermal fillers have garnered increasing attention in dentistry due to their diverse clinical applications and therapeutic benefits. This systematic review aimed to provide a comprehensive analysis of their efficacy and clinical indications in dental practice, comparing and contrasting with previous literature to elucidate their evolving roles in dentistry.
The systematic review highlighted the diverse applications of HA and BoNT-A in dentistry. HA demonstrated efficacy in promoting bone formation, reducing gingival inflammation, enhancing temporomandibular function, and improving outcomes in periodontal defects. It also showed benefits in post-operative swelling and trismus reduction, and variations in lip augmentation. BoNT-A proved effective for gummy smile treatment, reducing MFP, improving mouth opening, and enhancing facial aesthetics. Both HA and BoNT-A presented favorable safety profiles, though some dose-related concerns were noted. These findings underscore the therapeutic potential of HA and BoNT-A in dental practice.
The systematic review highlights the potential of HA in improving clinical outcomes and quality of life for patients with periodontitis. HA demonstrated efficacy in various areas, including bone repair post-dental extraction, gingivitis management, TMDs, postoperative swelling reduction, periodontal defect treatment, and lip augmentation [37, 38]. Particularly noteworthy were the improved clinical outcomes in periodontal defects with HA gel and debridement, as reported by Mamajiwala et al [21]. However, variations in the effectiveness of lip augmentation among different HA fillers, as noted by Steenen et al, suggest the importance of careful selection based on patient-specific factors [24].
The use of HA for both gummy smiles and chin augmentation underscores its versatility and effectiveness in addressing various aesthetic concerns. With its biocompatibility, long-lasting results, and low risk of adverse effects, HA remains a popular choice among patients and practitioners [31-35]. HA’s role extends beyond conventional treatments by addressing oxidative stress and inflammation associated with periodontal disease. Studies included in the review demonstrated HA’s efficacy in enhancing bone formation, reducing gingival inflammation, and managing TMDs, leading to improved mouth opening, pain reduction, and enhanced oral health-related quality of life [15-20]. These findings underscore HA’s therapeutic versatility in dentistry, suggesting its promise not only in treating periodontal defects but also in mitigating systemic inflammatory responses and promoting overall patient well-being. Further research into HA’s mechanisms and long-term effects in periodontal care could provide deeper insights into its clinical utility and broader applications in dental practice.
Across the reviewed studies, BoNT-A showed promising efficacy in managing various orofacial pain conditions like TMD and MFP by inducing muscle relaxation and alleviating muscular hyperactivity. BoNT-A also demonstrated diverse clinical applications, including gummy smile treatment, reduction of excessive gingival display, and lower facial contouring, consistent with previous researches [9, 39]. Additionally, BoNT-A injections showed benefits in functional purposes, such as reducing masseter muscle hypertrophy and improving mandibular range of motion, enhancing dental aesthetics and functional outcomes [40, 41]. However, concerns regarding dose-related adverse effects, as highlighted by De la Torre Canales et al, underscore the importance of cautious dosage administration in BoNT-A treatments [19].
While the reviewed studies generally reported favorable efficacy outcomes, addressing safety concerns is essential. Some studies reported minimal adverse effects, while others raised concerns about dose-related adverse effects, emphasizing the importance of cautious administration and monitoring [14, 17, 24]. Aligning with our findings, a meta-analysis also reported that the majority of adverse events associated with HA treatment were mild or moderate in nature, although a minority of cases experienced serious adverse effects [42, 43]. Additionally, variability in safety outcomes among different HA fillers warrants attention, necessitating thorough assessment and selection based on safety profiles. In line with these findings, previous published cohort study and consensus report on complications after BoNT-A and HA dermal filler injections also reported the incidence of overall complications [44-46].
Strengths and limitations
This systematic review offers several strengths including, adhering to PRISMA guidelines ensures methodological rigor and transparency, providing valuable insights into efficacy and safety profiles. Its focus on clinical relevance serves as a practical resource for dental professionals seeking evidence-based guidance. Additionally, it covers various dental applications, enhancing its utility for addressing diverse patient needs.
