| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 16, Number 5, May 2024, pages 243-250
Predictors of Premature Ventricular Contractions Development in Patients With SARS-CoV-2 Infection
Aida I. Tarzimanovaa , Anna E. Braginaa
, Ekaterina E. Sokolovaa, d
, Tatiana S. Varginaa
, Anna E. Pokrovskayaa
, Tatiana A. Safronovaa
, Irakli Zh. Loriyaa
, Igor V. Cherkesovb
, Alexander G. Cherepanovc
, Liubov A. Ponomarevaa
, Daria D. Vaninaa
, Kseniya E. Krylovaa
, Nadezhda K. Ziskinaa
, Valery I. Podzolkova
aThe Second Internal Medicine (Second Faculty Therapy) Department, N.V. Sklifosovskiy Institute of Clinical Medicine, Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia
bDepartment of Plastic Surgery, I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia
cInstitute of Linguistics and Intercultural Communication, Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia
dCorresponding Author: Ekaterina E. Sokolova, The Second Internal Medicine (Second Faculty Therapy) Department, N.V. Sklifosovskiy Institute of Clinical Medicine, Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russia
Manuscript submitted March 22, 2024, accepted May 16, 2024, published online May 29, 2024
Short title: Predictors of PVCs Development in COVID-19 Patients
doi: https://doi.org/10.14740/jocmr5160
| Abstract | ▴Top |
Background: Epidemiological studies have demonstrated that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)-positive patients often develop atrial fibrillation, premature ventricular contractions (PVCs), and conduction disorders. The manifestation of ventricular cardiac arrhythmias accentuates the risk of sudden cardiac death.
Methods: A retrospective study was conducted on the cohort of 1,614 patients admitted for coronavirus disease 2019 (COVID-19). Patients were categorized into two groups based on the occurrence of PVCs. Group I comprised 172 patients diagnosed with PVCs of Lown-Wolf class II - IV upon hospital admission; group II (control group) consisted of 1,442 patients without this arrhythmia. Each patient underwent comprehensive clinical, laboratory, and instrumental evaluations.
Results: The emergence of PVCs in individuals afflicted with COVID-19 was associated with a 5.879-fold heightened risk of lethal outcome, a 2.904-fold elevated risk of acute myocardial infarction, and a 2.437-fold increased risk of pulmonary embolism. Upon application of diagnostic criteria to evaluate the “cytokine storm”, it was discovered that the occurrence of the “cytokine storm” was notably more frequent in the group with PVCs, manifesting in six patients (3.5%), compared to 16 patients (1.1%) in the control group (P < 0.05). The mean extent of lung tissue damage in group I was significantly greater than that of patients in group II (P < 0.05). Notably, the average oxygen saturation level, as measured by pulse oximetry upon hospital admission was 92.63±3.84% in group I and 94.20±3.50% in group II (P < 0.05).
Conclusions: The presence of PVCs in COVID-19 patients was found to elevate the risk of cardiovascular complications. Significant independent predictors for the development of PVCs in patients with SARS-CoV-2 infection include: age over 60 years (risk ratio (RR): 4.6; confidence interval (CI): 3.2 - 6.5), a history of myocardial infarction (RR: 3.5; CI: 2.6 - 4.6), congestive heart failure (CHF) with reduced left ventricular ejection fraction (RR: 5.5; CI: 3.9 - 7.6), respiratory failure (RR: 2.3; CI: 1.7 - 3.1), and the presence of a “cytokine storm” (RR: 4.5; CI: 2.9 - 6.0).
Keywords: New SARS-CoV-2 coronavirus infection; Premature ventricular contractions; Cardiac arrhythmias; Predictors
| Introduction | ▴Top |
The global issue of coronavirus infection, caused by the SARS-CoV-2 (COVID-19) virus, continues to pose a significant challenge to the world community today. Although there have been notable advancements in the prevention and treatment of COVID-19 over the past 3 years, the emergence of new virus strains underscores the ongoing relevance of studying this infection. The progression of COVID-19 is intricately linked to the development of a diverse spectrum of complications, with particular emphasis on heart rhythm disorders. The prevalence of arrhythmias ranges from 6.9% in mild cases of COVID-19 to 44% in severe cases [1]. Epidemiological studies conducted from 2020 to 2023 demonstrate that patients with SARS-CoV-2 coronavirus infection most commonly develop atrial fibrillation (AF), premature ventricular contractions (PVCs), and conduction disorders. Notably, the emergence of ventricular cardiac arrhythmias escalates the risk of sudden cardiac death [2].
A meta-analysis conducted by Liao et al, encompassing 17,435 COVID-19 patients, revealed that 3.3% of patients experienced life-threatening ventricular arrhythmias [3]. Similarly, a study by Garcia-Zamora et al, involving 12,713 patients with SARS-CoV-2 infection, observed the diagnosis of frequent PVCs in 2.5% of patients upon admission [4].
