| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Case Report
Volume 16, Number 5, May 2024, pages 256-263
Efficacy and Safety of Upadacitinib Plus Intensive Granulocyte and Monocyte Adsorptive Apheresis as Induction for Intractable Ulcerative Colitis
Satoshi Tanidaa, b, d, Shun Sasohb, Takahiro Otanic, Yoshimasa Kubotab, Tesshin Banb, Tomoaki Andob, Makoto Nakamurab, Takashi Johb
aEducation and Research Center for Community Medicine, Nagoya City University Graduate School of Medical Sciences, Nagoya 467-8601, Japan
bDivision of Gastroenterology, Gamagori City Hospital, Gamagori 443-8501, Japan
cDepartment of Public Health, Nagoya City University Graduate School of Medical Sciences, Nagoya 467-8601, Japan
dCorresponding Author: Satoshi Tanida, Education and Research Center for Community Medicine, Nagoya City University Graduate School of Medical Sciences, Nagoya 467-8601, Japan
Manuscript submitted March 25, 2024, accepted April 26, 2024, published online May 29, 2024
Short title: UPA Plus Intensive GMA for Intractable UC
doi: https://doi.org/10.14740/jocmr5165
| Abstract | ▴Top |
Monotherapy with a selective Janus kinase (JAK) inhibitor or intensive granulocyte and monocyte adsorptive apheresis (GMA) has been limited to patients with intractable ulcerative colitis (UC). No previous reports have described the efficacy including histopathological evaluations and the safety of combination therapy with upadacitinib (UPA) plus intensive GMA (two sessions per week) for intractable UC showing resistance to conventional agents and adalimumab. This retrospective study evaluated the 10-week clinical and histopathological efficacy of induction combination therapy with UPA plus intensive GMA in patients with intractable UC. Among eight patients (moderate UC, n = 1; severe UC, n = 7) who received combination therapy with UPA plus intensive GMA, 50.0% had achieved clinical remission by 10 weeks. Percentages of patients with histological-endoscopic mucosal improvement and mucosal healing at 10 weeks were 62.5% and 12.5%, respectively. After excluding one patient who discontinued treatment by week 10 because of intolerance for UPA, mean full Mayo score, endoscopic subscore and C-reactive protein concentration at baseline were 11.43 ± 0.37, 3 ± 0 and 1.29 ± 0.70 mg/dL, respectively. Corresponding values at 10 weeks were 2.28 ± 0.77 (P < 0.03), 1.14 ± 0.34 (P < 0.03) and 0.03 ± 0.008 mg/dL (P < 0.05), respectively. Adverse events of herpes zoster, temporary increase in creatinine phosphokinase and anemia were observed in one patient each. One patient discontinued combination therapy at week 4 because of temporary taste abnormality due to UPA. Combination comprising UPA plus intensive GMA appears likely to achieve satisfactory induction of clinical remission and histopathological improvement for patients with intractable UC for whom conventional agents and anti-tumor necrosis factor-α antibody have failed.
Keywords: Ulcerative colitis; Upadacitinib; Intensive granulocyte and monocyte adsorptive apheresis; Histological improvement
| Introduction | ▴Top |
Ulcerative colitis (UC) is characterized by mucosal ulceration, rectal bleeding, diarrhea, and abdominal pain. Moderate-to-severe UC often shows repeated flares and a continuously active clinical course; such cases are considered intractable. UC is thought to occur in people with a genetic predisposition following environmental exposure, with defects in the gut epithelial barrier, microbiota, and dysregulated immune responses strongly implicated [1]. Treatments for moderate-to-severe UC include 5-aminosalicylic acid (5-ASA), corticosteroids, thiopurines, biologics including neutralizing antibodies against tumor necrosis factor (TNF)-α [2-4], interleukin (IL)-12 and/or IL-23 [5, 6], integrins [7], small-molecule antagonists targeting integrin-α4 [8] and Janus kinase (JAK) inhibitors [9, 10]. Upadacitinib (UPA), a JAK inhibitor that is selective for JAK1, was approved for the treatment of moderate-to-severe UC in Japan in 2022 [11]. UPA can play a vital role in treating UC by inhibiting JAK1-STAT signaling, which is associated with intestinal mucosal immunology and hemostasis, and plays roles in epithelial proliferation, differentiation and activation of B cells and differentiation and expansion of naive T helper cells into Th1, Th2, Th9, and Th17 effector lymphocytes regulated by IL-6, IL-11, and IL-13 [12]. UPA can therefore induce clinical remission and histopathological improvement during induction therapy. Moreover, UPA may have a milder adverse event profile compared to pan-JAK inhibitors like tofacitinib [13]. In addition, intensive granulocyte and monocyte adsorptive apheresis (GMA) preformed in two sessions a week with Adacolumn® (JIMRO, Takasaki, Japan) is also available in Europe and Japan for the treatment of patients with active UC that may or may not be refractory to standard pharmacotherapy, biologics and JAK inhibitors [14, 15]. GMA depletes elevated and activated myeloid lineage leucocytes and is associated with a marked downregulation of inflammatory cytokines