Despite the promising findings, the studies included in our review exhibited several limitations, echoing concerns raised in previous literature. These limitations include small sample sizes, short study durations, lack of control groups, incomplete safety reporting, and methodological shortcomings. Future research should address these limitations by conducting larger, well-designed studies with longer follow-up periods, comprehensive safety assessments, and standardized outcome measures to enhance the quality and reliability of evidence. Findings may not universally apply to all dental settings or patient populations, requiring careful interpretation and consideration of individual patient factors. Moreover, exploring optimal dosing regimens, formulations, and long-term efficacy and safety profiles of BoNT-A and HA dermal fillers would further advance our understanding and clinical utility of these interventions in dentistry. Additionally, investigating patient-reported outcomes, quality of life measures, and cost-effectiveness analyses would provide valuable insights into the holistic impact of BoNT-A and HA treatments on patient care and healthcare resource utilization.
Clinical implications and recommendations
The findings of our review have significant clinical implications for dental practice, consistent with prior literature. BoNT-A and HA dermal fillers present valuable adjunctive therapies for addressing diverse dental concerns, thereby improving patient outcomes and enhancing overall oral health and esthetics [13-24]. Dental practitioners can utilize these interventions to deliver tailored treatment approaches, optimize aesthetic outcomes as finishing an orthodontic treatment by harmonizing the patient’s profile and bolster patient satisfaction. These studies collectively shed light on the wide-ranging applications of HA in dentistry, spanning from bone repair and gingivitis treatment to the management of TMDs and periodontal defects. However, it is imperative for dental professionals to exercise caution, adhere to evidence-based guidelines, and prioritize patient safety when integrating BoNT-A and HA treatments into clinical practice. Close collaboration among dentists, oral surgeons, dermatologists, and other healthcare providers is essential to ensure comprehensive patient care and interdisciplinary management of complex dental conditions.
| Conclusion | ▴Top |
This systematic review provides valuable insights into the clinical applications and indications of BoNT-A and HA dermal fillers in dentistry, corroborating with previous literature. The review underscores BoNT-A and HA multifaceted role in dentistry, demonstrating its efficacy in enhancing bone formation, reducing gingival inflammation, and managing TMDs. These outcomes translate into improved clinical parameters such as enhanced mouth opening, pain reduction, and overall oral health-related quality of life for patients with periodontitis. While further research is needed to address existing limitations and optimize the clinical utility of BoNT-A and HA, their versatility offers tailored solutions in modern dental practice. Continued interdisciplinary collaboration and evidence-based practice are crucial to fully realize the potential benefits of these treatments, meeting the evolving needs of patients and advancing dental care standards.
| Supplementary Material | ▴Top |
Suppl 1. Efficacy and Advancements in Dentistry With HA and BoNT-A
Acknowledgments
The authors would like to express their gratitude to all individuals and organizations who contributed to this systematic review. Special thanks to independent consultant Ashish Verma and Shivani Chaudhary for their support in editing service and to all the researchers and clinicians whose work has been referenced in this paper.
Financial Disclosure
No financial assistance was received to support this study.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
Marta Maci, Carlotta Fanelli, and Mauro Lorusso contributed to the conception and design of the study. Donatella Ferrara, Marino Caroprese, and Michele Laurenziello contributed to data collection and analysis. Michele Tepedino and Domenico Ciavarella contributed to interpretation of data and critical revision of the manuscript. All authors approved the final version of the manuscript for submission.