The risk of experiencing sudden cardiac death in patients with SARS-CoV-2 infection varies significantly across different studies, ranging from 0.9% to 11% [5, 6]. Furthermore, a study conducted by Cho et al, which included 143 patients with severe COVID-19, revealed that 28.7% of patients experienced PVCs, 15.4% with unsustained ventricular tachycardia, 1.4% with sustained ventricular tachycardia, and 0.7% with ventricular fibrillation [7].
The comprehensive understanding of the nature and incidence of cardiac arrhythmias in patients with SARS-CoV-2 infection remains incomplete. Ongoing research is uncovering potential mechanisms underlying the development of ventricular arrhythmias in COVID-19 patients, including metabolic disorders and hypoxia, virus-induced hypokalemia via the renin-angiotensin-aldosterone system, and direct viral damage to cardiomyocytes resulting in viral myocarditis [8-14].
The identification of predictors for the development of PVCs in patients with SARS-CoV-2 coronavirus infection is a subject of ongoing debate, as it holds the potential to facilitate timely treatment administration and enhance patient prognosis.
The aim of this study was to ascertain the factors that may predict the occurrence of PVCs in patients with SARS-CoV-2 (COVID-19) infection.
| Materials and Methods | ▴Top |
This retrospective study involved a total of 1,614 patients (with an average age of 63.32 ± 13.82 years), who had been admitted for SARS-CoV-2 infection at Clinical Hospital No. 4 of the First Moscow State Medical University named after I.M. Sechenov (Sechenov University), operating as a medical facility designated to treat patients afflicted with the COVID-19, during the time period spanning from April to September 2020. The patients were categorized into two distinct groups based on the presence or absence of PVCs. Group I comprised 172 patients with COVID-19 who exhibited PVCs of classes II - IV according to Lown-Wolf upon admission, while group II (the control group) consisted of 1,442 patients who did not manifest this rhythm disorder. This study received approval from the Local Ethics Committee (protocol No. 04-21, dated February 18, 2021) and was conducted in compliance with the ethical standards of the responsible institution on human subjects, as well as with the Helsinki Declaration. The clinical characteristics of the patients are detailed in Table 1.
 Click to view | Table 1. Characteristics of the Cohort |
The prerequisites for patient categorization into group I encompassed the manifestation of class II-IV extrasystoles as defined by Laun-Wolf, which was validated via 24-h electrocardiogram (ECG) monitoring utilizing Holter. Furthermore, a confirmed diagnosis of coronavirus infection was required, as evidenced by the detection of SARS-CoV-2 RNA in swab samples procured from the nasopharynx and oropharynx using polymerase chain reaction (PCR). For comprehensive assessment, 24-h Holter ECG monitoring was implemented for all patients throughout the initial 3 days of their hospitalization period.
In contrast, those with a medical history of AF or end-stage renal failure (identified by a reduced glomerular filtration rate below 15 mL/min/1.73 m2), as well as those who were pregnant or breastfeeding, were categorically excluded from our study.
The treatment of COVID-19 for the study participants involved antiviral and anticoagulant therapies, as well as glucocorticosteroids and genetically engineered biological drugs (tocilizumab, netakimab, levilimab) that were also administered to halt the “cytokine storm”. There were no significant variations in the treatment of coronavirus infection between the two groups. Patients with a medical history of hypertension (HTN), coronary heart disease (CHD), and CHF were managed in accordance with clinical guidelines.
All patients underwent a comprehensive clinical, laboratory, and instrumental examination, encompassing a PCR test specifically targeting SARS-CoV-2 RNA, alongside a general and biochemical blood test, a coagulogram, the determination of inflammatory laboratory markers, echocardiographic examination, 24-h ECG monitoring, and multi-slice spiral computed tomography (MSCT) of the chest, as well as measurement of oxygen saturation with a pulse oximeter. The extent of pulmonary impairment was evaluated based on the established categorization: CT0 denotes the nonexistence of inflammation foci and infiltrates; CT1 signifies the manifestation of viral pneumonia symptoms, encompassing up to a quarter of the pulmonary tissue; CT2 corresponds to the breadth of lung impairment ranging from 25% to 50%; CT3 relates to the pulmonary tissue damage spanning from 50% to 75%; CT4 represents injury to over 75% of the pulmonary tissue.
