| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 16, Number 6, June 2024, pages 284-292
Battle of the Blocks: Which Pain Management Technique Triumphs in Gender-Affirming Bilateral Mastectomies?
Sengottaian Sivakumara, c, Aron Kresselb, Roni Mendoncaa, Michael Girshina
aDepartment of Anesthesia, Metropolitan Hospitals, New York, NY 10029, USA
bDepartment of Plastic Surgery, Metropolitan Hospitals, New York, NY 10029, USA
cCorresponding Author: Sengottaian Sivakumar, Department of Anesthesia, Metropolitan Hospitals, New York, NY 10029, USA
Manuscript submitted March 22, 2024, accepted May 8, 2024, published online June 18, 2024
Short title: Pain Management for Bilateral Mastectomies
doi: https://doi.org/10.14740/jocmr5159
| Abstract | ▴Top |
Background: Gender-affirming mastectomy, performed on transgender men and non-binary individuals, frequently leads to considerable postoperative pain. This pain can significantly affect both patient satisfaction and the overall recovery process. The study examines the efficacy of four analgesic techniques pectoral nerve (PECS) 2 block, erector spinae plane (ESP) block, thoracic wall local anesthesia infiltration (TWI), and systemic multimodal analgesia (SMA) in managing perioperative pain, with special consideration for the effects of chronic testosterone therapy on pain thresholds.
Methods: A retrospective analysis was conducted on patients aged 18 - 45 who underwent gender-affirming bilateral mastectomies at a New York City community hospital. The study compared intraoperative and post-anesthesia care unit (PACU) opioid consumption, postoperative pain scores, the interval to first rescue analgesia, and total PACU duration among the four analgesic techniques.
Results: The study found significant differences in intraoperative and PACU opioid consumption across the groups, with the PECS 2 block group showing the least opioid requirement. The PACU morphine milligram equivalent (MME) consumption was highest in the SMA group. Postoperative pain scores were significantly lower in the PECS and ESP groups at earlier time points post-surgery. However, by postoperative day 2, pain scores did not significantly differ among the groups. Chronic testosterone therapy did not significantly impact intraoperative opioid requirements.
Conclusion: The PECS 2 block is superior in reducing overall opioid consumption and providing effective postoperative pain control in gender-affirming mastectomies. The study underscores the importance of tailoring pain management strategies to the unique physiological responses of the transgender and non-binary community. Future research should focus on prospective designs, standardized block techniques, and the complex relationship between hormonal therapy and pain perception.
Keywords: Gender-affirming mastectomy; Chronic testosterone therapy; Pectoral nerve block; Erector spinae plane block; Thoracic wall infiltration; Non-binary individuals; Retrospective analysis; Analgesic efficacy
| Introduction | ▴Top |
In the realm of gender-affirming surgical procedures, top surgery clinically known as masculinization chest surgery stands as a pivotal intervention for transgender men and non-binary individuals assigned female at birth [1]. This surgical approach, primarily composed of mastectomy, is a cornerstone in alleviating gender dysphoria and enhancing the concordance between an individual’s physical appearance and gender identity, thereby significantly augmenting the quality of life [2]. Nevertheless, the perioperative period is frequently accompanied by considerable pain, a factor that can profoundly impact patient outcomes and recovery. Recognizing the necessity for efficacious pain management strategies is paramount in the context of such procedures. Historically, a variety of analgesic techniques have been employed, including the pectoral nerve (PECS) 2 block, erector spinae plane (ESP) block, thoracic wall local anesthesia infiltration (TWI), and systemic multimodal analgesia (SMA), supplemented by additional methods such as the serratus anterior block and intercostal nerve block [3-6]. A critical aspect often overlooked in the analgesic paradigm is the influence of chronic testosterone therapy, which is commonly prescribed for the masculinization of transgender and non-binary individuals. Chronic administration of testosterone is known to modulate pain thresholds, thus potentially altering the analgesic requirements for these patients [7, 8]. Considering this, our retrospective comparative analysis aims to elucidate the analgesic efficacy of four perioperative pain management strategies in gender-affirming bilateral mastectomies. Our primary outcome will involve a comparison of intraoperative and post-anesthesia care unit (PACU) opioid consumption, quantified in morphine milligram equivalent (MME). Secondary outcomes will encompass differences in pain scores between patients receiving chronic testosterone therapy, postoperative pain scores, the interval to first rescue analgesia, and the total duration within the PACU. Through this investigation, we aspire to refine pain management protocols to better serve the transgender and non-binary community by acknowledging and integrating their unique physiological responses to chronic testosterone therapy.