Data Availability
The data supporting the findings of this systematic review are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Srivastava S, Kharbanda S, Pal US, Shah V. Applications of botulinum toxin in dentistry: a comprehensive review. Natl J Maxillofac Surg. 2015;6(2):152-159.
doi pubmed pmc - Lighthall JG. Rejuvenation of the upper face and brow: neuromodulators and fillers. Facial Plast Surg. 2018;34(2):119-127.
doi pubmed - Hong SO. Cosmetic treatment using botulinum toxin in the oral and maxillofacial area: a narrative review of esthetic techniques. Toxins (Basel). 2023;15(2):82.
doi pubmed pmc - Bozca BC, Oztas P, Erbil AH. Botulinum toxin: a review of cosmetic applications and hyperhidrosis treatment. Health Sci. 2023;3(3):211-222.
- John HE, Price RD. Perspectives in the selection of hyaluronic acid fillers for facial wrinkles and aging skin. Patient Prefer Adherence. 2009;3:225-230.
doi pubmed pmc - Samizadeh S, Samizadeh S. Dermal fillers: understanding the fundamentals. In: Samizadeh S. (ed). Non-Surgical Rejuvenation of Asian Faces. Springer, Cham. 2022:253-265.
- Kumar R, Dhaliwal HP, Kukreja RV, Singh BR. The botulinum toxin as a therapeutic agent: molecular structure and mechanism of action in motor and sensory systems. Semin Neurol. 2016;36(1):10-19.
doi pubmed - Shah-Desai S, Sezgin B, Dhillon B, Walker L, Wu R, Trevidic P. Elevating aesthetics: patient-specific treatment with hyaluronic acid fillers to improve appearance and psychosocial wellbeing. EMJ. 2023;8(2):10-18.
- Serrera-Figallo MA, Ruiz-de-Leon-Hernandez G, Torres-Lagares D, Castro-Araya A, Torres-Ferrerosa O, Hernandez-Pacheco E, Gutierrez-Perez JL. Use of botulinum toxin in orofacial clinical practice. Toxins (Basel). 2020;12(2):112.
doi pubmed pmc - Vangelisti R, Pagnacco O, Erra C. Hyaluronic acid in the topical treatment of gingival inflammations: preliminary clinical trial. Attualita Terapeut Int. 1997;15:2-3.
- Saini RS, Ali Abdullah Almoyad M, Binduhayyim RIH, Quadri SA, Gurumurthy V, Bavabeedu SS, Kuruniyan MS, et al. The effectiveness of botulinum toxin for temporomandibular disorders: a systematic review and meta-analysis. PLoS One. 2024;19(3):e0300157.
doi pubmed pmc - Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100.
doi pubmed pmc - Alcantara CEP, Castro MAA, Noronha MS, Martins-Junior PA, Mendes RM, Caliari MV, Mesquita RA, et al. Hyaluronic acid accelerates bone repair in human dental sockets: a randomized triple-blind clinical trial. Braz Oral Res. 2018;32:e84.
doi pubmed - Boccalari E, Tadakamadla SK, Occhipinti C, Lanteri V, Maspero C. Evaluation of the effectiveness of a novel mouth rinse containing hyaluronic acid and hydrogen peroxide on gingivitis: a randomized pilot controlled trial. Clin Exp Dent Res. 2022;8(3):673-679.
doi pubmed pmc - Castano-Joaqui OG, Cano-Sanchez J, Campo-Trapero J, Munoz-Guerra MF. TMJ arthroscopy with hyaluronic acid: a 12-month randomized clinical trial. Oral Dis. 2021;27(2):301-311.
doi pubmed - Costa AB, Romansina D, Ramalho J, Pereira P, Tedesco TK, Morimoto S, Goncalves F, et al. Botulinum toxin A in the management of a gummy smile: a clinical controlled preliminary study. Aesthet Surg J. 2022;42(4):421-430.
doi pubmed - De la Torre Canales G, Alvarez-Pinzon N, Munoz-Lora VRM, Vieira Peroni L, Farias Gomes A, Sanchez-Ayala A, Haiter-Neto F, et al. Efficacy and safety of botulinum toxin type A on persistent myofascial pain: a randomized clinical trial. Toxins (Basel). 2020;12(6):395.