Statistical analysis
Statistical analysis of the study data was conducted using Statistica 10.0 software (StatSoft Inc., USA). The Kolmogorov-Smirnov test was utilized to evaluate the normal distribution of indicators. Descriptive statistics for digital results included the arithmetic mean M and its standard deviation. The mean and standard error of the mean were calculated for normally distributed parameters, whereas the quartile interval (25th, 50th, and 75th percentiles) was determined for non-normally distributed parameters. The nonparametric Mann-Whitney test was utilized for statistical analysis. The χ2 test was used to assess the statistical significance of differences between qualitative indicators. Statistical study results were deemed reliable at a probability of error P < 0.05. Multivariate logistic regression analysis was employed to identify prognostic markers for the development of events, and receiver operating characteristic (ROC) analysis was conducted to assess the sensitivity and specificity of the marker.
| Results | ▴Top |
Gender distribution and prevalence of obesity were comparable between the study groups. The patients in group I were notably older than those in group II. Furthermore, the incidence of HTN, CHD, CHF, and diabetes mellitus (DM) was higher in patients with PVCs compared to those in the control group. HTN was diagnosed in 140 (81%) patients in group I and in 831 (58%) patients in group II, while CHD was observed in 77 (81%) and 358 (26%) patients, and CHF was present in 78 (82%) and 428 (31%) patients, respectively (P < 0.05).
Through the utilization of a 24-h ECG assessment performed on patients pertaining to both group I and II, occurrences of atrial extrasystoles coupled with transient episodes of supraventricular tachycardia were detected. Nevertheless, the prevalence of supraventricular arrhythmias did not exhibit any significant divergences.
The study examined the incidence of cardiovascular complications, including cases of death from acute myocardial infarction (AMI), pulmonary embolism (PE), and acute cerebrovascular accident (ACVA), as well as the development of non-fatal AMI, PE, and ACVA. The characteristics of cardiovascular complications occurring upon hospital admission are summarized in Table 2.
 Click to view | Table 2. Cardiovascular Complications in the Study Groups, Observed During the Admission |
Using multivariate regression analysis, a comprehensive assessment was performed to determine the impact of PVCs on the development of cardiovascular complications in patients with SARS-CoV-2 infection. The occurrence of PVCs was associated with a 5.879-fold increased risk of lethal outcomes (confidence interval (CI): 3.640 - 9.496), a 2.904-fold increased risk of AMI (CI: 1.921 - 4.391), and a 2.437-fold increased risk of PE (CI: 1.597 - 3.720), as depicted in Figure 1.
