| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Review
Volume 16, Number 5, May 2024, pages 197-207
Male Breast Cancer: Imaging Considerations for Diagnosis and Surveillance
Mathew Thomasa, d, Hatem Al Kashroomb, Shilpa Reddyc, Daniel Zaccarinic, Katherine Willerb
aDepartment of Internal Medicine, State University of New York (SUNY) Upstate, Syracuse, NY, USA
bDepartment of Radiology, State University of New York (SUNY) Upstate, Syracuse, NY, USA
cDepartment of Pathology, State University of New York (SUNY) Upstate, Syracuse, NY, USA
dCorresponding Author: Mathew Thomas, Department of Internal Medicine, State University of New York (SUNY) Upstate, Syracuse, NY 13210, USA
Manuscript submitted April 5, 2024, accepted May 13, 2024, published online May 29, 2024
Short title: Radiological Evaluation in Male Breast Cancer
doi: https://doi.org/10.14740/jocmr5169
- Abstract
- Introduction
- Risk Factors and Pathological Characteristics
- Diagnosis
- When to Do a Mammogram in Men?
- Mammographic Appearance
- Ultrasound in Male Breast Cancer
- MRI in Male Breast Cancer
- Follow-Up Imaging in Breast Cancer Survivors
- Conclusions
- References
| Abstract | ▴Top |
Male breast cancer accounts for less than 1% of all breast cancer cases. The important risk factors for the development of male breast cancer are family history, genetic mutations, obesity, liver disease, alcoholism, exogenous estrogen administration, and radiation exposure to the chest area. Despite its rarity, numerous studies have investigated the data on imaging considerations (mammogram, ultrasound, and magnetic resonance imaging (MRI)), but have addressed only certain aspects of male breast cancer. A comprehensive approach on the imaging characteristics, timing of imaging, prognostication based on imaging characteristics, and follow-up strategies in male breast cancer are still lacking. The purpose of this review article was to provide a comprehensive overview of the imaging findings, optimal timing to obtain imaging, and the appropriate follow-up strategies in male breast cancer survivors. This article also describes how imaging modalities can aid in determining prognosis. By addressing this knowledge gap, the article provides valuable insights for clinicians managing this uncommon yet clinically significant disease.
Keywords: Male breast cancer; Mammogram; Sonogram; MRI in male breast cancer; Surveillance; Screening
| Introduction | ▴Top |
Male breast cancer is a rare clinical entity, accounting for less than 1% of all breast cancer cases [1-3]. In 2023, an estimated 2,800 new cases of invasive breast cancer are expected to be diagnosed in men in the United States [4].
The adult male breast comprises skin, subcutaneous fat, atrophic ducts, and stromal elements [1]. Lobular breast development stimulated by estrogen and progesterone is rare in men. Hence, breast conditions associated with lobular proliferation including invasive lobular cancer (ILC), lobular carcinoma in situ (LCIS), fibroadenoma, and phyllodes tumor are uncommon in men [1]. Conditions related to ductal proliferation including gynecomastia, invasive ductal carcinoma (IDC), and ductal carcinoma in situ (DCIS) occur in men [1].
| Risk Factors and Pathological Characteristics | ▴Top |
The risk of male breast cancer increases with age, and cases tend to occur at an older age compared to female breast cancer [5-7]. According to the Surveillance Epidemiology and End Results (SEER) database, the median age at diagnosis of male breast cancer is 67 years, compared to 61 years in women [8]. Approximately 15-20% of men with breast cancer report a family history of breast or ovarian cancer [7]. About 10% of male breast cancers have a genetic predisposition, with BRCA2 being the most common genetic mutation [9-11]. Other genetic factors predisposing to male breast cancer are BRCA1, PTEN, TP53, PALB2, CHEK2 mutations, and Klinefelter syndrome [12-17]. Klinefelter syndrome is a rare genetic disorder characterized by an additional X chromosome (47, XXY karyotype), resulting in a spectrum of clinical features including behavioral difficulties, azoospermia, small testes, gynecomastia, and androgen