including IL-1β, IL-6, IL-8, and TNF-α and expression of leukocyte-related adhesion molecules [16, 17]. The availability of these therapeutic options can ameliorate short- and long-term disease activity, but monotherapy using these therapeutic options has been limited to patients with intractable UC. Moreover, the efficacy of intensive GMA in terms of the induction of clinical remission in patients with severe UC has not been satisfactory [18]. Thus, ingenuity in trialing therapeutic combinations that are currently available in clinical settings is needed. A very recent report showed that combination therapy with UPA and intensive GMA appeared effective as a therapeutic option for a patient with flare-up of severe UC associated with new-onset active pyoderma gangrenosum [19]. With this background in mind, the present retrospective study focused on the 10-week clinical and histopathological efficacy of combination therapy with UPA and intensive GMA for patients with intractable UC.
| Case Report | ▴Top |
Investigations
Eight consecutive patients with moderate or severe UC who received combination therapy with UPA plus intensive GMA in Gamagori City Hospital, Japan between January 2023 and January 2024 were included in this study. The aim of the study was to evaluate the efficacy and safety of combination treatment with UPA plus intensive GMA. The following inclusion criteria were applied: 1) UC that was refractory or dependent on corticosteroids or showing loss of response to TNF-α antagonists and/or immunosuppressants; and 2) moderate-to-severe active UC, with a full Mayo score of 6 - 12 at baseline (including an endoscopic subscore of 2 - 3) despite concurrent treatment with 5-ASA, corticosteroids, azathioprine (AZA) and/or TNF-α antagonists.
Only dosages of corticosteroids were tapered off as appropriate during the 10-week clinical course. UPA was administered at 45 mg once a day for 8 weeks as induction therapy, then at 30 mg once a day as maintenance therapy. A total of 10 sessions of GMA were simultaneously provided in two sessions a week (intensive procedure) during UPA induction therapy. UPA was decreased to 30 mg/day during induction therapy or 15 mg/day during maintenance therapy when adverse events occurred for which UPA administration was considered contributory.
Exclusion criteria were: 1) diagnosis of Crohn’s disease, indeterminate colitis, fulminant colitis, toxic megacolon, or disease limited to the rectum; 2) active infection; 3) previous exposure to JAK inhibitors (tofacitinib, filgotinib and UPA); 4) exposure to conventional or intensive GMA within 56 days prior to combination therapy; and 5) allergy to UPA. Prohibited concomitant therapies included biologics, cyclosporine, tacrolimus, live vaccines, AZA, and 6-mercaptopurine. The study protocol was approved by the Institutional Review Board of Gamagori City Hospital (approval no. GH #160-5). The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Treatment and assessments
Disease activities and severities were assessed using the full Mayo score [20] at baseline and at 10 weeks, and a partial Mayo score for points of treatment discontinuation due to worsening rectal bleeding or increased stool frequency because of insufficient response to UPA and addition of another treatment within the 10-week study period, or when adverse events due to UPA treatment were observed and UPA was discontinued. The stool frequency subscore of the Mayo score was: 0: normal number of stools; 1: 1 - 2 stools more than normal; 2: 3 - 4 stools more than normal; or 3: ≥ 5 stools more than normal. The rectal bleeding subscore of the Mayo score was: 0: no blood seen; 1: streaks of blood seen with stool less than half of the time; 2: obvious blood seen with stool most of the time; or 3: blood alone passed. The primary outcome was the clinical remission rate after 10 weeks of UPA (induction UPA at 45 mg/day for 8 weeks plus intensive GMA, then maintenance UPA at 30 mg/day for 2 weeks). Clinical remission was defined as a full Mayo score ≤ 2 with no individual subscore > 1. Other assessments at 10 weeks included clinical response (decrease in full Mayo score ≥ 3 from baseline and ≥ 30% plus ≥ 1-point decrease from baseline in rectal bleeding score (RBS) or an absolute RBS of 0 or 1) and histological-endoscopic mucosal improvement (endoscopic score ≤ 1 without friability and Geboes score ≤ 3.1) [11, 21]. Histopathological findings in the epithelium and submucosa of the colon in biopsy specimens taken from the area of most severe inflammation were evaluated using the Geboes score [22]. Briefly, the score was structured as a six-grade classification system for UC inflammation as follows: 0: structural change only; 1: chronic inflammation; 2: neutrophils present in lamina propria; 3: neutrophils present in epithelium; 4: crypt destruction; and 5: erosions or ulcers. Histological-endoscopic mucosal improvement was defined as an endoscopic score ≤ 1 without friability and Geboes score ≤ 3.1, while mucosal healing was defined as an endoscopic score of 0 and a Geboes score < 2 [11].
