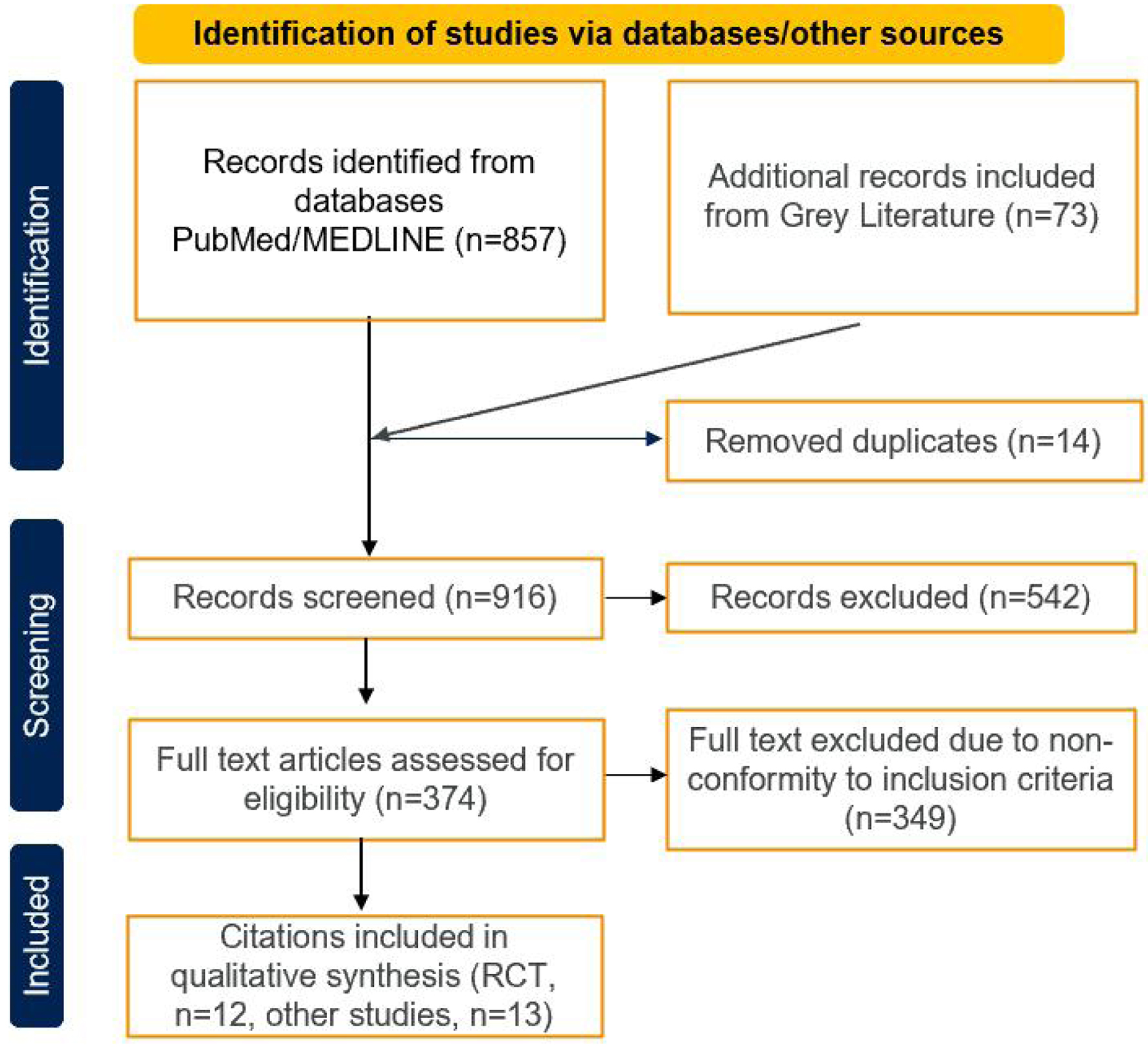
Figure 1. PRISMA flowchart.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Review
Volume 16, Number 6, June 2024, pages 273-283
Botulinum Toxin Type A and Hyaluronic Acid Dermal Fillers in Dentistry: A Systematic Review of Clinical Application and Indications
Figures

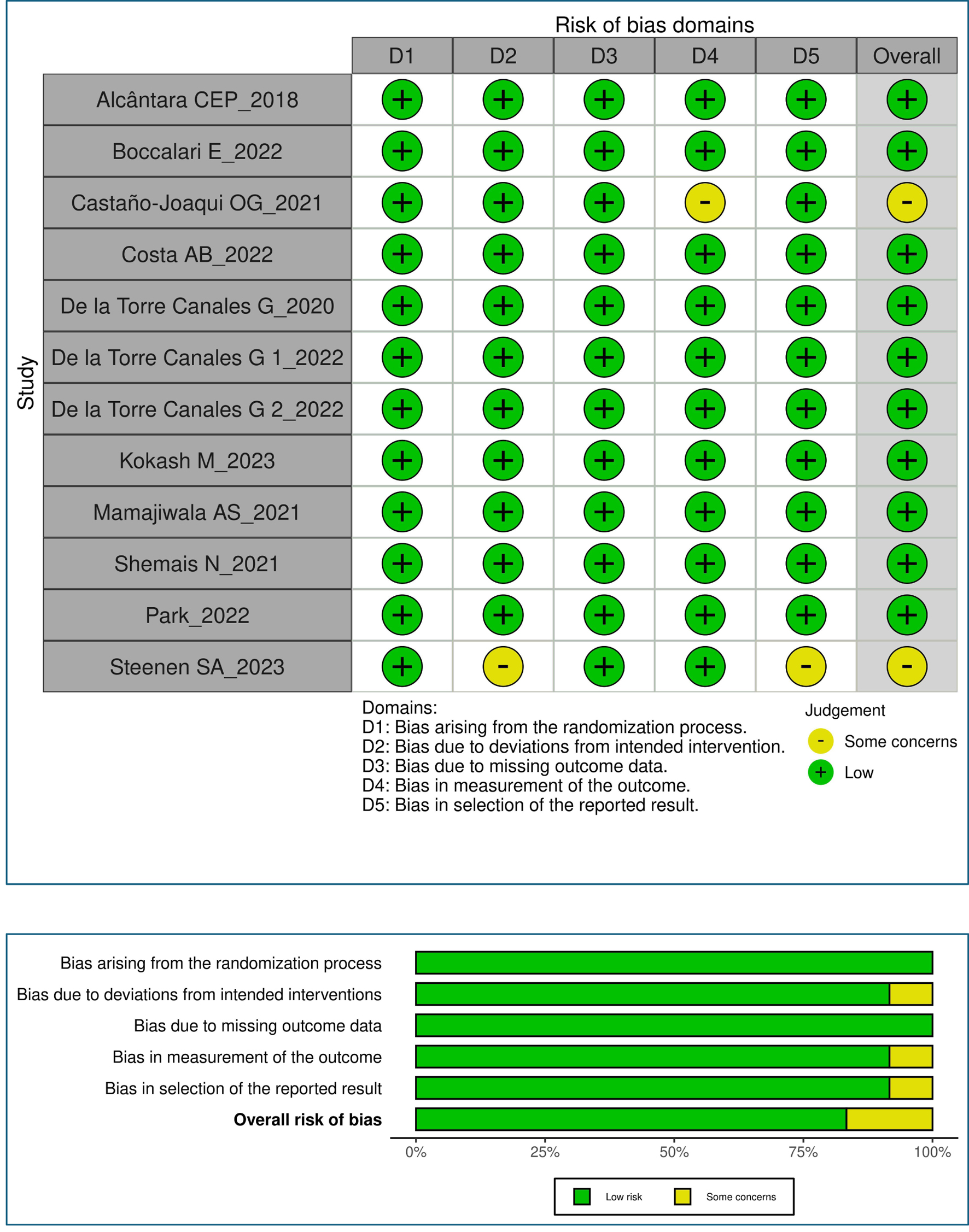
Tables
| Treatment goal | Area of injection | Product | Treatment overtime |
|---|---|---|---|
| BoNT-A: botulinum toxin type A; TMD: temporomandibular disorder. | |||
| Bruxism, facial pain and TMD | Masseter and temporal muscles | BoNT-A | 4 - 6 months |
| Mentalis muscle relaxation | Mental muscle | BoNT-A | 4 - 6 months |
| Orbicularis oris muscle relaxation | Orbicularis oris | BoNT-A | 4 - 6 months |
| Gummy smile | Levator labii superioris and depressor septi nasi | BoNT-A or hyaluronic acid | 4 - 6 months |
| Harmonization profile of class III malocclusions | Upper lip and cheekbone | Hyaluronic acid | 6 - 8 months |
| Harmonization profile of class II malocclusions | Chin | Hyaluronic acid | 9 - 12 months |
| Increase lip volume and hydration | Upper and lower lips | Hyaluronic acid | 6 - 8 months |
| Promoting tissue regeneration in periodontal and implant defect | In situ | Hyaluronic acid | Depending on the defect |
| Gingival recession | Periodontal tissues | Hyaluronic acid | Depending on the defect |
| # | Study | Year | Location | Type | NCT number | Duration and follow-up | Sample size | Age (mean ± SD) | Gender | Objective | Application in dentistry |
|---|---|---|---|---|---|---|---|---|---|---|---|