deficiency [18]. Apart from genetic risks, the other important risk factors are conditions that alter the estrogen/androgen ratio (obesity, liver disease, testicular conditions, alcoholism, exogenous estrogen administration), and radiation exposure to the chest area [19-21]. Gynecomastia is a benign breast condition affecting approximately 40-65% of males [19]. While some studies have suggested gynecomastia as a potential risk factor for male breast cancer, it is important to note that gynecomastia is usually associated with obesity and high estrogen states, such as alcoholism or liver disease [19]. These associations could potentially confound the results, leading to false positives in determining the true impact of gynecomastia on male breast cancer.
Invasive ductal carcinoma is the most common histological subtype accounting for 85-90% of male breast cancer cases [22, 23], and ductal carcinoma in situ (DCIS) accounts for about 5% of the cases [24]. On histopathology, most cases of DCIS demonstrate a solid or cribriform pattern and is less common to see DCIS independent of invasive carcinoma of no special type [25]. Compared to female breast cancer, male breast cancer has higher rates of estrogen and progesterone receptor expression and lower rates of human epidermal growth factor receptor 2 (HER2)-neu expression [26-28]. Male breast cancer usually presents as a painless unilateral subareolar mass, often eccentric to the nipple [27]. It is also important to exclude the possibility of metastatic tumors affecting the male breast, particularly one originating from prostatic adenocarcinoma or squamous cell carcinoma of the head and neck [29]. The pathological images of IDC are depicted in Figures 1, 2, and DCIS in Figure 3.
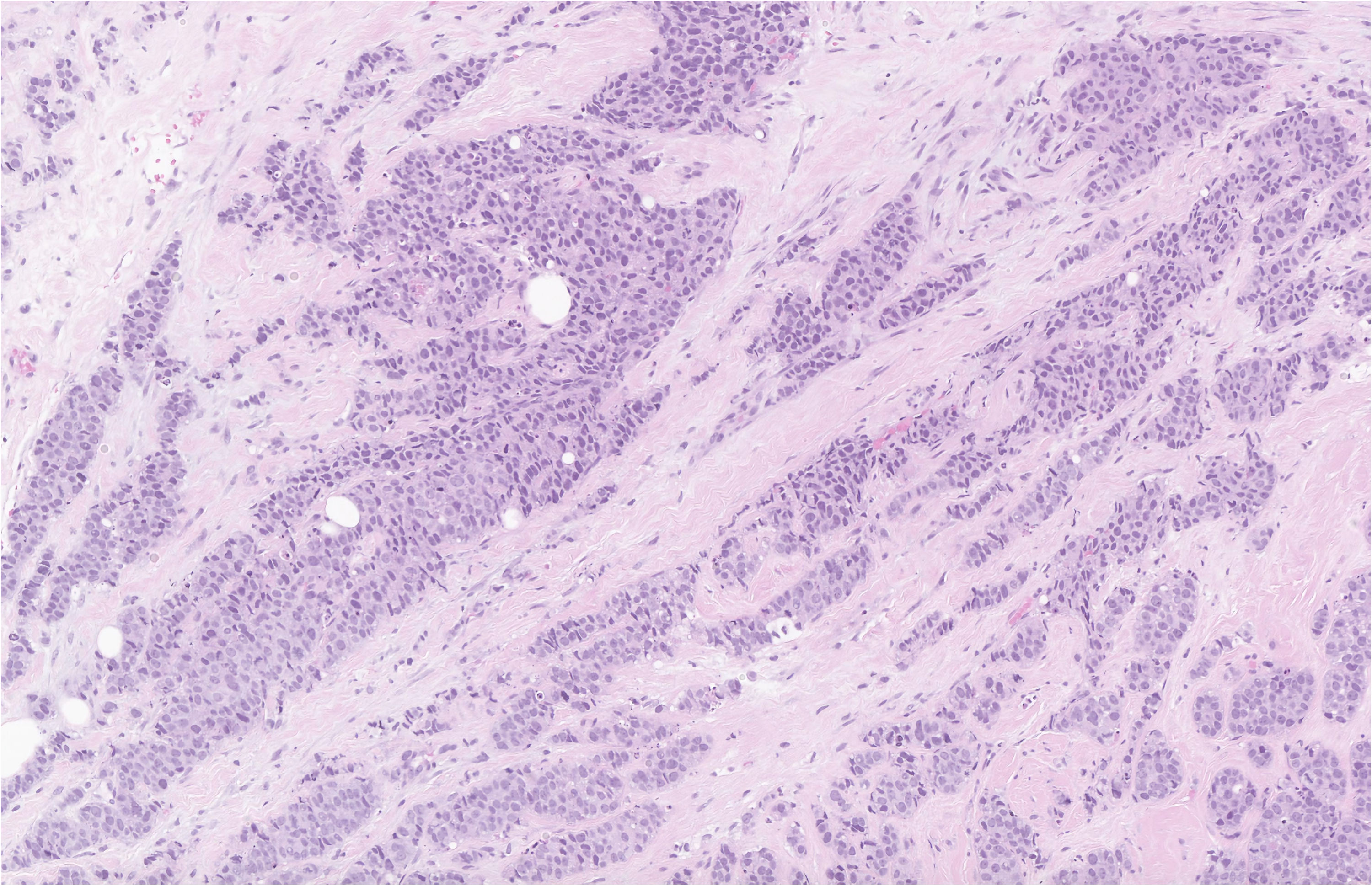 Click for large image | Figure 1. Hematoxylin and eosin stain of the breast biopsy demonstrating IDC with a nuclear grade of 3 at low power. The myoepithelial layer is lost and the tubuloglandular architecture seen in benign breast tissue is not preserved. |
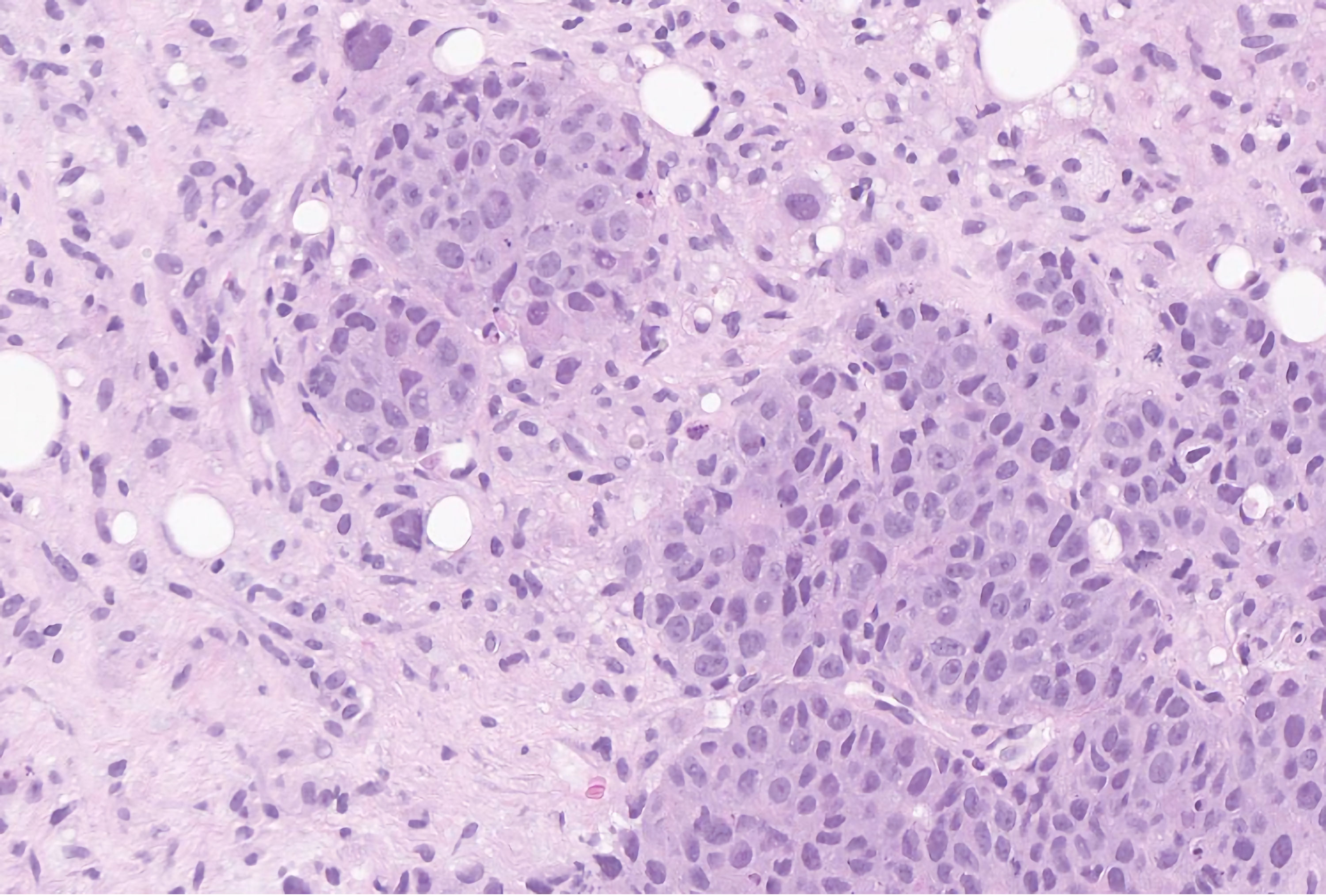 Click for large image | Figure 2. Hematoxylin and eosin stain of the breast biopsy demonstrating invasive carcinoma of no special type, with a nuclear grade of 3 at high power. The cells have bizarre shapes and sizes with prominent nuclei and hyperchromatic chromatin. GATA-3 and mammaglobin stained positively in the tumor cells, consistent with a breast primary. |
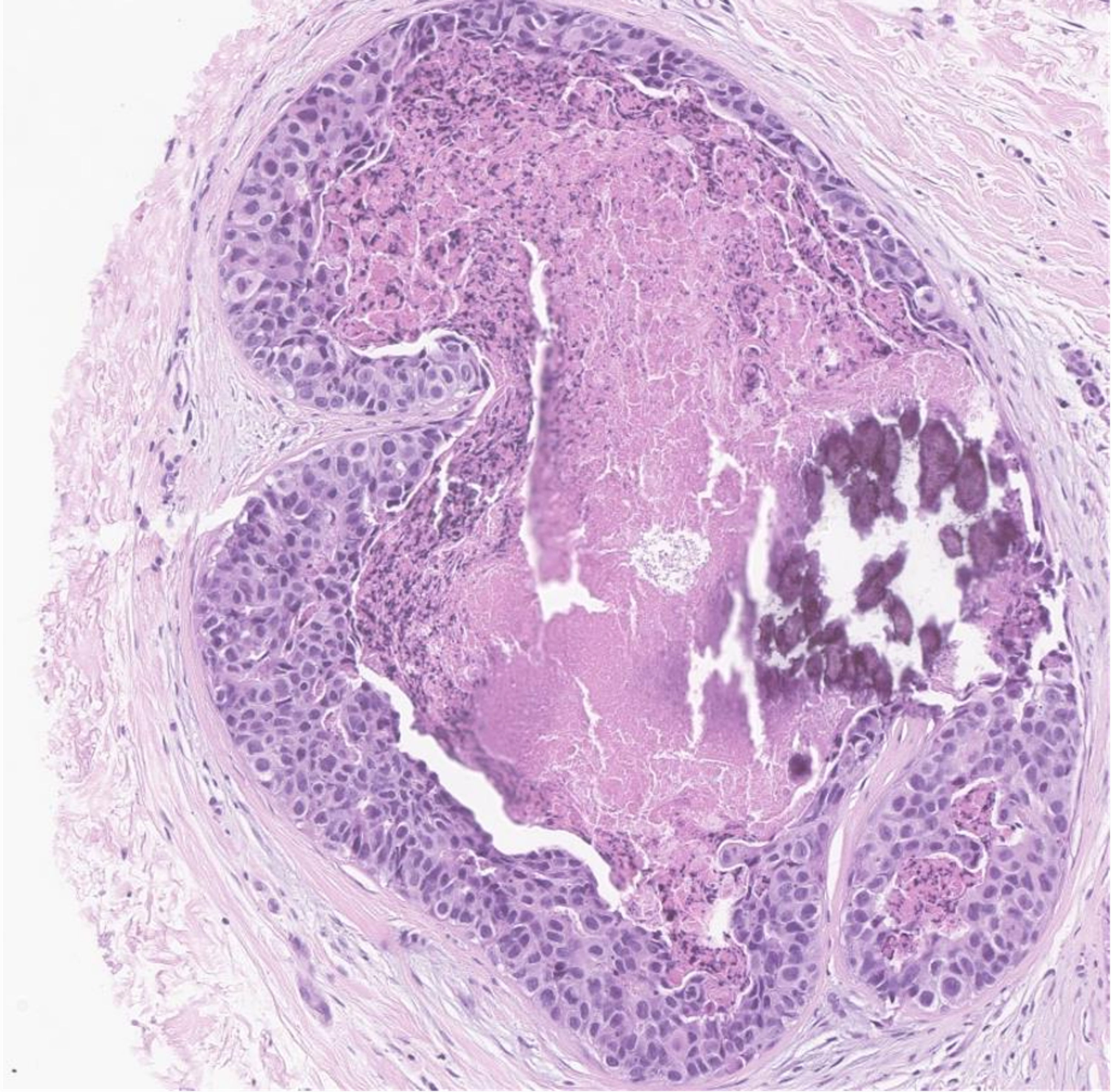 Click for large image | Figure 3. Hematoxylin and eosin stain of the breast biopsy demonstrating ductal carcinoma in situ with nuclear grade 3 at medium power. This patient had carcinoma with comedo and solid histological patterns, in addition to comedonecrosis and calcifications. The myoepithelial layer is maintained. The cells themselves are moderately pleomorphic with hyperchromatic chromatin. |
| Diagnosis | ▴Top |
As mentioned above, male breast cancer tends to occur at an older age compared to female breast cancer [5-7]. The rarity of the disease, coupled with the low index of suspicion and lack of screening techniques, contributes to male breast cancer often being detected at a later stage compared to female breast cancer [8]. History and physical examination play a crucial role in the diagnosis. Increasing awareness among clinicians and utilizing imaging modalities can enhance the early detection of male breast cancer.
Male breast cancer poses diagnostic imaging challenges as benign breast conditions like gynecomastia must be differentiated from breast cancer. On a mammogram, gynecomastia appears as a flame-shaped density (without calcifications), gradually blending into the surrounding fat (Fig. 4) [3]. Other benign breast conditions arising from the skin and subcutaneous tissue like lipoma, epidermal inclusion cyst, pseudo-gynecomastia, and retro-areolar abscess should also be differentiated from male breast cancer. Data on the utilization of magnetic resonance imaging (MRI) in the evaluation of gynecomastia are limited, but its role in male breast cancer is discussed later. Figure 5a, b represents MRI findings suggestive of gynecomastia.