Among the seven patients remaining after excluding one discontinued case, changes in full Mayo scores, Mayo endoscopic subscores and C-reactive protein (CRP) concentrations at baseline and 10 weeks were also evaluated. Data on any adverse events were recorded, including date of onset, severity, outcome, and relationship of such events to the administered therapies.
Data are presented as mean ± standard error of the mean, and comparisons were made using the Wilcoxon signed-rank test for paired data. In addition, 95% confidence intervals (CIs) were also assessed using the paired t-test. Statistical analyses were performed using SPSS version 27 software (IBM, Chicago, IL, USA). A significance level of 0.05 was used for all statistical tests, with two-tailed tests applied when appropriate.
Outcomes
The demographic characteristics of included patients are shown in Table 1. The mean age of included patients was 46.3 years (range, 18 - 78 years), and mean disease duration was 13.6 years (range, 1.3 - 26 years). Regarding colonic involvement, two had extensive colitis, five had left-sided colitis and one had distal colitis. Concurrent medications included 5-ASA, corticosteroids, AZA and adalimumab (TNF-α antagonist). Of the seven patients, five were corticosteroid-refractory, two were corticosteroid-dependent, and one had experienced loss of response to AZA and adalimumab. Mean full Mayo score and CRP levels at baseline were 11.5 (range, 9 - 12) and 1.83 mg/dL (range, 0.03 - 5.57 mg/dL), respectively (Table 1). The scores of stool frequency and rectal bleeding at baseline were also high (Table 2).
 Click to view | Table 1. Baseline Demographic Data of Eight Cases That Received Combination Therapy With UPA and Intensive GMA for Intractable UC |
 Click to view | Table 2. Clinical Course Through 10 Weeks |
Among the eight patients, including one case that has recently been described (case 3) [19], who received combination therapy with UPA and intensive GMA, one patient discontinued combination therapy within 4 weeks after reporting adverse events of taste abnormality and subsequently received induction infusion of mirikizumab (an anti-IL-23 antibody) at 300 mg on three occasions at 4-week intervals. Clinical remission and response rates at 10 weeks were 50.0% and 75.0%, respectively (Table 2). Moreover, percentages of patients with histological-endoscopic mucosal improvement (endoscopic score ≤ 1 without friability and Geboes score ≤ 3.1) and mucosal healing (endoscopic score of 0 and Geboes score < 2) [11] at 10 weeks were 62.5% and 12.5%, respectively (Table 2). In addition, all six patients who received corticosteroids and completed 10-week combination therapy with UPA and intensive GMA and one patient who was switched from this combination therapy to mirikizumab were able to discontinue corticosteroids within 10 weeks (100%) (Table 2). Of the seven patients who completed the full 10-week combination therapy with UPA and intensive GMA, mean full Mayo score decreased from 11.43 ± 0.37 at baseline to 2.28 ± 0.72 at 10 weeks (P < 0.03) and mean endoscopic subscore decreased from 3 ± 0 at baseline to 1.14 ± 0.34 at 10 weeks (P < 0.03) (Fig. 1a, b). Mean CRP level decreased from 1.29 ± 0.70 mg/dL at baseline to 0.034 ± 0.008 mg/dL at 10 weeks (P < 0.05) (Fig. 1c). These improvements were significant (Fig. 1). In addition, paired t-testing was performed to analyze the 95% CI. The difference in mean Mayo score between 10 weeks and baseline was -9.0 (95% CI: -11.7 to -6.3; P < 0.001), the difference in mean CRP concentration between 10 weeks and baseline was -1.3 (95% CI: -3.2 to 0.7; P = 0.076), and the difference in mean endoscopy subscore between 10 weeks and baseline was -1.7 (95% CI: -2.7 to -0.7; P = 0.007). Outcomes of paired t-tests were consistent with those of Wilcoxon signed-rank tests except for the difference in mean CRP concentration.
