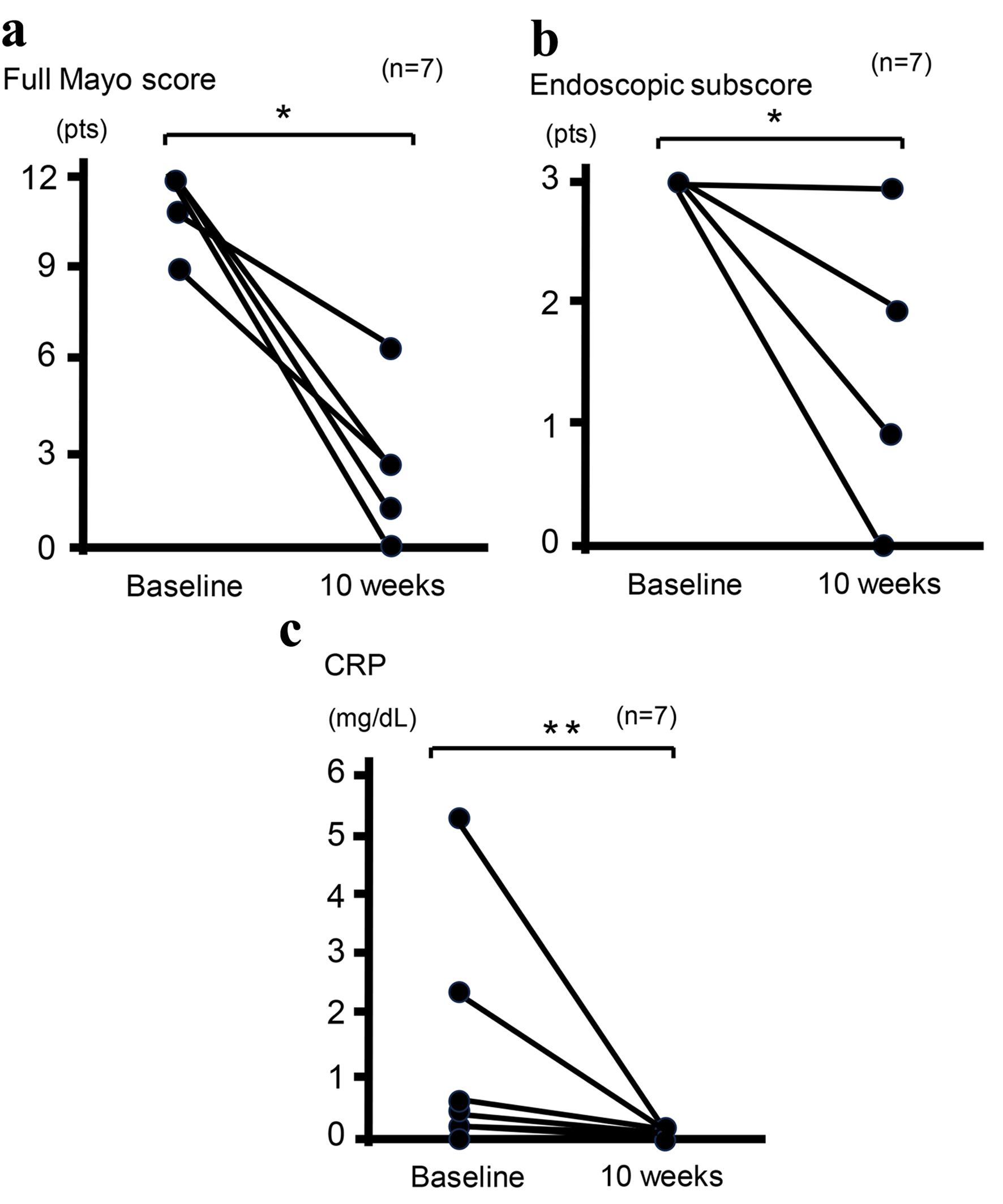
Figure 1. Clinical outcomes at baseline and 10 weeks in seven patients who completed 10-week combination therapy with UPA plus intensive GMA. Mean full Mayo scores (P < 0.03) (a), endoscopic subscores (P < 0.03) (b), and CRP concentrations (P < 0.05) (c) show significant differences between baseline and 10 weeks after analysis using the Wilcoxon signed-rank test for paired data. *P < 0.03; **P < 0.05. CRP: C-reactive protein; GMA: granulocyte and monocyte adsorptive apheresis; UPA: upadacitinib.