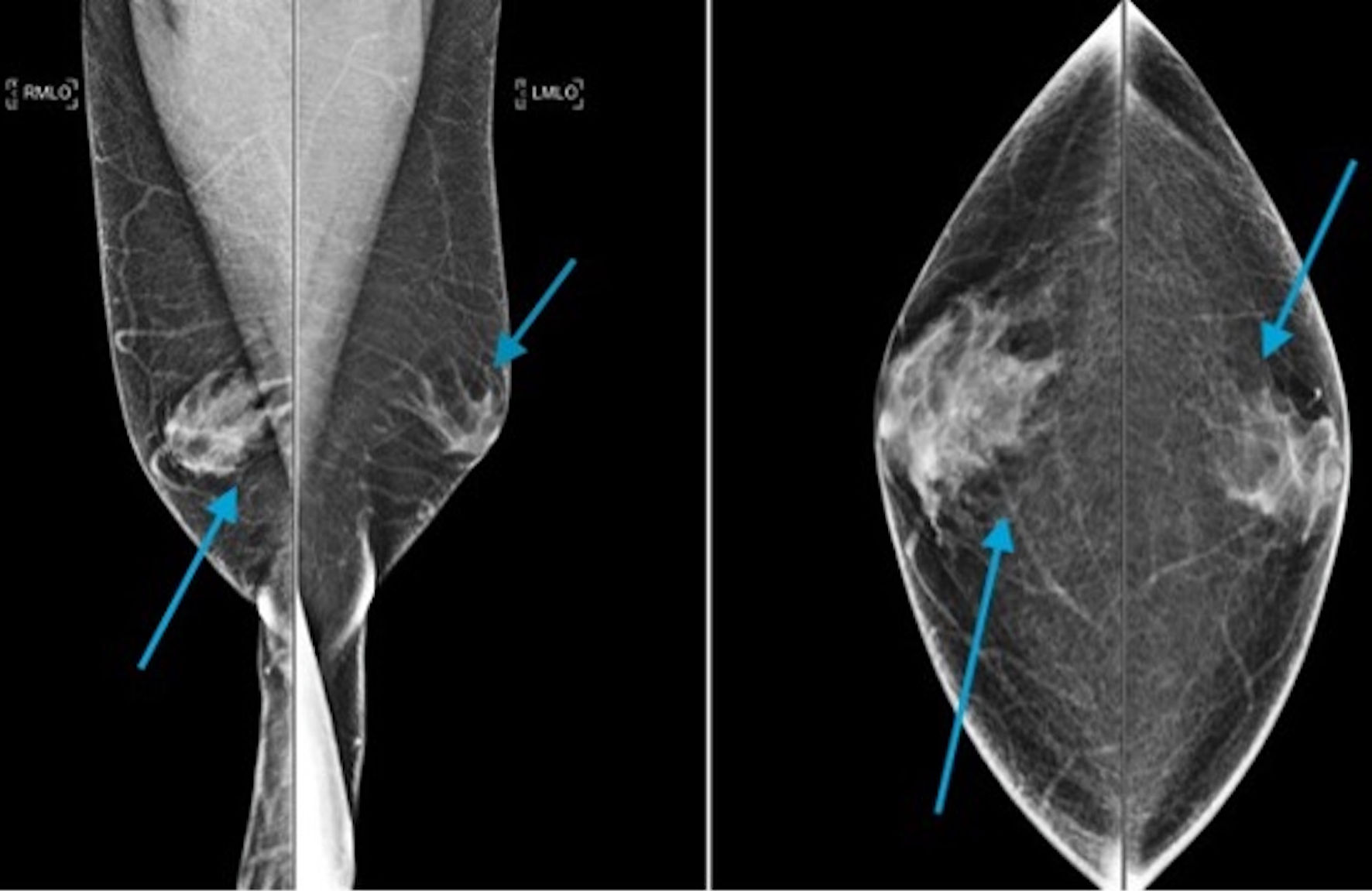 Click for large image | Figure 4. A mediolateral oblique digital mammogram demonstrating irregular flamed shaped structure within the subareolar region of the right breast extending into the adjacent fat, characteristic of gynecomastia (arrow heads). This is greater on the right than on the left side. |
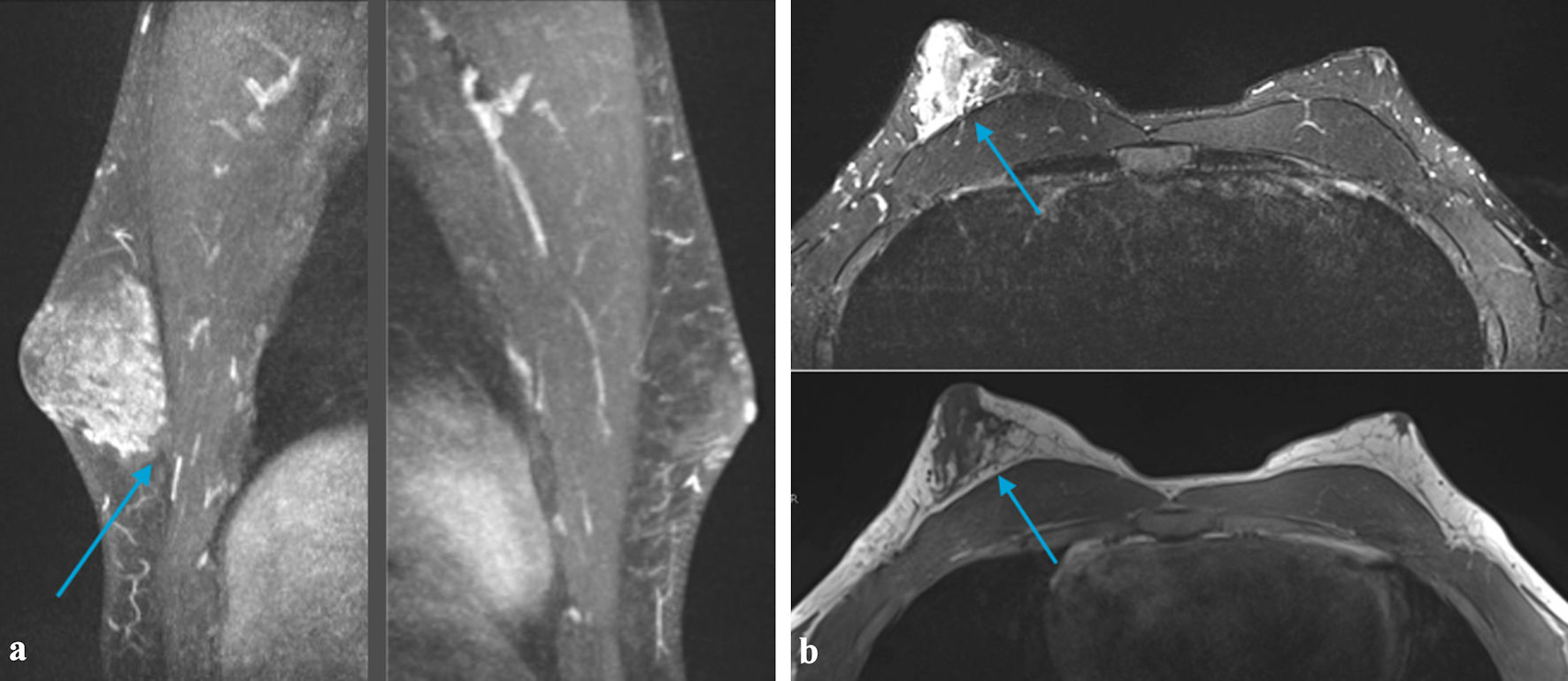 Click for large image | Figure 5. (a) MRI of the breast demonstrating a small area of heterogeneous tissue beneath the left areola. There is an asymmetrically larger amount of heterogeneous tissue within the subareolar region of the right breast extending into the central breast (arrow). These findings are suggestive of gynecomastia (This could be related to an underlying germ cell tumor and/or medications). No suspicious area of mass was identified within either breast. (b) MRI of the breast demonstrating small area of heterogeneous tissue beneath the left areola. There is an asymmetrically larger amount of heterogeneous tissue within the subareolar region of the right breast extending into the central breast (arrow). These findings are suggestive of gynecomastia (This could be related to an underlying germ cell tumor and/medications). No suspicious area of mass was identified within either breast. MRI: magnetic resonance imaging. |
| When to Do a Mammogram in Men? | ▴Top |
Mammography screening is highly successful in reducing mortality by early detection of cancer in women, but its role in male breast cancer is less well understood [30]. In general, a screening mammogram is not routinely recommended due to the low incidence of male breast cancer, and hence mammography is used as a diagnostic tool in men.
Symptomatic men above 25 years old should be evaluated with a diagnostic mammogram initially followed by an ultrasound if a mammogram is abnormal or inconclusive. For men less than 25 years, ultrasound followed by a mammogram is preferred [31]. A bilateral mammogram is preferred over a unilateral mammogram, as it can assess the symmetry and can also detect contralateral breast pathologies [31]. The sensitivity and specificity of mammogram in detecting male breast cancer is about 92-95% and 90-95%, respectively [30, 32, 33].
The National Comprehensive Cancer Network (NCCN) guidelines recommend that men with BRCA mutations get training for breast self-examination and start receiving yearly clinical breast examinations from 35 years of age [34]. The guidelines do not recommend screening mammography or MRI in male BRCA mutation carriers [34]. The 2020 American Society of Clinical Oncology (ASCO) guideline for the management of male breast cancer recommends ipsilateral annual mammogram be offered to men with a history of breast cancer treated with lumpectomy regardless of the genetic predisposition, and contralateral annual mammogram may be offered to men with a history of breast cancer and a genetic predisposition mutation [35].