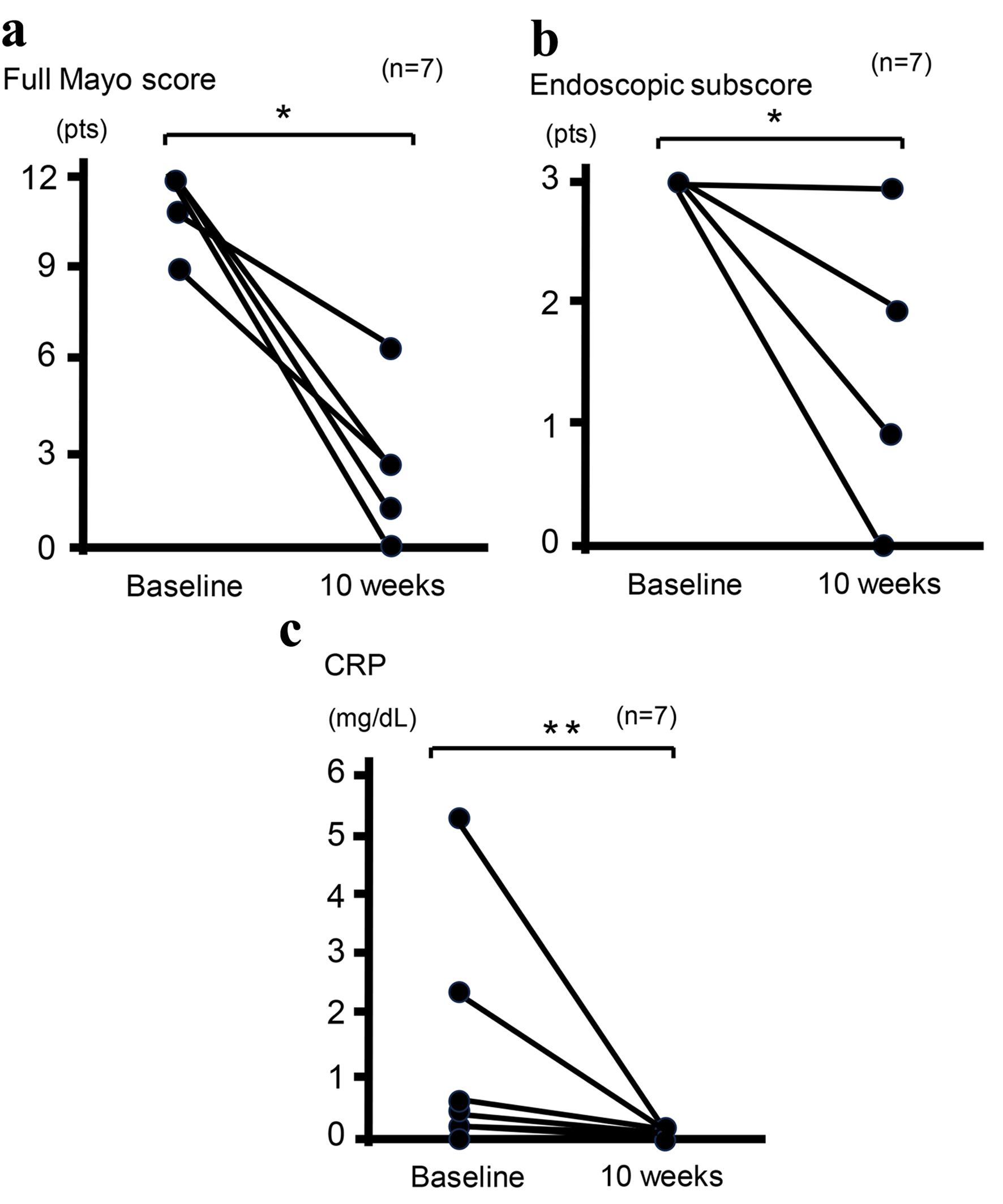 Click for large image | Figure 1. Clinical outcomes at baseline and 10 weeks in seven patients who completed 10-week combination therapy with UPA plus intensive GMA. Mean full Mayo scores (P < 0.03) (a), endoscopic subscores (P < 0.03) (b), and CRP concentrations (P < 0.05) (c) show significant differences between baseline and 10 weeks after analysis using the Wilcoxon signed-rank test for paired data. *P < 0.03; **P < 0.05. CRP: C-reactive protein; GMA: granulocyte and monocyte adsorptive apheresis; UPA: upadacitinib. |
Adverse events were observed in three patients, comprising herpes zoster and transient increase in creatinine phosphokinase (CK) due to intense physical exercise in one case (case 1) and mild anemia in one case (case 3). In case 1, UPA was not discontinued and all 10 sessions of intensive GMA were completed. In case 3, UPA was decreased to 30 mg/day and all 10 sessions of intensive GMA were completed. Anemia quickly resolved. One patient experienced taste abnormality within 4 weeks; UPA was discontinued and taste abnormality rapidly improved. In general, combination therapy with UPA plus intensive GMA appeared safe and well tolerated.