| RCT: randomized controlled trial; SD: standard deviation; HA: hyaluronic acid; TMJ: temporomandibular joint; BTX-A/BoNT-A: botulinum toxin type A; TMD: temporomandibular disorder; NR: not reported; MFP: myofascial pain; OFD: open flap debridement; OA: oral appliance. | |||||||||||
| 1 | Alcantara et al [13] | 2018 | Brazil | RCT | NCT02709525 | 90 days | 16 | 18.67 (SD = 7.95) | NR | Evaluate effects of HA on bone repair of dental sockets | Bone repair of dental sockets |
| 2 | Boccalari et al [14] | 2022 | Italy | RCT | NCT04438421 | Up to 21 days | 50 | H2O2/HA: 35 (12.5), placebo: 40 (13.4) | M: 23, F: 27 | Compare effectiveness of H2O2/HA mouth rinse vs. placebo on gingivitis | Gingivitis improvement |
| 3 | Castano-Joaqui et al [15] | 2021 | Spain | RCT | NCT04110587 | Up to 12 months | 51 | 41 (18 to 76) | M: 4, F: 47 | Determine effects of HA as adjunct to TMJ arthroscopy | HA adjunct in TMJ arthroscopy |
| 4 | Costa et al [16] | 2022 | Brazil | RCT | NCT03812965 | Up to 25 weeks | 17 | Group 1: 28.8 (4.6), group 2: 27.4 (5.7) | F: 18, M: 2 | Evaluate effects of BTX-A on excessive gingiva display reduction | Gummy smile treatment |
| 5 | De la Torre Canales et al [17] | 2020 | Brazil | RCT | ReBEC RBR-2d4vvv | Up to 24 weeks | 100 | 36.8 ± 5.6 | F: 100 | Assess safety and efficacy of different BoNT-A doses for persistent myofascial pain | Management of persistent MFP |
| 6 | De la Torre Canales et al [18] | 2022 | Brazil | RCT | ReBEC RBR-2d4vvv | Up to 180 days | 80 | NR | F: 80 | Evaluate effects of BoNT-A on mandibular range of motion and muscle tenderness in MFP patients | Mandibular range of motion and muscle tenderness in MFP patients |