A retrospective study conducted by Marino et al [36] investigated the utility of screening mammograms in men at high risk of breast cancer (personal history and/or family history of breast cancer, gynecomastia, etc.). The study included 163 men who underwent 806 screening mammograms. Seventy-seven percent (125/163) of the men had a personal history of breast cancer, and 44% (72/163) had a family history of breast cancer. Fifteen percent (24/163) were known mutation carriers; 17% (4/24) and 83% (20/24) were positive for BRCA1 and BRCA2, respectively. The study yielded a cancer detection rate of 4.9/1,000 mammograms, which is similar to that of screening mammograms in women (5.4/1,000) in population-based screening. This study concluded that screening mammograms can be considered in men at high risk of breast cancer.
| Mammographic Appearance | ▴Top |
In general, male breast cancer usually occurs in a subareolar location with nipple involvement in 40-50% of cases [37]. It can also occur eccentric to the nipple [3]. The mammogram findings suggestive of cancer include irregular mass with spiculated or indistinct margins [3, 31, 38, 39]. Oval or round masses with well-circumscribed margins are also not uncommon [3, 31, 38, 39]. Calcifications are present in 9-31% of male breast cancer cases but are fewer in number, coarser, and less frequently rod-shaped compared to female breast cancer [3, 40, 41]. Calcifications with typical benign features should be considered suspicious in men [38-40, 42-44]. Secondary signs of breast cancer, including nipple retraction, skin thickening, and axillary lymphadenopathy can also be detected with a mammogram.
A retrospective single-institution study conducted by Mathew et al investigated the clinical, imaging, and pathological abnormalities in male breast cancer [42]. Two hundred forty-four men with breast cancer were identified, out of which 57 patients underwent either mammogram or ultrasound, 49 patients underwent both mammography and ultrasound, six patients underwent only mammogram, and two patients underwent only ultrasound. The important mammogram findings observed were noncalcified mass alone (69%), a mass with microcalcifications (29%), and microcalcifications without a mass (2%). The masses were either irregular (50%), lobular (20%), oval (11%), or round (17%). Margins were frequently spiculated (33%), indistinct (32%), circumscribed (15%), or microlobulated (6%). Pleomorphic calcifications were present in 47% of the cases.
Dershaw et al conducted a retrospective study to identify the mammographic findings in male breast cancer. The study included 102 male breast cancer patients, out of which a mammogram was performed in 23 patients [44]. The findings observed were mass without calcifications (17 cases (74% of the cases)) and mass with microcalcifications (two cases (9% of the cases)). One case had microcalcifications (punctate, not pleomorphic type) without the presence of an underlying mass. Most of the tumors were subareolar. Secondary signs of malignancy including nipple inversion and axillary lymphadenopathy were also identified by a mammogram. Benign incidental findings like gynecomastia and skin calcifications were detected in four men.
Appelbaum et al evaluated the mammographic appearances and pathologic features in 97 cases of histologically proven male breast disease [3]. Twelve cases of male breast cancer were detected by mammogram. In 10 cases, the carcinoma manifested as a nodular lesion, with six lesions being eccentric, three central, and one distant relative to the nipple. The lesions were well-defined in six cases and ill-defined in four cases. The lesions were lobulated, round, and ovoid in shape. Primary breast cancer was not visible in one case as it was obscured by concomitant gynecomastia. Calcifications were fewer in number and coarser compared to female breast cancer. Secondary features including skin thickening, nipple retraction, and axillary lymphadenopathy were also detected by mammogram.
From a histopathological perspective, DCIS typically appears as microcalcifications on mammogram, which can present as amorphous, heterogenous, or coarse [45]. DCIS can appear as a mass on mammogram in approximately 10% of the cases and as architectural distortion in approximately 7-13% of the cases [45].
The mammographic appearance in male breast cancer is depicted in Figures 6-8.
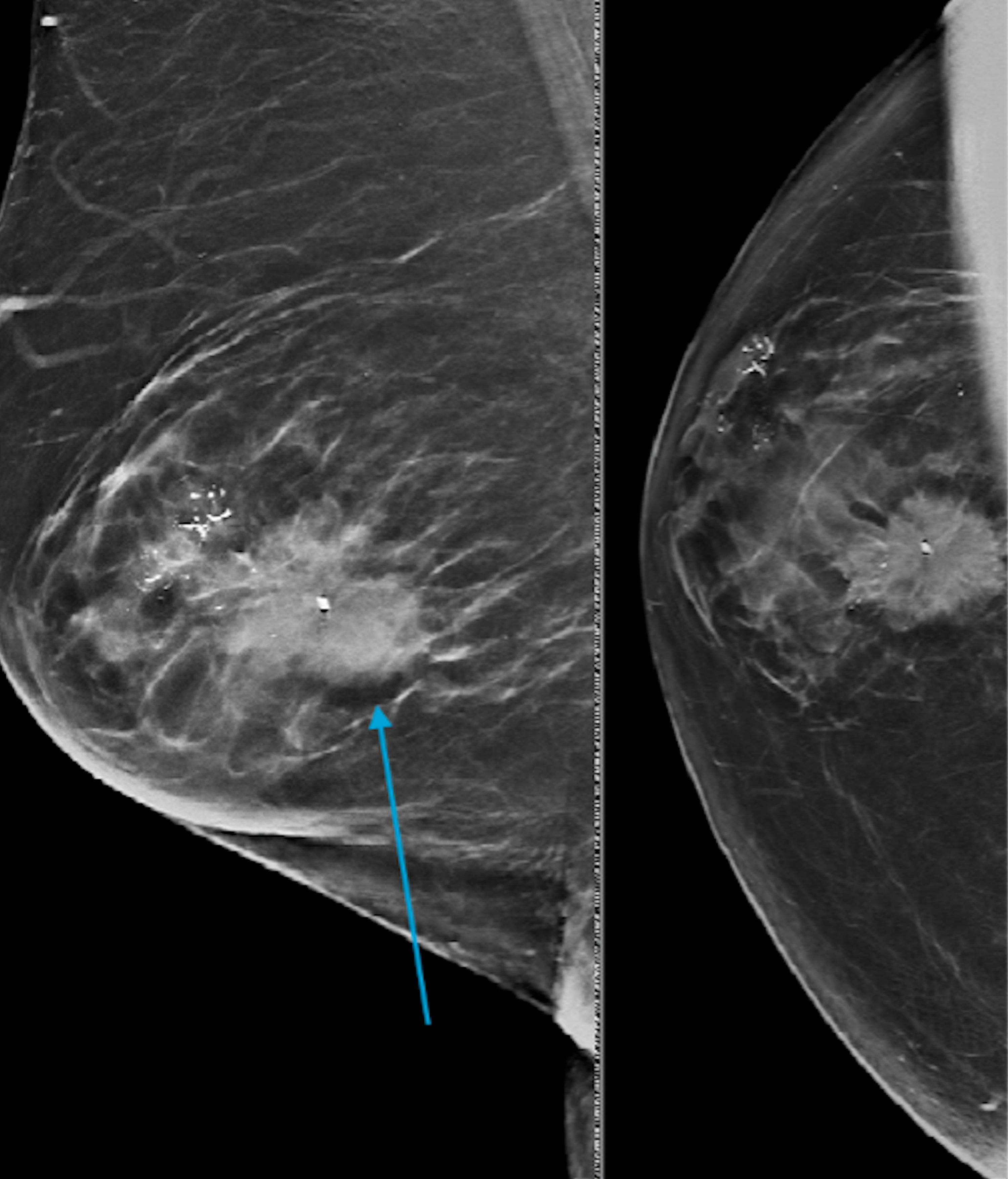 Click for large image | Figure 6. Bilateral diagnostic mammogram demonstrating an irregular mass with pleomorphic calcification in the right breast at 10:00 o’clock position (arrow) and 12:00 o’clock position. Moderate gynecomastia is present in the left breast. Biopsy of the mass at 10:00 o’clock revealed ductal carcinoma in situ and 12:00 o’clock revealed invasive ductal carcinoma. |