| Materials and Methods | ▴Top |
This retrospective analysis was conducted upon approval by the Institutional Review Board (IRB committee approval number 22-12-226-182). The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration. It entailed a comprehensive review of electronic medical records for patients who underwent gender-affirming mastectomies at a community hospital in New York City from October 2021 to October 2022. Inclusion criteria were confined to patients aged 18 - 45 years who underwent gender-affirming bilateral mastectomies under general anesthesia and were managed with one of four perioperative pain strategies: PECS 2 block, ESP block, TWI, or SMA. It is imperative to note that thoracic infiltration refers to administering a local anesthetic solution to incision lines, utilizing either 0.25% bupivacaine or 0.2% ropivacaine, 20 mL on each side for both the types of blocks. Additionally, systemic analgesia comprised the use of opioids and NSAIDs, including ketorolac and intravenous acetaminophen. Exclusion criteria encompassed patients with chronic pain disorders or opioid dependency, those who had revision or reconstructive surgeries in addition to the mastectomy, those with allergies or contraindications to any study analgesics, or those with significant comorbidities affecting pain management strategy safety or efficacy. Importantly, as this was a single-centered study, all surgical procedures were performed by the same surgeon, ensuring consistency in surgical techniques and reducing variability in the surgical component of the study.
In our study, patients were presented with the choice of two primary nerve block techniques for their analgesic management: the ESP block and the PECS block. The ESP block was typically performed with the patient in a sitting position prior to the induction of anesthesia, whereas the PECS block was usually administered with the patient in a supine position after anesthesia had been initiated. The choice between these techniques was offered to patients based on their comfort with being conscious during the block procedure and their preference for body positioning. Those who opted for the nerve block while awake and in a sitting position were allocated to the ESP group. Conversely, patients who preferred to be anesthetized and thus unaware of the block procedure were assigned to the PECS block group. Both the blocks were performed under ultrasound guidance. This patient-centered approach to the selection of analgesic technique allowed for individualized care, but also introduced a potential selection bias into our study, as patients’ preferences may have been influenced by factors not accounted for in our analysis, such as previous experiences with anesthesia, anxiety levels, and expectations of postoperative pain and recovery.
Sample size calculation
A power analysis using Altiparmak et al’s [9] effect size indicated that 16 subjects per group needed to achieve 80% power at a 0.05 alpha level. Our study enrolled 22 per group, summing to 88, surpassing the recommendation for robust statistical power.
Statistical analysis
In this study, we extracted a wide range of data, including patient demographics, surgical specifics, pain management approaches, perioperative pain levels, analgesic use, and various time-related measures. Patients were categorized into four groups based on their pain management technique. Our primary evaluation focused on intraoperative and PACU opioid use, measured in MME, and secondary evaluations included numeric rating scale (NRS) pain scores, timing of initial rescue analgesic, and PACU duration. We used analysis of variance (ANOVA) and Kruskal-Wallis tests for our dual-faceted statistical approach, accommodating both parametric and non-parametric data, with a significance cut-off at P < 0.05. Analyses were performed using SPSS software 27.0.
| Results | ▴Top |
Our retrospective analysis showed that the demographic characteristics - age (mean 26.99 ± 5.588 years) as shown in Table 1, body mass index, and American Society of Anesthesiology physical status - were consistent across the groups, thus allowing for a valid comparison of the interventions.
 Click to view | Table 1. Age Distribution of Participants by Group |
The ANOVA suggests that there was a significant difference in the means of intraoperative MME across the groups (PECS 17.77 ± 8.986 vs. ESP 24.45 ± 11.887 vs. SMA 24.91 ± 8.372 vs. TWI 23.78 ± 7.804, P = 0.044), with PECS group having the least and SMA group having the maximum intraoperative MME requirement. However, the post-hoc Tukey honestly significant difference (HSD) test did not show any pairwise group comparison of statistical significance (Fig. 1).