| Discussion | ▴Top |
We have reported here that combination therapy with UPA plus intensive GMA could reduce the 10-week clinical activity comprising CRP concentrations and endoscopic activity in addition to histopathological improvement in patients with intractable UC.
UPA is a potent, orally active and selective JAK1 inhibitor that suppresses JAK signal transducers and activators of the transcription pathway, thereby downregulating expressions of multiple immune-relevant mediators and inflammatory cytokines that are implicated in the pathogenesis of inflammatory bowel disease [23, 24] by competing with ATP and by blocking nucleotide binding to inhibit kinase activity and the phosphorylation of downstream effectors, then suppressing signal transducer and activator of transcription dimer formation, its translocation to the nucleus and promotor binding [25]. Moreover, UPA has the potential to improve the benefit-to-risk profile in patients with refractory immune-mediated diseases by minimizing the inhibitory effects on other JAK isoforms compared to less selective JAK inhibitors [26, 27]. In two multicenter, double-blinded placebo-controlled trials of patients with moderate-to-severe active UC presenting with a mean adapted Mayo score of 7.0 (moderately active UC), significantly more patients achieved clinical remission by week 8 in the group with UPA at 45 mg/day than in placebo groups (26% vs. placebo 5% and 34% vs. placebo 4%; P < 0.0001) and more patients also achieved clinical response with UPA at 45 mg/day than in placebo groups (73% vs. placebo 27% and 74% vs. placebo 25%; P < 0.0001). In addition, rates of patients achieving histologic-endoscopic mucosal improvement (Mayo endoscopic subscore ≤ 1; Geboes score ≤ 3.1) at 8 weeks with UPA at 45 mg/day were significantly greater than in placebo groups (30% vs. placebo 6% and 36% vs. placebo 6%, respectively; P < 0.001). Moreover, rates of patients achieving mucosal healing (Mayo endoscopic subscore 0; Geboes score < 2.0) at 8 weeks were also significantly greater with UPA at 45 mg/day than with placebo (11% vs. placebo 1% and 13% vs. placebo 2%, respectively; P < 0.001) [28]. UPA monotherapy is thus clinically and histopathologically effective, but is considered to have limited effect on intractable UC. Additional treatment would be necessary for active UC patients who are biologically naive and have lost response to TNF-α antagonists. In the present study investigating the efficacies of combination therapy with UPA plus intensive GMA on eight patients consisting of seven patients with severely active UC in large part (one patient presenting with an adapted Mayo score of 8, and six patients presenting with an adapted Mayo score of 9) and one patient with moderately active UC, rates of clinical remission and histological-endoscopic mucosal improvement at 10 weeks were 50.0% and 62.5%, respectively. Based on these outcomes, the addition of intensive GMA to UPA monotherapy appears effective for inducing clinical remission and histological-endoscopic mucosal improvement among patients with severely active UC. Additional positive investigations in a larger cohort of patients are warranted to verify this finding.
Next, GMA with Adacolumn® (JIMRO, Takasaki, Japan) offers an efficacious and safe therapeutic option for patients with immune-mediated diseases such as mild-to-moderate UC and pyoderma gangrenosum that prove refractory to pharmacotherapy [29, 30]. The column in GMA selectively traps activated granulocytes by binding Mac-1 expressed on the granulocytes to iC3b on beads [31]. In addition, immunoglobulin G on cellulose acetate beads mediates adsorption through Fcγ receptors on myeloid-lineage cells. GMA also removes CD11b+ activated neutrophils, thereby reducing the infiltration into inflamed regions [32, 33]. A decrease in peripheral CD14+ CD16+monocytes (pro-inflammatory phenotype) and an increase in circulating levels of the CD4+ CD25high+/FOXP3 phenotype (functional regulatory T cells) are observed in the blood of patients after GMA treatment. An increased level of regulatory T cells thus actively suppresses inflammatory responses by producing anti-inflammatory cytokines such as IL-10, IL-35 and transforming growth factor β [34]. Moreover, in patients with active UC, downregulation of the leukocyte adhesion molecule L-selectin and neutrophil adhesion to endothelial cells activated by IL-1β were observed after in vitro exposure of peripheral blood to cellulose acetate beads that comprise a main part of Adacolumn [35]. Such findings suggest that combination therapy works by drastically downregulating circulating inflammatory cytokines and expressions of adhesive molecules on activated granulocytes, as an effect of GMA, and by downregulating local inflammatory cytokines at microenvironmental sites in the gut mucosa, as an effect of UPA [14, 36]. These downregulations combined induced rapid clinical remission and histological-endoscopic mucosal improvement. In addition, serious adverse side effects have been rare among patients receiving GMA [18].