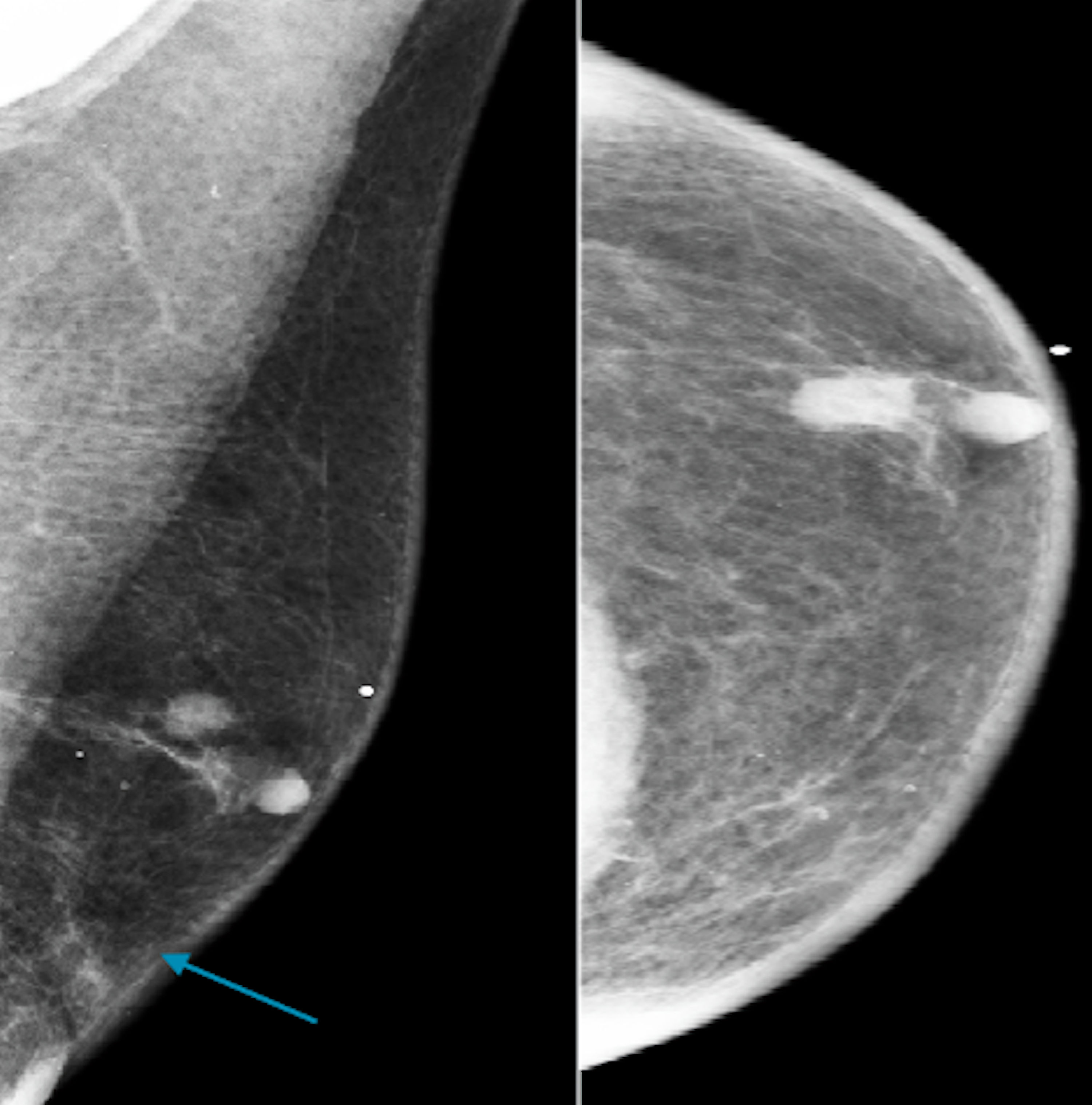 Click for large image | Figure 7. Craniocaudal and mediolateral oblique digital mammogram demonstrating scattered areas of fibro-glandular density and a suspicious mass (blue arrow) at 12:00 o’clock of the left breast according to BI-RADS 4. BIRADS: the Breast Imaging Reporting and Data System. |
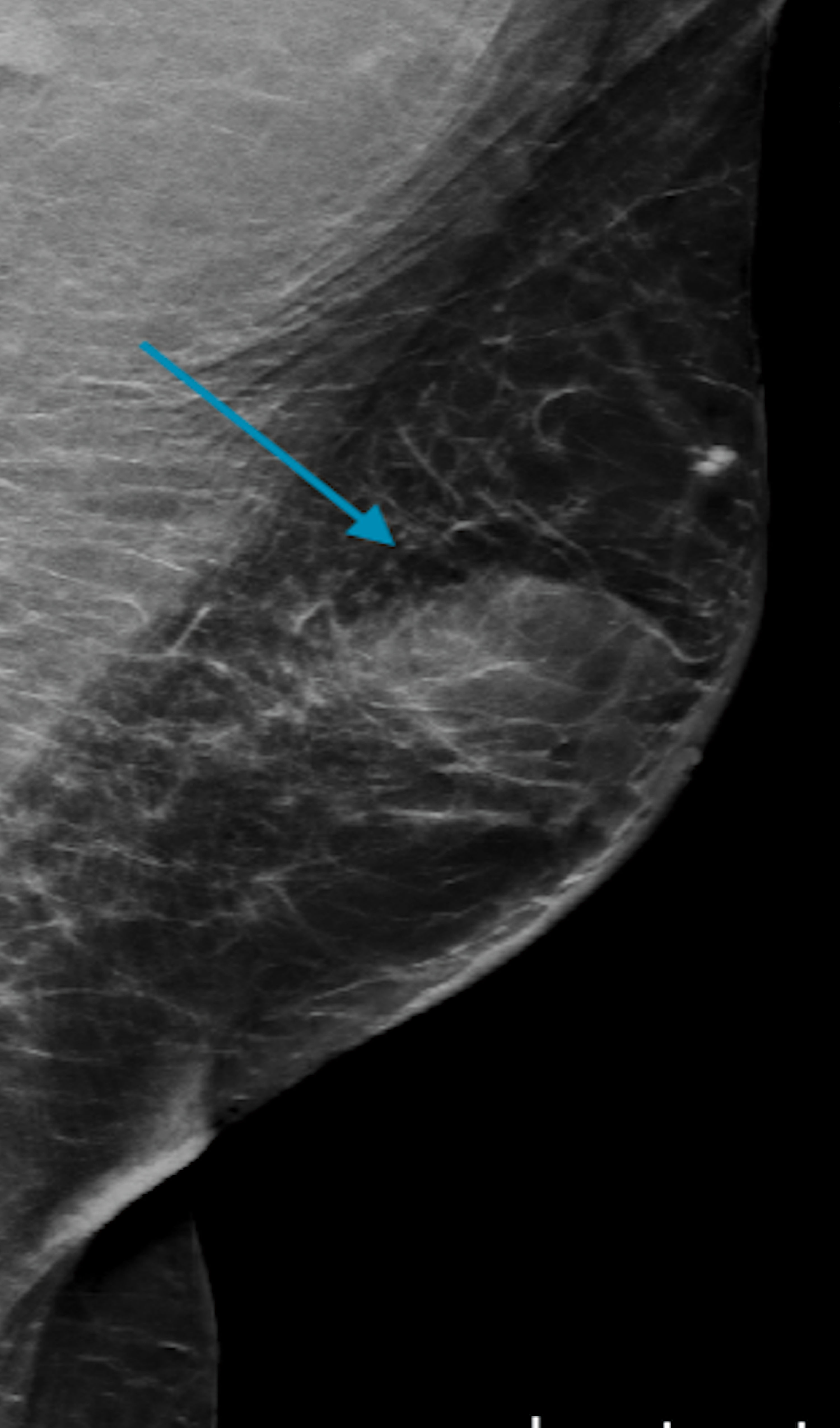 Click for large image | Figure 8. A mediolateral oblique digital mammogram illustrating a partially circumscribed indistinct mass in the central left breast (blue arrow). |
| Ultrasound in Male Breast Cancer | ▴Top |
In general, on ultrasound male breast cancer appears as hypoechoic lesions with angulated, microlobulated, or spiculated margins [46-48]. Ultrasound with Doppler studies can be used to identify the intra-lesional blood flow to assess the posterior extent of the disease. The sensitivity and specificity of ultrasound in detecting male breast cancer are about 88-100% and 95-97%, respectively [33, 49]. Ultrasound is more sensitive in detecting axillary lymphadenopathy than mammography in male breast cancer [33, 47].
As described above, the retrospective single-institution study conducted by Mathew et al also evaluated the sonographic findings in male breast cancer [42]. In this study, a total of 51 patients were evaluated by ultrasound and were able to demonstrate masses in 90% and architectural distortion in 10% of patients with cancer. Of the visible masses, 35 (69%) were solid, and 11 (22%) were complex with mixed solid and cystic components. The masses were irregular and hypoechoic in more than 50% of the patients. The margins were microlobulated, spiculated, circumscribed, or annular in nature. This study also assessed the vascularity of the lesions using power Doppler and demonstrated increased vascularity in 32 (70%) of the cases with cancer.
A retrospective study was conducted by Munoz Carrasco et al to assess the clinical variables useful in differentiating gynecomastia from carcinoma and also to analyze the contribution of mammography and ultrasound in the evaluation of male breast disease [33]. A total of 628 patients were included in the study, out of which 518 patients underwent mammograms, and 423 patients underwent ultrasounds. The imaging findings were classified according to the Breast Imaging Reporting and Data System (BI-RADS) lexicon [50]. Gynecomastia and pseudo-gynecomastia were placed in BI-RADS category 2. This study was able to detect 19 (2.9%) carcinomas, 526 (80.4%) gynecomastia, 84 other benign conditions, and 25 (3.8%) normal cases. The other benign breast conditions identified included epidermal inclusion cyst, lipoma, fibromatosis, post-surgical changes, and fat necrosis. Of the 423 ultrasound examinations, 388 cases (91.7%) were classified as BI-RADS 1 or 2 (0.5% were false-negative results) and 35 cases (8.3%) were classified as BI-RADS 3, 4, or 5 (54.2% were false-positive results). This study concluded that mammography was the most sensitive (94.7%) and ultrasound was the most specific (95.3%) for the detection of malignancy (P > 0.05).
Yang et al [48] evaluated the sonographic appearance in male breast cancer. Eight patients with a mean age of 69 years were included in the study, out of which four (50%) of the study population had complex cystic mass, and the remaining four patients had a solid heterogeneous lesion. In all the patients, masses were identified in the subareolar region eccentric to the nipple. The margins of the lesions were irregular in three patients, indistinct in three, microlobulated in one, and smooth in one patient. Posterior acoustic enhancement was visible in three patients, mild shadowing was visible in three and no acoustic phenomenon was visible in the remaining two patients.
From a histopathological perspective, DCIS is rarely seen on ultrasound [45]. However, it usually appears as a microlobulated mass with mild hypoechogenicity, ductal extension, and normal acoustic transmission [45]. Ultrasound is also used to evaluate the severity of cancer. Historically, malignant breast cancer was thought to show posterior acoustic shadowing on ultrasound [51]. However, it is now accepted that IDC may exhibit variable posterior acoustic appearances ranging from shadowing to enhancement to remaining unchanged. A study conducted by Aho et al [51] demonstrated that the association of posterior acoustic properties was impacted by age. Posterior shadowing was associated with lower histological grade and estrogen receptor (ER)+ status, especially in older adults, while posterior acoustic enhancement was more commonly associated with ER- status, especially in younger patients [51]. Malignant breast masses were also traditionally considered to exhibit ill-defined or spiculated margins, indicating a poor prognosis. However, subsequent studies have since demonstrated that well-defined margins are more likely to represent high-grade tumors [51].
The sonographic appearance of male breast cancer is described in Figures 9-12.