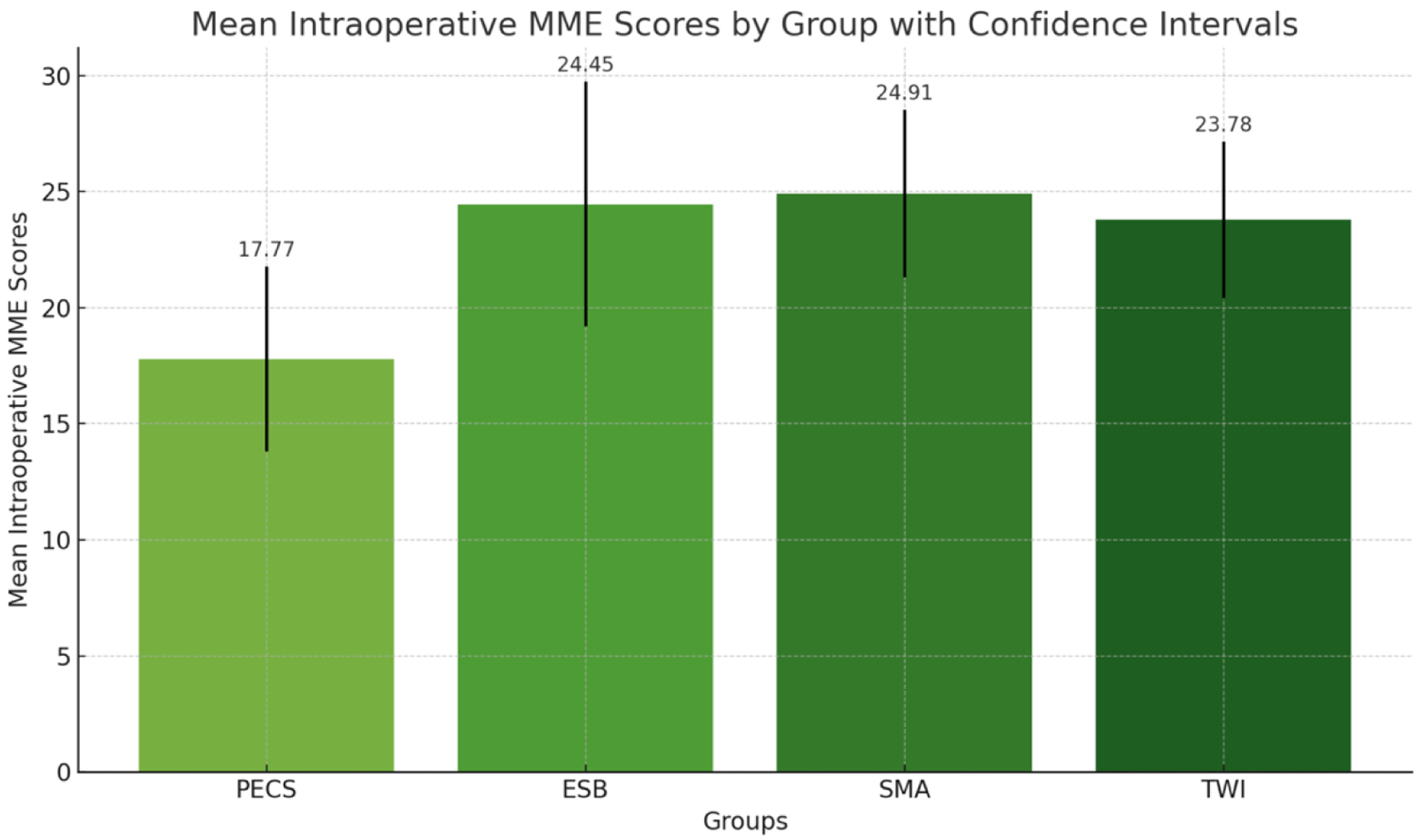 Click for large image | Figure 1. The mean intraoperative morphine milligram equivalent (MME) scores for each group (PECS, ESP, SMA, TWI) with 95% confidence intervals. Each bar represents the average MME score for a group, visually comparing the anesthesia requirements across the different groups. PECS: pectoral nerve; ESP: erector spinae plane; TWI: thoracic wall local anesthesia infiltration; SMA: systemic multimodal analgesia. |
The ANOVA suggests that the PACU MME in various groups was statistically significant (PECS group 2.95 ± 3.078 vs. ESP 2.18 ± 2.889 vs. SMA 5.09 ± 2.372 vs. TWI 4.87 ± 1.842, P < 0.05), with SMA group having the highest requirement of analgesic in terms of MME in the PACU, and ESP the least. Pairwise group comparison showed a significant difference in PACU MME between PECS vs. SMA, ESP vs. SMA, ESP vs. TWI, and TWI vs. ESP (P < 0.05). There was no significant difference in PACU analgesic requirement between SMA and TWI (Fig. 2).