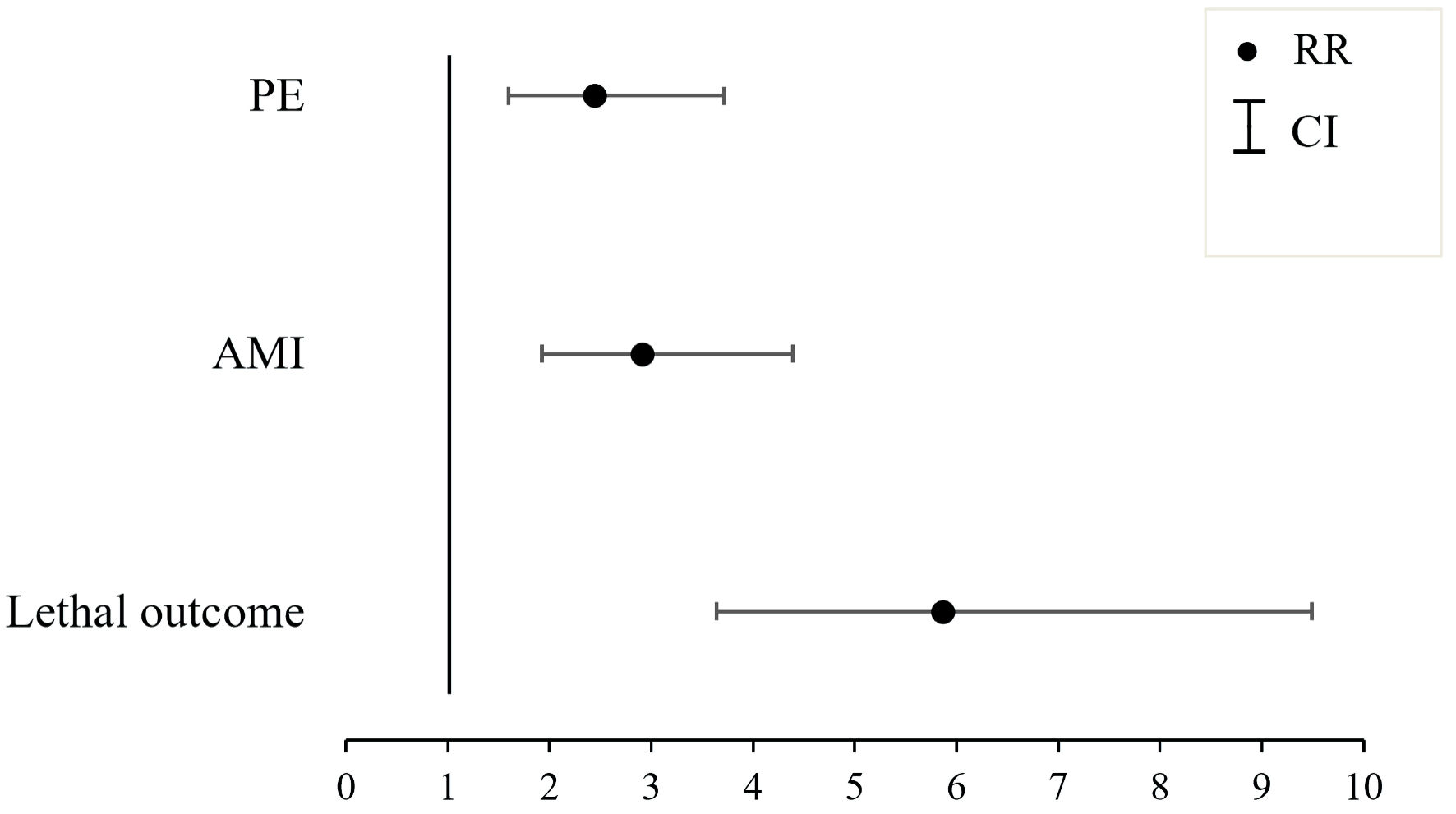 Click for large image | Figure 1. The influence of the presence of premature ventricular contractions (PVCs) on the onset of cardiovascular complications within the studied cohort. AMI: acute myocardial infarction, PE: pulmonary embolism; RR: risk ratio; CI: confidence interval. |
In patients with PVCs the mean extent of lung tissue damage, as revealed by MSCT of the chest, was significantly higher (40.55% in group I and 36.81% in group II, P < 0.05). Additionally, the average oxygen saturation measured by pulse oximetry upon hospital admission was significantly lower in patients with PVCs (92.63±3.84% in group I and 94.20±3.50% in group II, P < 0.05), indicating more severe respiratory failure in this patient group.
The findings from the biochemical blood test analysis conducted on patients with COVID-19 and PVCs revealed a notable increase in inflammatory markers compared to the control group (Table 3).
 Click to view | Table 3. Inflammation Markers in Group I and II Patients (Median (Q1 - Q3)) |
In our study, we evaluated the occurrence of the “cytokine storm” utilizing the diagnostic criteria [15], which encompassed an escalation in the concentration of one of the inflammation markers, the manifestation of respiratory failure, bilateral pneumonia, and a progressive rise in the need for noninvasive respiratory support. Additionally, increased levels of D-dimer, lactate dehydrogenase, lymphopenia, and heightened aspartate aminotransferase (AST) levels were considered. We uncovered a significantly higher frequency of “cytokine storm” in group I, occurring in six (3.5%) patients, in contrast to the control group, where it was observed in 16 (1.1%) patients (P < 0.05).
Patients with PVCs exhibited a notably higher occurrence of reduced left ventricular ejection fraction (LVEF), elevated systolic pulmonary artery pressure (sPAP), increased left atrial (LA) volume, and left ventricular end-systolic volume (LV ESV) in comparison to patients in the control group (P < 0.05). The detailed echocardiography results can be found in Table 4.
 Click to view | Table 4. Parameters of Echocardiography in Group I and II Patients (Median (Q1 - Q3)) |
Through the execution of multivariate regression analysis, independent predictors for the onset of PVCs of classes II - IV according to Lown-Wolf among SARS-CoV-2-positive patients were identified. Risk ratio (RR) and CI were calculated for each parameter. Variables encompassing gender, body mass index, AMI, and coexisting disorders were pragmatically controlled as per the specifications outlined in Table 5.
 Click to view | Table 5. Predictors of PVCs Development in Severe Cases of SARS-CoV-2 Infection |
To establish the threshold values of the predictors, we conducted ROC analysis (Figs. 2, 3).