Regarding safety in the present study, herpes zoster and increased levels of CK were seen in one patient, and mild anemia was seen in one patient. In addition, taste abnormality requiring rapid UPA discontinuation was observed within 4 weeks in one patient. These findings were consistent with adverse events reported from early post-marketing phase pharmacovigilance regarding safety and adverse events associated with UPA in Japanese patients with UC [37]. Increased risks for hematological disorders, opportunistic infections (including herpes zoster), and elevations in CK or lipid levels have been reported with the use of UPA. Taste abnormality has also been reported, albeit less frequently, in two of 1,647 patients administered UPA in a clinical setting (reduced sense of taste in one patient, taste abnormality in one patient). Proposed interventions include discontinuing strenuous physical exercise and administering drugs for hypercholesterolemia and/or hypertriglyceridemia as appropriate during elevations in CK or lipid levels, respectively, and decreasing doses of UPA while hematological disorders such as anemia, leukocytopenia or neutropenia are present. Treatments for herpes zoster in addition to temporary discontinuation of UPA are required while herpes zoster is present. Moreover, discontinuation of UPA is recommended immediately after detecting taste abnormality or reduced sense of taste [11, 37].
The main limitations of this study were the retrospective study design, the small sample size (N = 8) and the limited exposure time, which was likely too short to allow characterization of the safety of combination therapy with UPA and intensive GMA. Whether this combination therapy is more effective than either UPA or GMA monotherapy thus remains unclear due to the limited number of cases and the non-comparative design of the study. Our view is that the therapeutic outcomes in the present case studies need to be validated by additional comparative, controlled studies in larger cohorts of patients.
Learning points
Combination therapy comprising UPA plus intensive GMA was well tolerated and appears effective as a therapeutic option to induce clinical remission and histological-endoscopic mucosal improvement instead of UPA monotherapy for patients with intractable UC.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
Satoshi Tanida has received speaker fees from Kissei, Mitsubishi Tanabe Pharma, JIMRO, AbbVie, and Janssen. None of the other authors have any conflict of interest to declare.
Informed Consent
Informed consent was obtained in the form of opt-out on the website during inclusion.
Author Contributions
ST designed and performed the study. ST and TA assisted with and supported sample collection. TO statistically analyzed the collected data. ST and YK drafted the manuscript and SS, YK, TB, and TA performed critical editing of the manuscript. ST prepared and wrote manuscript. MN and TJ carefully supervised this study.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
5-ASA: 5-aminosalicylic acid; AZA: azathioprine; CI: confidence interval; CK: creatinine phosphokinase; CRP: C-reactive protein; GMA: granulocyte and monocyte adsorptive apheresis; IL: interleukin; JAK: Janus kinase; RBS: rectal bleeding score; TNF: tumor necrosis factor; UC: ulcerative colitis; UPA: upadacitinib
| References | ▴Top |
- Le Berre C, Honap S, Peyrin-Biroulet L. Ulcerative colitis. Lancet. 2023;402(10401):571-584.
doi pubmed - Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, et al. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2005;353(23):2462-2476.
doi pubmed - Sandborn WJ, van Assche G, Reinisch W, Colombel JF, D'Haens G, Wolf DC, Kron M, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142(2):257-265.e251-253.
doi pubmed - Nielsen OH, Ainsworth MA. Tumor necrosis factor inhibitors for inflammatory bowel disease. N Engl J Med. 2013;369(8):754-762.
doi pubmed - Sands BE, Sandborn WJ, Panaccione R, O'Brien CD, Zhang H, Johanns J, Adedokun OJ, et al. Ustekinumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2019;381(13):1201-1214.
doi pubmed - D'Haens G, Dubinsky M, Kobayashi T, Irving PM, Howaldt S, Pokrotnieks J, Krueger K, et al. Mirikizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2023;388(26):2444-2455.