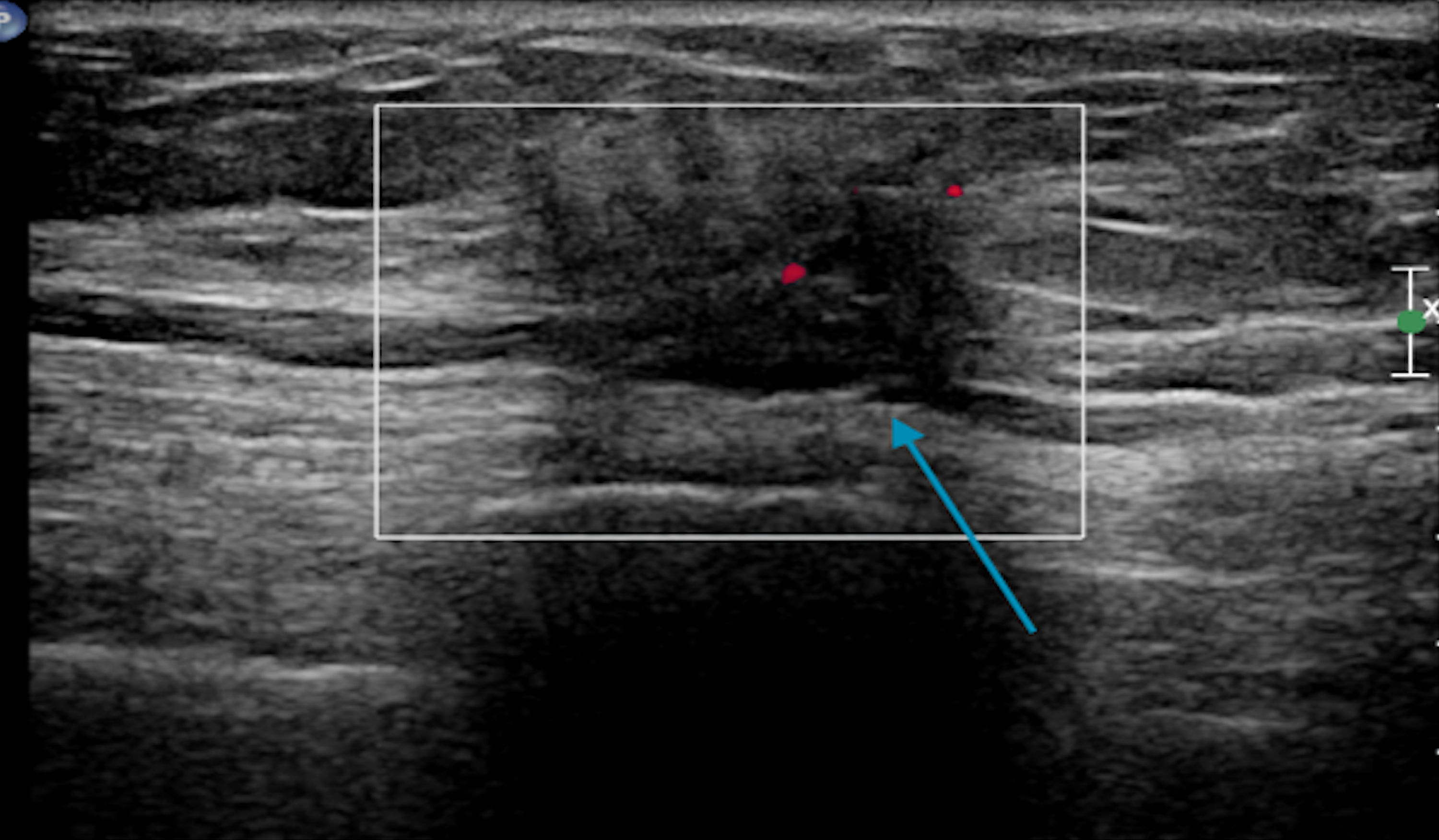 Click for large image | Figure 9. Targeted sonogram of the left breast showing a 1.1 × 0.7 × 0.8 cm irregular nonparallel spiculated hypoechoic mass with posterior shadowing (blue arrow). |
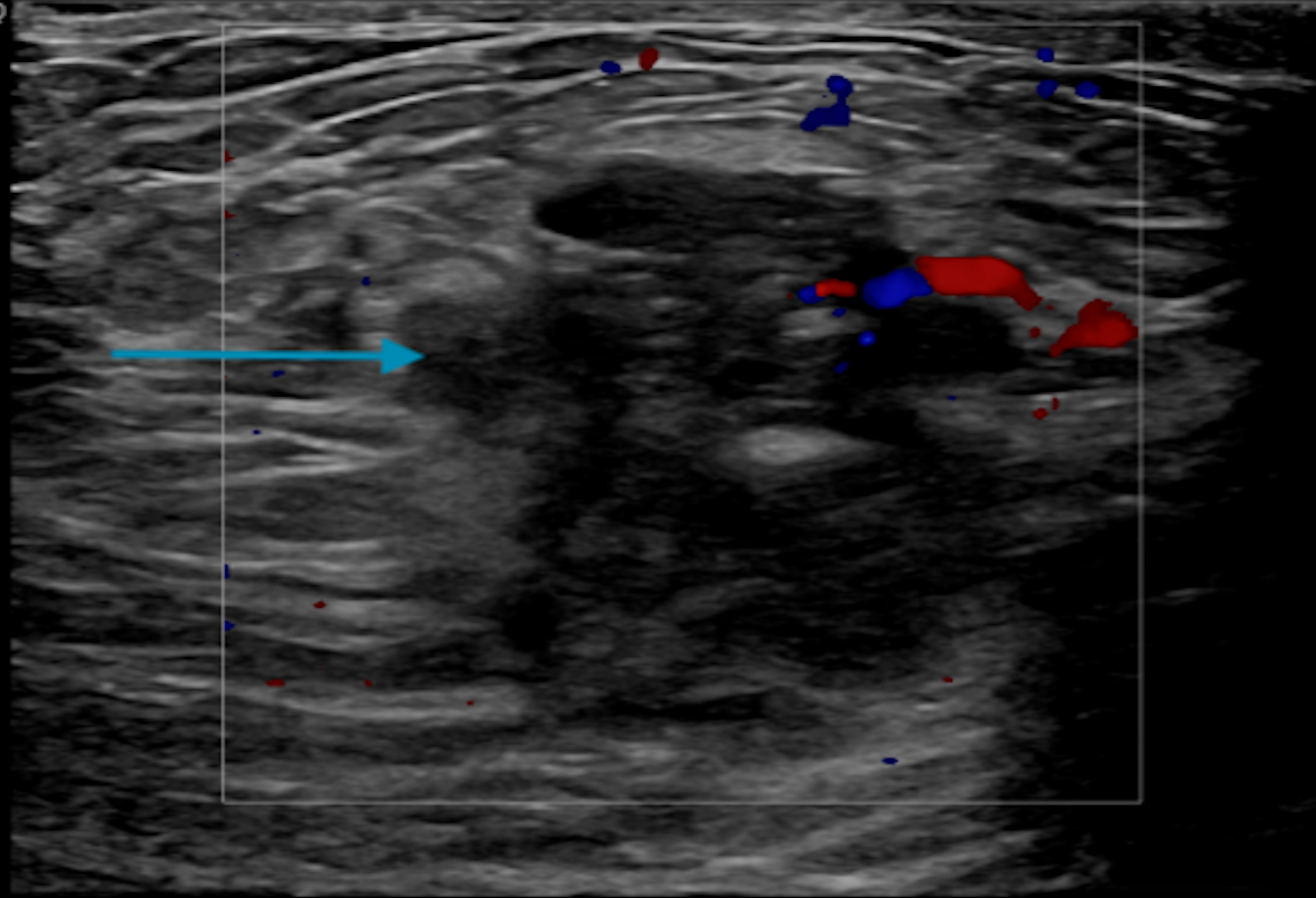 Click for large image | Figure 10. Single transverse image ultrasound of the right breast demonstrating an irregular 2.5 × 1.3 × 3 cm mass at 12:00 o’clock position and a 3.1 × 0.5 × 1.2 cm hypoechoic mass with calcifications at 10:00 o’clock position (blue arrow). |
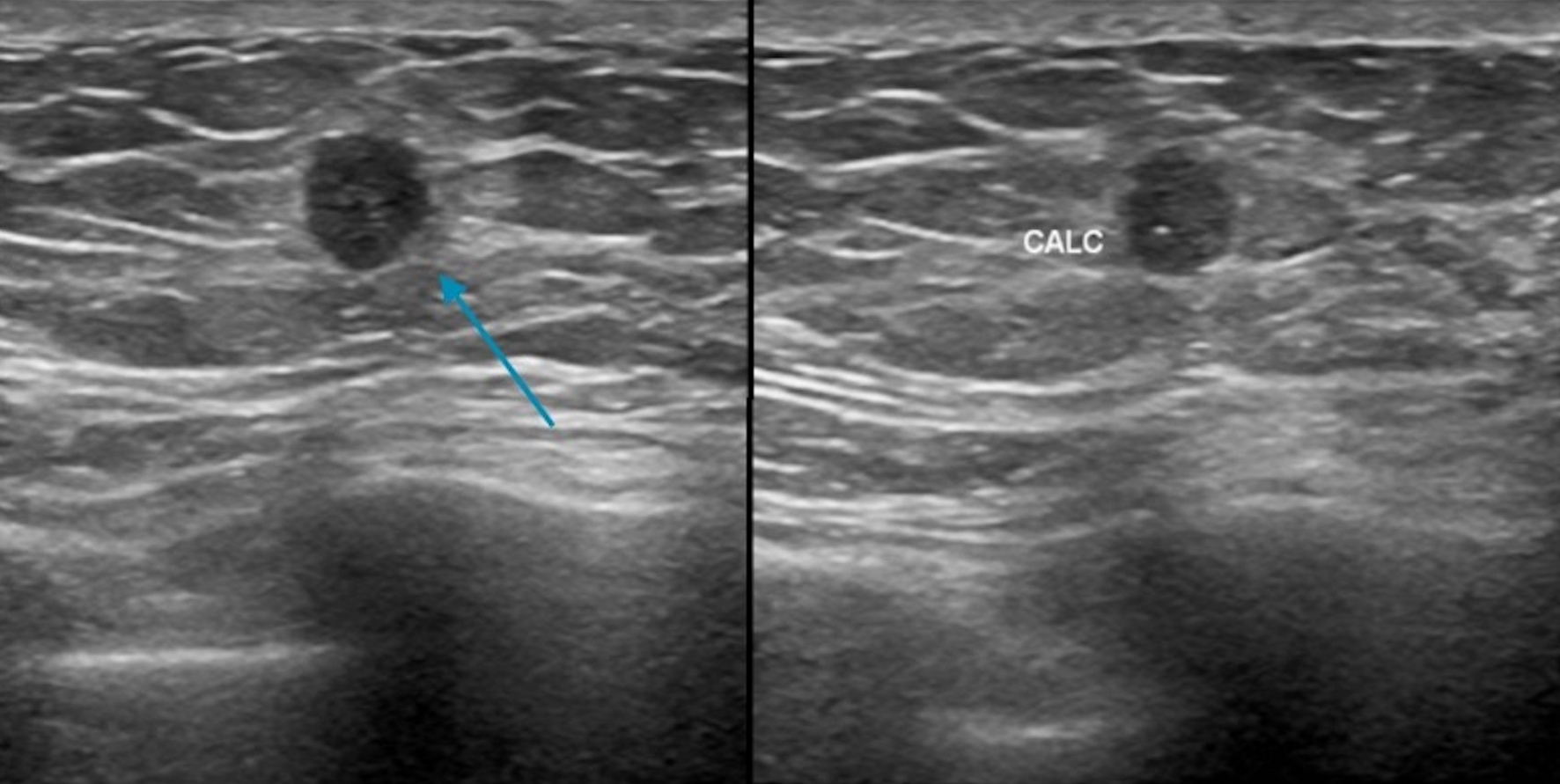 Click for large image | Figure 11. Ultrasound demonstrating a circumscribed mass (blue arrow) in the 12:00 o’clock position with calcifications. CALC: calcification. |
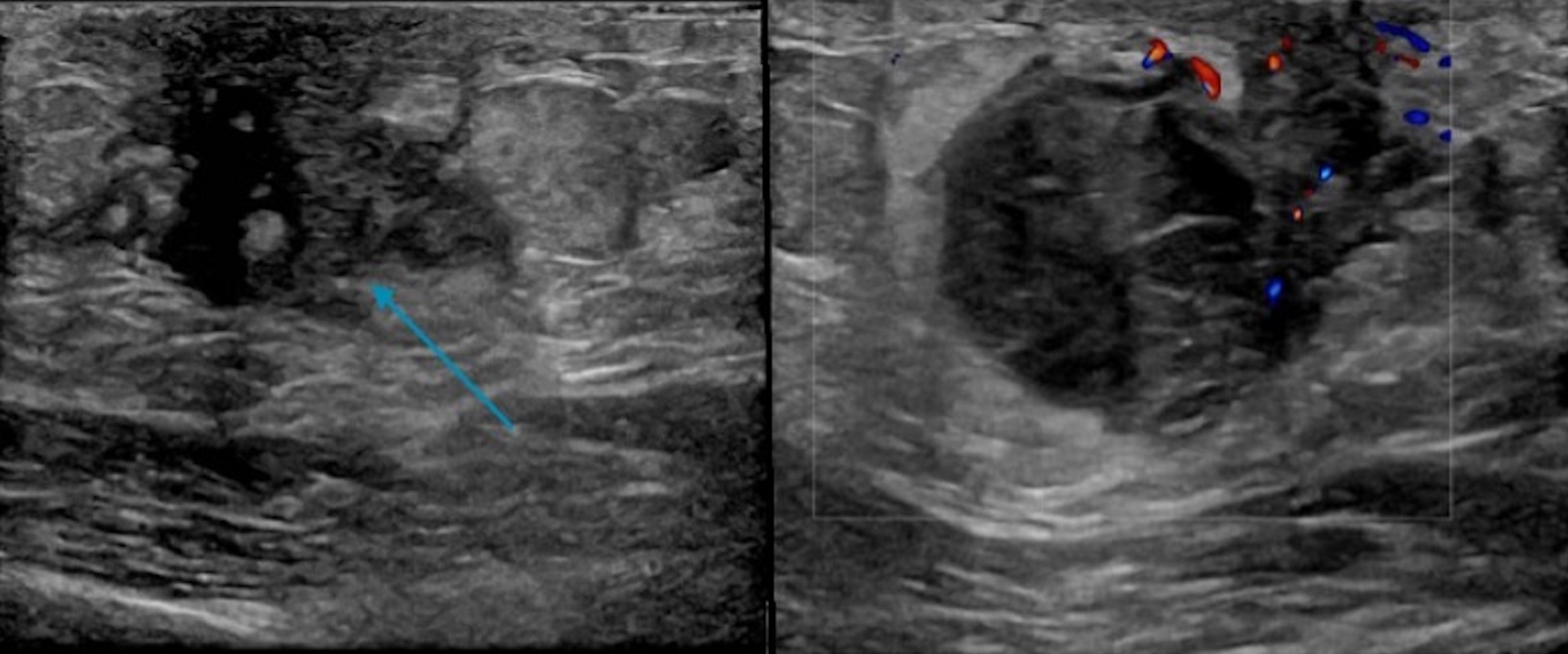 Click for large image | Figure 12. Targeted ultrasound displaying a 3.1 × 1.8 × 2.7 cm hypoechoic mainly circumscribed mass (blue arrow) behind the left nipple with mild surrounding edema (no left axillary lymphadenopathy). |
| MRI in Male Breast Cancer | ▴Top |
Breast MRI has been shown to have the highest sensitivity in detecting male breast cancer compared to ultrasound and mammogram [52, 53]. However, breast MRI is not routinely recommended for male breast cancer but can be helpful in a subset of patients (Table 1) [52, 54]. MRI to evaluate for the residual disease has to be done immediately in the post-surgical period before the formation of granulation tissue, which could make the interpretation difficult [52].
 Click to view | Table 1. Indications for MRI in Male Breast Cancer |
| Follow-Up Imaging in Breast Cancer Survivors | ▴Top |
The absolute risk of second breast cancer in male breast cancer is less than 2%, and hence, in general, a follow-up mammogram is not routinely recommended for the early-stage disease [55].
| Conclusions | ▴Top |