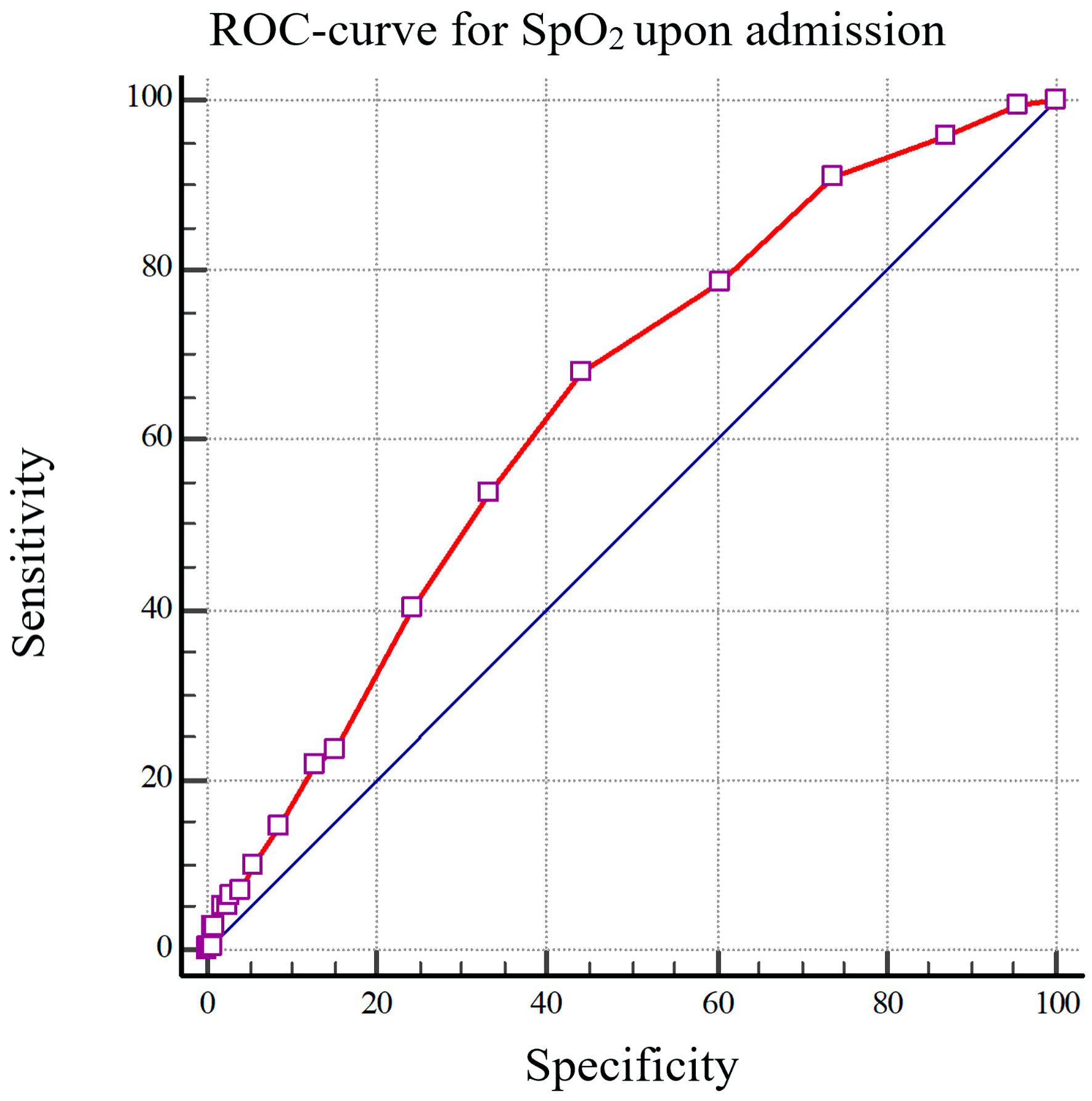 Click for large image | Figure 2. The ROC curve depicting the reduction in peripheral capillary oxygen saturation (SpO2) upon admission, specifically less than 95%, as a predictive measure for the onset of premature ventricular contractions (PVCs) in individuals afflicted with COVID-19 (AUC: 0.641). SpO2: the average value of oxygen saturation measured by a pulse oximeter; ROC: receiver operating characteristic; COVID-19: coronavirus disease 2019; AUC: area under the curve. |
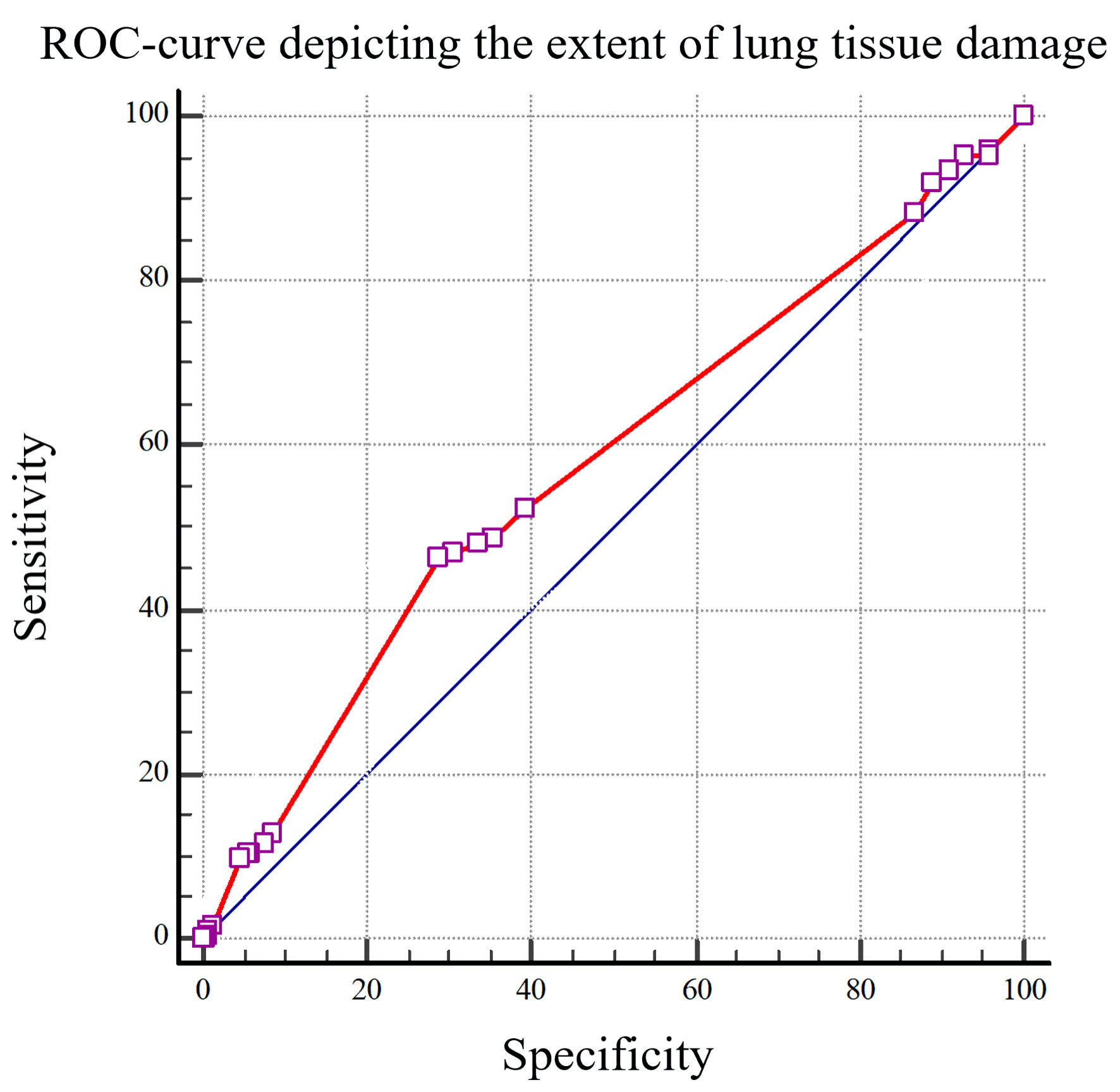 Click for large image | Figure 3. The ROC curve depicting the extent of lung tissue damage to predict the development of premature ventricular contractions (PVCs) in patients afflicted with COVID-19 (AUC: 0.576). ROC: receiver operating characteristic; COVID-19: coronavirus disease 2019; AUC: area under the curve. |
Oxygen saturation levels upon admission of less than 95%, with a sensitivity of 68.05% and specificity of 55.77% (area under the curve (AUC): 0.641), along with pulmonary parenchymal damage exceeding 50%, featuring a sensitivity of 46.51% and a specificity of 71.08%, (AUC: 0.576) indicate an elevated risk of developing PVCs in patients afflicted with coronavirus infection.
| Discussion | ▴Top |
During the multivariate regression analysis, an observed correlation indicated that the presence of PVCs in patients with COVID-19 results in an elevated risk of developing cardiovascular complications, signifying damage to the cardiovascular system in the context of SARS-CoV-2 infection, the pathogenesis of which remains intricate and poorly understood.