doi pubmed pmc - De la Torre Canales G, Poluha RL, Pinzon NA, Da Silva BR, Almeida AM, Ernberg M, Manso AC, et al. Efficacy of botulinum toxin type-A I in the improvement of mandibular motion and muscle sensibility in myofascial pain TMD subjects: a randomized controlled trial. Toxins (Basel). 2022;14(7):441.
doi pubmed pmc - De la Torre Canales G, Camara-Souza MB, Poluha RL, de Figueredo OMC, Nobre BBS, Ernberg M, Conti PCR, et al. Long-term effects of a single application of botulinum toxin type A in temporomandibular myofascial pain patients: a controlled clinical trial. Toxins (Basel). 2022;14(11):741.
doi pubmed pmc - Kokash M, Darwich K, Ataya J. The effect of hyaluronic acid addition to collagen in reducing the trismus and swelling after surgical extraction of impacted lower third molars: a split-mouth, randomized controlled study. Clin Oral Investig. 2023;27(8):4659-4666.
doi pubmed - Mamajiwala AS, Sethi KS, Raut CP, Karde PA, Mamajiwala BS. Clinical and radiographic evaluation of 0.8% hyaluronic acid as an adjunct to open flap debridement in the treatment of periodontal intrabony defects: randomized controlled clinical trial. Clin Oral Investig. 2021;25(9):5257-5271.
doi pubmed - Park Y, Ku SK, Lee DH, Kim ST. Combined effects of botulinum toxin injection and oral appliance therapy on lower facial contouring: a randomized controlled trial. J Clin Med. 2022;11(14):4092.
doi pubmed pmc - Shemais N, Elarab AE, ElNahass H. The effect of botulinum toxin A in patients with excessive gingival display with and without zinc supplementation: randomized clinical trial. Clin Oral Investig. 2021;25(11):6403-6417.
doi pubmed - Steenen SA, Bauland CG, van der Lei B, Su N, van Engelen MDG, Anandbahadoer-Sitaldin R, Koeiman W, et al. Head-to-head comparison of 4 hyaluronic acid dermal fillers for lip augmentation: a multicenter randomized, quadruple-blind, controlled clinical trial. J Am Acad Dermatol. 2023;88(4):932-935.
doi pubmed - Shome D, Khare S, Kapoor R. Efficacy of botulinum toxin in treating Asian Indian patients with masseter hypertrophy: a 4-year follow-up study. Plast Reconstr Surg. 2019;144(3):390e-396e.
doi pubmed - Li Z, Gao J, Ma X, Zhang H. Efficacy of botulinum toxin in treating Asian Indian patients with masseter hypertrophy: a 4-year follow-up study. Plast Reconstr Surg. 2020;145(6):1105e.
doi pubmed - Cengiz AF, Goymen M, Akcali C. Efficacy of botulinum toxin for treating a gummy smile. Am J Orthod Dentofacial Orthop. 2020;158(1):50-58.
doi pubmed - Alimohammadi M, Punga AR. Neurophysiological measures of efficacy and safety for botulinum toxin injection in facial and bulbar muscles: special considerations. Toxins (Basel). 2017;9(11):352.
doi pubmed pmc - Blitzer A, Binder WJ, Aviv JE, Keen MS, Brin MF. The management of hyperfunctional facial lines with botulinum toxin. A collaborative study of 210 injection sites in 162 patients. Arch Otolaryngol Head Neck Surg. 1997;123(4):389-392.
doi pubmed - von Lindern JJ. Type A botulinum toxin in the treatment of chronic facial pain associated with temporo-mandibular dysfunction. Acta Neurol Belg. 2001;101(1):39-41.
pubmed - Germani Vieira M, Rogerio V, Roschel P, Rabelo V, Teixeira T, Munoz-Lora VRM. Myomodulation using hyaluronic acid fillers as an efficient and innovative treatment for gummy smile: A case report. J Oral Biol Craniofac Res. 2022;12(3):376-380.