doi pubmed - Feagan BG, Rutgeerts P, Sands BE, Hanauer S, Colombel JF, Sandborn WJ, Van Assche G, et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2013;369(8):699-710.
doi pubmed - Matsuoka K, Watanabe M, Ohmori T, Nakajima K, Ishida T, Ishiguro Y, Kanke K, et al. AJM300 (carotegrast methyl), an oral antagonist of alpha4-integrin, as induction therapy for patients with moderately active ulcerative colitis: a multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Gastroenterol Hepatol. 2022;7(7):648-657.
doi pubmed - Sandborn WJ, Su C, Sands BE, D'Haens GR, Vermeire S, Schreiber S, Danese S, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376(18):1723-1736.
doi pubmed - Feagan BG, Danese S, Loftus EV, Jr., Vermeire S, Schreiber S, Ritter T, Fogel R, et al. Filgotinib as induction and maintenance therapy for ulcerative colitis (SELECTION): a phase 2b/3 double-blind, randomised, placebo-controlled trial. Lancet. 2021;397(10292):2372-2384.
doi pubmed - Danese S, Vermeire S, Zhou W, Pangan AL, Siffledeen J, Greenbloom S, Hebuterne X, et al. Upadacitinib as induction and maintenance therapy for moderately to severely active ulcerative colitis: results from three phase 3, multicentre, double-blind, randomised trials. Lancet. 2022;399(10341):2113-2128.
doi pubmed - Ernest-Suarez K, Panaccione R. Update on the role of upadacitinib in the treatment of adults with moderately to severely active ulcerative colitis. Therap Adv Gastroenterol. 2023;16:17562848231158235.
doi pubmed pmc - Nielsen OH, Boye TL, Gubatan J, Chakravarti D, Jaquith JB, LaCasse EC. Selective JAK1 inhibitors for the treatment of inflammatory bowel disease. Pharmacol Ther. 2023;245:108402.
doi pubmed - Tanida S, Mizoshita T, Nishie H, Ozeki K, Katano T, Kubota E, Kataoka H, et al. Combination therapy with adalimumab plus intensive granulocyte and monocyte adsorptive apheresis in patients with refractory ulcerative colitis. J Clin Med Res. 2015;7(11):884-889.
doi pubmed pmc - Tanida S, Ozeki K, Katano T, Tanaka M, Shimura T, Kubota E, Kataoka H, et al. Induction therapy with a combination of weekly adalimumab plus intensive granulocyte and monocyte adsorptive apheresis in patients with ulcerative colitis and failure of conventional agents, Biologics and Janus Kinase inhibitor. J Clin Med Res. 2023;15(3):181-186.
doi pubmed pmc - Saniabadi AR, Hanai H, Takeuchi K, Umemura K, Nakashima M, Adachi T, Shima C, et al. Adacolumn, an adsorptive carrier based granulocyte and monocyte apheresis device for the treatment of inflammatory and refractory diseases associated with leukocytes. Ther Apher Dial. 2003;7(1):48-59.
doi pubmed - Nishise S, Abe Y, Nomura E, Sato T, Sasaki Y, Iwano D, Yoshizawa K, et al. Relationship between tumor necrosis factor-alpha release and granulocyte and monocyte adsorption to cellulose acetate beads. Ther Apher Dial. 2014;18(3):252-257.
doi pubmed - Hanai H, Watanabe F, Yamada M, Sato Y, Takeuchi K, Iida T, Tozawa K, et al. Adsorptive granulocyte and monocyte apheresis versus prednisolone in patients with corticosteroid-dependent moderately severe ulcerative colitis. Digestion. 2004;70(1):36-44.
doi pubmed - Tanida S, Kubo R, Yoshii S, Takahama T, Sasoh S, Kubota Y, Ban T, et al. Upadacitinib plus intensive granulocyte and monocyte adsorptive apheresis for ulcerative colitis achieved ulcer healing for pyoderma gangrenosum. J Clin Med Res. 2023;15(10-11):446-455.
doi pubmed pmc - Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317(26):1625-1629.
doi pubmed - Reinisch W, Sandborn WJ, Panaccione R, Huang B, Pollack PF, Lazar A, Thakkar RB. 52-week efficacy of adalimumab in patients with moderately to severely active ulcerative colitis who failed corticosteroids and/or immunosuppressants. Inflamm Bowel Dis. 2013;19(8):1700-1709.
doi pubmed - Geboes K, Riddell R, Ost A, Jensfelt B, Persson T, Lofberg R. A reproducible grading scale for histological assessment of inflammation in ulcerative colitis. Gut. 2000;47(3):404-409.