| 7 | De la Torre Canales et al [19] | 2022 | Brazil | RCT | ReBEC RBR-2d4vvv | Up to 72 months | 14 | NR | F: 14 | Assess long-term effects of BoNT-A on pain and muscle thickness in MFP-TMD patients | Pain improvement and muscle adverse effects |
| 8 | Kokash et al [20] | 2023 | Syria | RCT | ISRCTN16820104 | Up to 7 days | 20 | 22.7 ± 3.079 | F: 75%, M: 25% | Study efficacy of HA addition to collagen on swelling and trismus post-molar surgery | Postoperative swelling and mouth-opening limitation |
| 9 | Mamajiwala et al [21] | 2021 | India | RCT | CTRI/2018/03/012334 | Up to 12 months | 20 | 39.9 ± 4.18 | M: 55%, F: 45% | Compare HA gel with OFD vs. OFD alone in periodontal intrabony defect treatment | Treatment of periodontal intrabony defects |
| 10 | Park et al [22] | 2022 | Korea | RCT | NR | 24 weeks | 30 | 31.8 | M: 5, F: 25 | Evaluate combined effects of BoNT and OA therapy on lower facial contouring | Esthetically effective lower facial contouring |
| 11 | Shemais et al [23] | 2021 | Egypt | RCT | NCT03717987 | Up to 24 weeks | 25 | Control: 25.3, test: 25 | M: control 8.3%, test 7.7%; F: control 91.7%, test 92.3% | Evaluate effect of oral zinc supplement on BTX-A efficacy for excessive gingival display | Excessive gingival display reduction |
| 12 | Steenen et al [24] | 2023 | Netherlands | RCT | NCT04362891 | Up to 13 weeks | 143 | NR | F: 143 | Assess superiority among HA dermal fillers for lip augmentation | Lip augmentation durability and safety |