doi pubmed pmc - Miglani A, Vishnani R, Reche A, Buldeo J, Wadher B. Hyaluronic acid: exploring its versatile applications in dentistry. Cureus. 2023;15(10):e46349.
doi pubmed pmc - Cooper H, Gray T, Fronek L, Witfill K. Lip augmentation with hyaluronic acid fillers: a review of considerations and techniques. J Drugs Dermatol. 2023;22(1):23-29.
doi pubmed - Safran T, Swift A, Cotofana S, Nikolis A. Evaluating safety in hyaluronic acid lip injections. Expert Opin Drug Saf. 2021;20(12):1473-1486.
doi pubmed - Go BC, Frost AS, Friedman O. Using injectable fillers for chin and jawline rejuvenation. World J Otorhinolaryngol Head Neck Surg. 2023;9(2):131-137.
doi pubmed pmc - Mansour A, Acharya AB, Alliot C, Eid N, Badran Z, Kareem Y, Rahman B. Hyaluronic acid in dentoalveolar regeneration: biological rationale and clinical applications. J Oral Biol Craniofac Res. 2024;14(2):230-235.
doi pubmed pmc - Pascali M, Quarato D, Carinci F. Filling procedures for lip and perioral rejuvenation: a systematic review. Rejuvenation Res. 2018;21(6):553-559.
doi pubmed - Taylor SC, Downie JB, Shamban A, Few J, Weichman BM, Schumacher A, Gallagher CJ. Lip and perioral enhancement with hyaluronic acid dermal fillers in individuals with skin of color. Dermatol Surg. 2019;45(7):959-967.
doi pubmed - Maas C, Kane MA, Bucay VW, Allen S, Applebaum DJ, Baumann L, Cox SE, et al. Current aesthetic use of abobotulinumtoxinA in clinical practice: an evidence-based consensus review. Aesthet Surg J. 2012;32(1 Suppl):8S-29S.
doi pubmed - Fedorowicz Z, van Zuuren EJ, Schoones J. Botulinum toxin for masseter hypertrophy. Cochrane Database Syst Rev. 2013;2013(9):CD007510.
doi pubmed pmc - Etemad-Moghadam S. Future perspectives of botulinum toxin application in dentistry. In: Jabbari B, editor. Botulinum toxin treatment in surgery, dentistry, and veterinary medicine. 2020; p. 359-389.
- Stojanovic L, Majdic N. Effectiveness and safety of hyaluronic acid fillers used to enhance overall lip fullness: a systematic review of clinical studies. J Cosmet Dermatol. 2019;18(2):436-443.
doi pubmed - Czumbel LM, Farkasdi S, Gede N, Miko A, Csupor D, Lukacs A, Gaal V, et al. Hyaluronic acid is an effective dermal filler for lip augmentation: a meta-analysis. Front Surg. 2021;8:681028.
doi pubmed pmc - Steenen SA, Bauland CG, de Lange J, van der Lei B. Complications after botulinum neurotoxin type A and dermal filler injections: data from a large retrospective cohort study. Aesthet Surg J. 2023;43(1):NP56-NP63.
doi pubmed pmc - Alam M, Kakar R, Nodzenski M, Ibrahim O, Disphanurat W, Bolotin D, Borovicka JH, et al. Multicenter prospective cohort study of the incidence of adverse events associated with cosmetic dermatologic procedures: lasers, energy devices, and injectable neurotoxins and fillers. JAMA Dermatol. 2015;151(3):271-277.
doi pubmed - Signorini M, Liew S, Sundaram H, De Boulle KL, Goodman GJ, Monheit G, Wu Y, et al. Global aesthetics consensus: avoidance and management of complications from hyaluronic acid fillers-evidence- and opinion-based review and consensus recommendations. Plast Reconstr Surg. 2016;137(6):961e-971e.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