doi pubmed pmc - Lovato P, Brender C, Agnholt J, Kelsen J, Kaltoft K, Svejgaard A, Eriksen KW, et al. Constitutive STAT3 activation in intestinal T cells from patients with Crohn's disease. J Biol Chem. 2003;278(19):16777-16781.
doi pubmed - Schreiber S, Rosenstiel P, Hampe J, Nikolaus S, Groessner B, Schottelius A, Kuhbacher T, et al. Activation of signal transducer and activator of transcription (STAT) 1 in human chronic inflammatory bowel disease. Gut. 2002;51(3):379-385.
doi pubmed pmc - Mohamed MF, Bhatnagar S, Parmentier JM, Nakasato P, Wung P. Upadacitinib: Mechanism of action, clinical, and translational science. Clin Transl Sci. 2024;17(1):e13688.
doi pubmed pmc - Ghaffari S, Kitidis C, Fleming MD, Neubauer H, Pfeffer K, Lodish HF. Erythropoiesis in the absence of janus-kinase 2: BCR-ABL induces red cell formation in JAK2(-/-) hematopoietic progenitors. Blood. 2001;98(10):2948-2957.
doi pubmed - Mohamed MF, Beck D, Camp HS, Othman AA. Preferential inhibition of JAK1 relative to JAK3 by upadacitinib: exposure-response analyses of Ex Vivo data from 2 phase 1 clinical trials and comparison to tofacitinib. J Clin Pharmacol. 2020;60(2):188-197.
doi pubmed pmc - Peyrin-Biroulet L, Siegel C, Tanida S, Bossuyt P, Torres E, Dubinsky M, Baert F, et al. P522 Upadacitinib promotes histologic and endoscopic mucosal healing: results from the Upadacitinib ulcerative colitis phase 3 program. J Crohn's and Colitis. 2022;16(Supplement_1):i477-i478.
- Ljung T, Thomsen OO, Vatn M, Karlen P, Karlsen LN, Tysk C, Nilsson SU, et al. Granulocyte, monocyte/macrophage apheresis for inflammatory bowel disease: the first 100 patients treated in Scandinavia. Scand J Gastroenterol. 2007;42(2):221-227.
doi pubmed - Gnesotto L, Mioso G, Alaibac M. Use of granulocyte and monocyte adsorption apheresis in dermatology (Review). Exp Ther Med. 2022;24(2):536.
doi pubmed pmc - Kanekura T. Clinical and immunological effects of adsorptive myeloid lineage leukocyte apheresis in patients with immune disorders. J Dermatol. 2018;45(8):943-950.
doi pubmed - Cuadrado E. Granulocyte/monocyte apheresis as immunotherapic tool: cellular adsorption and immune modulation. Autoimmun Rev. 2009;8(4):292-296.
doi pubmed - Hanai H, Takeda Y, Eberhardson M, Gruber R, Saniabadi AR, Winqvist O, Lofberg R. The mode of actions of the Adacolumn therapeutic leucocytapheresis in patients with inflammatory bowel disease: a concise review. Clin Exp Immunol. 2011;163(1):50-58.
doi pubmed pmc - Yokoyama Y, Fukunaga K, Fukuda Y, Tozawa K, Kamikozuru K, Ohnishi K, Kusaka T, et al. Demonstration of low-regulatory CD25High+CD4+ and high-pro-inflammatory CD28-CD4+ T-Cell subsets in patients with ulcerative colitis: modified by selective granulocyte and monocyte adsorption apheresis. Dig Dis Sci. 2007;52(10):2725-2731.
doi pubmed - Kashiwagi N, Sugimura K, Koiwai H, Yamamoto H, Yoshikawa T, Saniabadi AR, Adachi M, et al. Immunomodulatory effects of granulocyte and monocyte adsorption apheresis as a treatment for patients with ulcerative colitis. Dig Dis Sci. 2002;47(6):1334-1341.
doi pubmed - Tanida S, Mizoshita T, Ozeki K, Katano T, Tanaka M, Nishie H, Shimura T, et al. Combination therapy with intensive granulocyte and monocyte adsorptive apheresis plus ustekinumab in patients with refractory Crohn's disease. Ther Apher Dial. 2018;22(3):295-300.
doi pubmed - Early post-marketing phase vigilance regarding safety and adverse events of upadacitinib in Japanese patients with ulcerative colitis. https://a-connectabbviecojp/-/media/assets/pdf/products/rinvoq/clinical/pms/UC_immediately_after_marketingpdf. [Accessed Mar 12, 2024].
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