Male breast cancer is a rare clinical entity and poses unique pathological characteristics compared to female breast cancer. A male breast can be affected by different benign and malignant pathologies, with gynecomastia being the most common pathology. Due to its rarity, screening techniques are not routinely recommended but can be considered in high-risk men. Imaging techniques in the male breast are done as a diagnostic tool. There can be an overlap in the clinical and imaging features of benign and malignant breast pathologies, and hence familiarizing the imaging features helps in the radiological diagnoses. All imaging modalities have their unique advantages and applications in the diagnosis of male breast cancer. According to the literature to date, a routine mammogram is not recommended for follow-up in male breast cancer survivors due to the absolute low risk of second male breast cancer.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Author Contributions
Mathew Thomas: study conception, design, and drafting the manuscript. Hatem Al Kashroom: data collection (radiology images) and interpretation of the results. Shilpa Reddy and Daniel Zaccarini: data collection (pathology images) and interpretation of the results. Katherine Willer reviewed the manuscript and supervised the project. All authors reviewed the manuscript and approved the final version of the manuscript.
Data Availability
The authors declare that the data supporting the findings of this study are available within the article.
| References | ▴Top |
- Nguyen C, Kettler MD, Swirsky ME, Miller VI, Scott C, Krause R, Hadro JA. Male breast disease: pictorial review with radiologic-pathologic correlation. Radiographics. 2013;33(3):763-779.
doi pubmed - Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67(1):7-30.
doi pubmed - Appelbaum AH, Evans GF, Levy KR, Amirkhan RH, Schumpert TD. Mammographic appearances of male breast disease. Radiographics. 1999;19(3):559-568.
doi pubmed - BREASTCANCER.ORG. U.S. Breast Cancer Statistics. 2022. Available from: https://www.breastcancer.org/symptoms/understand_bc/statistics#:∼:text=About%202,620%20new%20cases%20of%20invasive%20breast%20cancer,expected%20to%20die%20in%202020%20from%20breast%20cancer.