Elderly age emerged as the most significant risk factor for PVCs occurrence. Specifically, in our study, patients in group I exhibited a notably advanced age compared to those in group II. Subsequent multivariate regression analysis revealed that individuals aged over 60 years demonstrate a 4.576-fold increase in the probability of developing PVCs (CI: 3.224 - 6.495). The manifestation of severe cardiovascular pathology, accompanied by structural and functional changes in the myocardium, notably contributes to the onset of ventricular arrhythmias during SARS-CoV-2 infection. This was evidenced by significantly more frequent observations of decreased LVEF, increased LV ESV, LA volume, and elevated mean pulmonary artery pressure (mPAP) based on ECHO-CG data in patients with PVCs compared to those in the control group, aligning with findings in the study by Mouram et al [16]. These identified alterations reflect myocardial remodeling and significantly influence the occurrence of heart rhythm disorders, both in the general population and in patients with SARS-CoV-2 infection.
The role of hypoxia in cardiovascular damage during COVID-19 is actively discussed [17, 18]. Our study highlighted that oxygen saturation of under 95% serves as an independent predictor for PVCs development in individuals with SARS-CoV-2 infection. In a hypoxic state, reduced energy supply to cellular metabolism and increased anaerobic fermentation lead to intracellular acidosis and the release of oxygen free radicals, which disrupt the phospholipid layer of the cell membrane. Severe hypoxia hampers intercellular cardiomyocyte interaction, induces dephosphorylation of the gap junction protein connexin-43, diminishes electrical coupling and myocardial anisotropy, thus contributing to arrhythmia onset. The significance of severe respiratory failure in the emergence of cardiac arrhythmias in COVID-19 has been corroborated in select clinical studies [17, 18].
C-reactive protein emerges as one of the most highly sensitive inflammatory markers in routine clinical practice, with its elevation indicating the severity of the inflammatory response and directly correlating with the patient’s condition severity in COVID-19 [19]. In our study, patients with arrhythmias exhibited notably higher average values of C-reactive protein and interleukin-6 (IL-6) compared to those in the control group. There is a probability that the occurrence of PVCs in severe SARS-CoV-2 infection stems from a pronounced inflammatory response. Elevated levels of inflammatory cytokines contribute to alterations in the function of specific ion channels and connexins that form intercellular gap junctions, increasing cardiomyocyte excitability and predisposing individuals to arrhythmias [20-23].
The emergence of PVCs in patients with COVID-19 is attributed to various contributing factors. Our study revealed that the incidence of arrhythmias was impacted by factors such as advanced age, existing cardiovascular conditions, hypoxia, and elevated inflammatory markers in the patients. An evaluation of the patient’s clinical condition and the identification of potential predictors for the occurrence of PVCs enable us to deliberate upon tailored approaches for managing individuals afflicted with the SARS-CoV-2 infection, aligning with contemporary concepts of personalized medicine.
Conclusions
The occurrence of PVCs in COVID-19 patients was associated with a 5.879-fold increased risk of lethal outcome, a 2.904-fold increased risk of AMI, and a 2.437-fold increased risk of PE.
Significant independent predictors for the development of PVCs in patients with SARS-CoV-2 infection include: age over 60 years, a history of myocardial infarction, CHF with reduced LVEF, respiratory failure, and the presence of a “cytokine storm”.
Acknowledgments
None to declare.
Financial Disclosure
This research received no external funding.
Conflict of Interest
The authors confirm that there is no potential conflict of interest to disclose in relation to this article.
Informed Consent
Completed written informed consent was obtained.
Author Contributions
Conceptualization: AT, AB, VP and ES; methodology: AT, AB, VP and ES; software: ES; validation: AT, AB and ES; formal analysis: ES; investigation: AT and ES; data curation: ES, TV, AP, TS, IL, AC, IC, LP, DV, KK and NZ; writing - original draft preparation: AT and ES; writing - review and editing: AT, VP and AB; writing - literacy search: AC; supervision: VP; project administration: VP. All authors have read and agreed to the published version of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
ACVA: acute cerebrovascular accident; AF: atrial fibrillation; AMI: acute myocardial infarction; AST: aspartate aminotransferase; CHF: congestive heart failure; CHD: coronary heart disease; CI: confidence interval; COVID-19: coronavirus disease 2019; ECG: electrocardiogram; HTN: hypertension; IL-6: interleukin-6; IVST: interventricular septum thickness; LA: left atrium; LV: left ventricle; LV EDV: left ventricular end-diastolic volume; LVEF: left ventricular ejection fraction; LV ESV: left ventricular end-systolic volume; LVPWT: left ventricular posterior wall thickness; MSCT: multi-slice spiral computed tomography; PE: pulmonary embolism; PCR: polymerase chain reaction; PVCs: premature ventricular contractions; RR: risk ratio; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; SpO2: the average value of oxygen saturation measured with a pulse oximeter
| References | ▴Top |
- Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, Wang B, et al. Clinical Characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061-1069.
doi pubmed pmc - Kumar A, Kapoor M, Beyong T. Autonomic nervous system involvement in COVID 19 and risk of Sudden cardiac death. Journal of Cardiovascular Disease Research. 2021;12(3);548-553.
doi - Liao SC, Shao SC, Cheng CW, Chen YC, Hung MJ. Incidence rate and clinical impacts of arrhythmia following COVID-19: a systematic review and meta-analysis of 17,435 patients. Crit Care. 2020;24(1):690.
doi pubmed pmc - Garcia-Zamora S, Lee S, Haseeb S, Bazoukis G, Tse G, Alvarez-Garcia J, Gul EE, et al. Arrhythmias and electrocardiographic findings in Coronavirus disease 2019: A systematic review and meta-analysis. Pacing Clin Electrophysiol. 2021;44(6):1062-1074.
doi pubmed pmc - Kuck KH, Schluter M, Vogler J, Heeger CH, Tilz RR. Has COVID-19 changed the spectrum of arrhythmias and the incidence of sudden cardiac death? Herz. 2023;48(3):212-217.