- Anderson WF, Althuis MD, Brinton LA, Devesa SS. Is male breast cancer similar or different than female breast cancer? Breast Cancer Res Treat. 2004;83(1):77-86.
doi pubmed - O'Malley CD, Prehn AW, Shema SJ, Glaser SL. Racial/ethnic differences in survival rates in a population-based series of men with breast carcinoma. Cancer. 2002;94(11):2836-2843.
doi pubmed - Giordano SH, Buzdar AU, Hortobagyi GN. Breast cancer in men. Ann Intern Med. 2002;137(8):678-687.
doi pubmed - Korde LA, Zujewski JA, Kamin L, Giordano S, Domchek S, Anderson WF, Bartlett JM, et al. Multidisciplinary meeting on male breast cancer: summary and research recommendations. J Clin Oncol. 2010;28(12):2114-2122.
doi pubmed pmc - Couch FJ, Farid LM, DeShano ML, Tavtigian SV, Calzone K, Campeau L, Peng Y, et al. BRCA2 germline mutations in male breast cancer cases and breast cancer families. Nat Genet. 1996;13(1):123-125.
doi pubmed - Haraldsson K, Loman N, Zhang QX, Johannsson O, Olsson H, Borg A. BRCA2 germ-line mutations are frequent in male breast cancer patients without a family history of the disease. Cancer Res. 1998;58(7):1367-1371.
pubmed - Wooster R, Bignell G, Lancaster J, Swift S, Seal S, Mangion J, Collins N, et al. Identification of the breast cancer susceptibility gene BRCA2. Nature. 1995;378(6559):789-792.
doi pubmed - Brose MS, Rebbeck TR, Calzone KA, Stopfer JE, Nathanson KL, Weber BL. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. J Natl Cancer Inst. 2002;94(18):1365-1372.
doi pubmed - Meijers-Heijboer H, van den Ouweland A, Klijn J, Wasielewski M, de Snoo A, Oldenburg R, Hollestelle A, et al. Low-penetrance susceptibility to breast cancer due to CHEK2(*)1100delC in noncarriers of BRCA1 or BRCA2 mutations. Nat Genet. 2002;31(1):55-59.
doi pubmed - Fackenthal JD, Marsh DJ, Richardson AL, Cummings SA, Eng C, Robinson BG, Olopade OI. Male breast cancer in Cowden syndrome patients with germline PTEN mutations. J Med Genet. 2001;38(3):159-164.
doi pubmed pmc - Ding YC, Steele L, Kuan CJ, Greilac S, Neuhausen SL. Mutations in BRCA2 and PALB2 in male breast cancer cases from the United States. Breast Cancer Res Treat. 2011;126(3):771-778.
doi pubmed pmc - Silvestri V, Rizzolo P, Zanna I, Falchetti M, Masala G, Bianchi S, Papi L, et al. PALB2 mutations in male breast cancer: a population-based study in Central Italy. Breast Cancer Res Treat. 2010;122(1):299-301.
doi pubmed - Lattin GE, Jr., Jesinger RA, Mattu R, Glassman LM. From the radiologic pathology archives: diseases of the male breast: radiologic-pathologic correlation. Radiographics. 2013;33(2):461-489.
doi pubmed - Herlihy AS, Halliday JL, Cock ML, McLachlan RI. The prevalence and diagnosis rates of Klinefelter syndrome: an Australian comparison. Med J Aust. 2011;194(1):24-28.
doi pubmed - Humphries MP, Jordan VC, Speirs V. Obesity and male breast cancer: provocative parallels? BMC Med. 2015;13:134.
doi pubmed pmc - Brinton LA, Cook MB, McCormack V, Johnson KC, Olsson H, Casagrande JT, Cooke R, et al. Anthropometric and hormonal risk factors for male breast cancer: male breast cancer pooling project results. J Natl Cancer Inst. 2014;106(3):djt465.
doi pubmed pmc - Ruddy KJ, Winer EP. Male breast cancer: risk factors, biology, diagnosis, treatment, and survivorship. Ann Oncol. 2013;24(6):1434-1443.
doi pubmed - Giordano SH, Cohen DS, Buzdar AU, Perkins G, Hortobagyi GN. Breast carcinoma in men: a population-based study. Cancer. 2004;101(1):51-57.
doi pubmed - Fentiman IS, Fourquet A, Hortobagyi GN. Male breast cancer. Lancet. 2006;367(9510):595-604.
doi pubmed - Hittmair AP, Lininger RA, Tavassoli FA. Ductal carcinoma in situ (DCIS) in the male breast: a morphologic study of 84 cases of pure DCIS and 30 cases of DCIS associated with invasive carcinoma—a preliminary report. Cancer. 1998;83(10):2139-2149.
doi pubmed - Krawczyk N, Fehm T, Banys-Paluchowski M. DCIS in male and aged women with comorbidities. Chirurgia (Bucur). 2021;116(5 Suppl):S120-S127.
doi pubmed - Cardoso F, Costa A, Norton L, Senkus E, Aapro M, Andre F, Barrios CH, et al. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Breast. 2014;23(5):489-502.
doi pubmed - Deb S, Jene N, Kconfab I, Fox SB. Genotypic and phenotypic analysis of familial male breast cancer shows under representation of the HER2 and basal subtypes in BRCA-associated carcinomas. BMC Cancer. 2012;12:510.
doi pubmed pmc - Yu E, Stitt L, Vujovic O, Joseph K, Assouline A, Younus J, Perera F, et al. Male breast cancer prognostic factors versus female counterparts with propensity scores and matched-pair analysis. Cureus. 2015;7(10):e355.
doi pubmed pmc - Kumar PP, Good RR, Newland JR. Immunodiagnosis by prostatic acid phosphatase to differentiate primary male breast cancer from metastatic prostate cancer. J Natl Med Assoc. 1986;78(8):782-784, 786.
pubmed pmc - Gao Y, Heller SL. Breast Cancer Screening in Men. J Breast Imaging. 2023;5(2):104-111.
doi pubmed - Mainiero MB, Lourenco AP, Barke LD, Argus AD, Bailey L, Carkaci S, D'Orsi C, et al. ACR appropriateness criteria evaluation of the symptomatic male breast. J Am Coll Radiol. 2015;12(7):678-682.
doi pubmed - Evans GF, Anthony T, Turnage RH, Schumpert TD, Levy KR, Amirkhan RH, Campbell TJ, et al. The diagnostic accuracy of mammography in the evaluation of male breast disease. Am J Surg. 2001;181(2):96-100.
doi pubmed - Munoz Carrasco R, Alvarez Benito M, Munoz Gomariz E, Raya Povedano JL, Martinez Paredes M. Mammography and ultrasound in the evaluation of male breast disease. Eur Radiol. 2010;20(12):2797-2805.
doi pubmed - NCCN. Clinical Practise Guidellines in Oncology 2019. Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
- Hassett MJ, Somerfield MR, Baker ER, Cardoso F, Kansal KJ, Kwait DC, Plichta JK, et al. Management of male breast cancer: ASCO guideline. J Clin Oncol. 2020;38(16):1849-1863.
doi pubmed - Marino MA, Gucalp A, Leithner D, Keating D, Avendano D, Bernard-Davila B, Morris EA, et al. Mammographic screening in male patients at high risk for breast cancer: is it worth it? Breast Cancer Res Treat. 2019;177(3):705-711.
doi pubmed pmc - Goss PE, Reid C, Pintilie M, Lim R, Miller N. Male breast carcinoma: a review of 229 patients who presented to the Princess Margaret Hospital during 40 years: 1955-1996. Cancer. 1999;85(3):629-639.
doi pubmed - Adibelli ZH, Oztekin O, Gunhan-Bilgen I, Postaci H, Uslu A, Ilhan E. Imaging characteristics of male breast disease. Breast J. 2010;16(5):510-518.
doi pubmed - Michels LG, Gold RH, Arndt RD. Radiography of gynecomastia and other disorders of the male breast. Radiology. 1977;122(1):117-122.
doi pubmed - Madhukar M, Chetlen A. Multimodality imaging of benign and malignant male breast disease. Clin Radiol. 2013;68(12):e698-706.
doi pubmed - Shi AA, Georgian-Smith D, Cornell LD, Rafferty EA, Staffa M, Hughes K, Kopans DB. Radiological reasoning: male breast mass with calcifications. AJR Am J Roentgenol. 2005;185(6 Suppl):S205-210.
doi pubmed - Mathew J, Perkins GH, Stephens T, Middleton LP, Yang WT. Primary breast cancer in men: clinical, imaging, and pathologic findings in 57 patients. AJR Am J Roentgenol. 2008;191(6):1631-1639.
doi pubmed - Doyle S, Steel J, Porter G. Imaging male breast cancer. Clin Radiol. 2011;66(11):1079-1085.
doi pubmed - Dershaw DD, Borgen PI, Deutch BM, Liberman L. Mammographic findings in men with breast cancer. AJR Am J Roentgenol. 1993;160(2):267-270.
doi pubmed - Greenwood HI, Heller SL, Kim S, Sigmund EE, Shaylor SD, Moy L. Ductal carcinoma in situ of the breasts: review of MR imaging features. Radiographics. 2013;33(6):1569-1588.
doi pubmed - Reis LO, Dias FG, Castro MA, Ferreira U. Male breast cancer. Aging Male. 2011;14(2):99-109.
doi pubmed - Gunhan-Bilgen I, Bozkaya H, Ustun E, Memis A. Male breast disease: clinical, mammographic, and ultrasonographic features. Eur J Radiol. 2002;43(3):246-255.
doi pubmed - Yang WT, Whitman GJ, Yuen EH, Tse GM, Stelling CB. Sonographic features of primary breast cancer in men. AJR Am J Roentgenol. 2001;176(2):413-416.
doi pubmed - Adibelli ZH, Oztekin O, Postaci H, Uslu A. The diagnostic accuracy of mammography and ultrasound in the evaluation of male breast disease: a new algorithm. Breast Care (Basel). 2009;4(4):255-259.
doi pubmed pmc - Vanel D. The American College of Radiology (ACR) Breast Imaging and Reporting Data System (BI-RADS): a step towards a universal radiological language? Eur J Radiol. 2007;61(2):183.
doi pubmed - Aho M, Irshad A, Ackerman SJ, Lewis M, Leddy R, Pope TL, Campbell AS, et al. Correlation of sonographic features of invasive ductal mammary carcinoma with age, tumor grade, and hormone-receptor status. J Clin Ultrasound. 2013;41(1):10-17.
doi pubmed pmc - Shin K, Martaindale S, Whitman GJ. Male breast magnetic resonance imaging: when is it helpful? Our experience over the last decade. Curr Probl Diagn Radiol. 2019;48(3):196-203.
doi pubmed - Bassett LW, Dhaliwal SG, Eradat J, Khan O, Farria DF, Brenner RJ, Sayre JW. National trends and practices in breast MRI. AJR Am J Roentgenol. 2008;191(2):332-339.
doi pubmed - Shaw A, Smith B, Howlett D. Male breast carcinoma and the use of MRI. Radiol Case Rep. 2011;6(3):455.
doi pubmed pmc - Giordano SH. Breast cancer in men. N Engl J Med. 2018;378(24):2311-2320.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