doi pubmed pmc - Sandoval Y, Januzzi JL, Jr., Jaffe AS. Cardiac troponin for assessment of myocardial injury in COVID-19: JACC review topic of the week. J Am Coll Cardiol. 2020;76(10):1244-1258.
doi pubmed pmc - Cho JH, Namazi A, Shelton R, Ramireddy A, Ehdaie A, Shehata M, Wang X, et al. Cardiac arrhythmias in hospitalized patients with COVID-19: A prospective observational study in the western United States. PLoS One. 2020;15(12):e0244533.
doi pubmed pmc - Ministry of Health of the Russian Federation. Temporary guidelines: Prevention, diagnosis and treatment of new coronavirus infection (COVID-19). Version 18 (10/26/2023). Available at: https://static-0.minzdrav.gov.ru/system/attachments/attaches/000/064/610/original/ВМР_COVID-19_V18.pdf. Accessed January 12, 2024.
- Chazova IE, Mironova OI. [COVID-19 and cardiovascular diseases]. Ter Arkh. 2020;92(9):4-7.
doi pubmed - Kochi AN, Tagliari AP, Forleo GB, Fassini GM, Tondo C. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol. 2020;31(5):1003-1008.
doi pubmed pmc - Romiti GF, Corica B, Lip GYH, Proietti M. Prevalence and impact of atrial fibrillation in hospitalized patients with COVID-19: a systematic review and meta-analysis. J Clin Med. 2021;10(11):2490.
doi pubmed pmc - Bhatia KS, Sritharan HP, Chia J, Ciofani J, Nour D, Chui K, Vasanthakumar S, et al. Cardiac complications in patients hospitalised with COVID-19 in Australia. Heart Lung Circ. 2021;30(12):1834-1840.
doi pubmed pmc - Grinevich VB, Gubonina IV, Doshchitsin VL, Kotovskaya YuV, Kravchuk YuA, Ped VI, Sas EI, et al. Management of patients with comorbidity during novel coronavirus (COVID-19) pandemic. National Consensus Statement 2020. Cardiovascular Therapy and Prevention. 2020;19(4):2630.
doi - Shlyakhto EV, Parmon EV, Berngardt ER, Zhabina ES. Features of electrocardiographic changes in non-coronarogenic syndromes in patients with COVID-19. Russian Journal of Cardiology. 2020;25(7):4019.
doi - Fajgenbaum DC, June CH. Cytokine storm. N Engl J Med. 2020;383(23):2255-2273.
doi pubmed pmc - Mouram S, Pannone L, Gauthey A, Sorgente A, Vergara P, Bisignani A, Monaco C, et al. Incidence and predictors of cardiac arrhythmias in patients with COVID-19. Front Cardiovasc Med. 2022;9:908177.
doi pubmed pmc - Denegri A, Sola M, Morelli M, Farioli F, Alberto T, D'Arienzo M, Savorani F, et al. Arrhythmias in COVID-19/SARS-CoV-2 pneumonia infection: prevalence and implication for outcomes. J Clin Med. 2022;11(5):1-7.
doi pubmed pmc - Peltzer B, Manocha KK, Ying X, Kirzner J, Ip JE, Thomas G, Liu CF, et al. Outcomes and mortality associated with atrial arrhythmias among patients hospitalized with COVID-19. J Cardiovasc Electrophysiol. 2020;31(12):3077-3085.
doi pubmed pmc - Podzolkov VI, Tarzimanova AI, Bragina AE, Shvedov II, Bykova EE, Ivannikov AA, Vasilyeva LV. Damage to the cardiovascular system in patients with SARS-CoV-2 coronavirus infection. Part 1: predictors of the development of an unfavorable prognosis. Rational Pharmacotherapy in Cardiology. 2021;17(6);825-830.
doi - Zylla MM, Merle U, Vey JA, Korosoglou G, Hofmann E, Muller M, Herth F, et al. Predictors and prognostic implications of cardiac arrhythmias in patients hospitalized for COVID-19. J Clin Med. 2021;10(1):133.
doi pubmed pmc - Mahdi M, Bezawada V, Ozer M, De Deyne P, Nagra B, Kantharia B. Cardiac arrhythmias and COVID-19: correlation with disease severity. Cureus. 2021;13(12):e20507.
doi pubmed pmc - Peltzer B, Manocha KK, Ying X, Kirzner J, Ip JE, Thomas G, Liu CF, et al. Arrhythmic complications of patients hospitalized with COVID-19: incidence, risk factors, and outcomes. Circ Arrhythm Electrophysiol. 2020;13(10):e009121.
doi pubmed pmc - Podzolkov VI, Tarzimanova AI, Bragina AE, Loriya IZh, Pokrovskaya AE, Bykova EE, Ivannikov AA, et al. Predictors of atrial fibrillation in patients with COVID-19. Russian Journal of Cardiology. 2022;27(7):5095.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