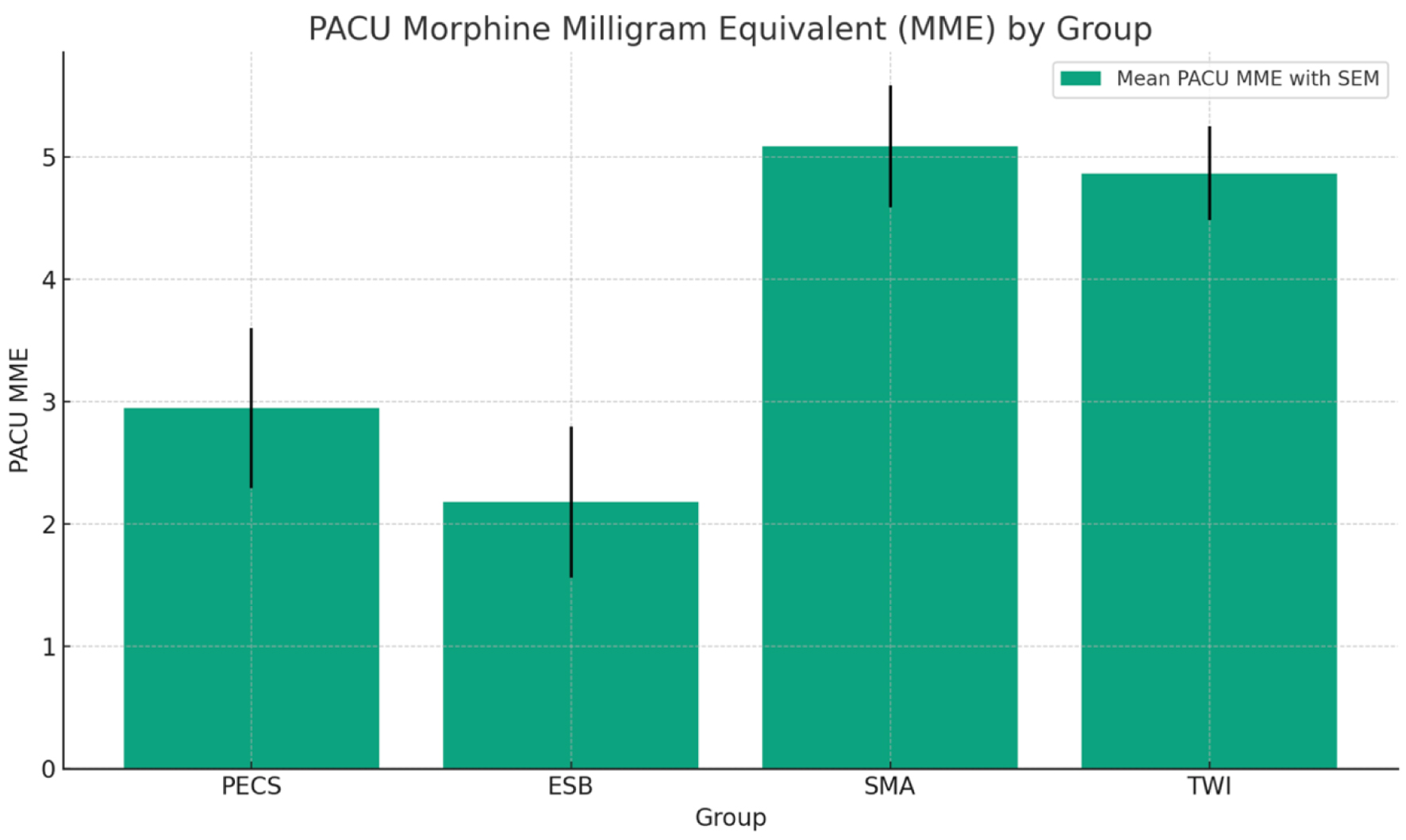 Click for large image | Figure 2. Bar graph representing the post-anesthesia care unit (PACU) morphine milligram equivalent (MME) for each group, complete with a legend indicating the mean PACU MME with standard error of the mean (SEM). |
The study assessed postoperative pain levels at different time intervals (30, 60, 90, 120 min, and day 2) among the four groups: PECS, ESP, SMA, and TWI, as shown in Figure 3. At 30 min post-surgery, SMA and TWI groups reported the highest NRS pain levels (8.13 and 7.96, respectively), much higher than PECS (4.27) and ESP (4.73). This trend continues at 60 min, where SMA reports an average pain of 5.04 and TWI 4.74, compared to PECS and ESP, which report significantly lower values. By 90 min, the pain levels started to reduce for all groups, with the highest mean pain reported by SMA (2.52). At the 120-min mark, the pain levels across all groups are notably reduced, with SMA still reporting the highest mean pain of 1.35. ANOVA results corroborate these findings, with significant group differences observed at 30, 60, and 90 min (P < 0.05). However, by 120 min, the P-value suggests that the group differences are not statistically significant. Tukey HSD post-hoc analysis for pain at 30 and 60 min indicates significant differences between the SMA PECS and ESP groups. At the 90-min mark, the SMA group’s pain differs significantly from the PECS group, but other pairwise comparisons are not consistently significant. The non-parametric Kruskal-Wallis test also supports these observations, with significant differences in pain scores across the groups at 30, 60, and 90 min but not at 120 min. Pain levels are significantly different across the groups at earlier postoperative intervals (30, 60, and 90 min), particularly between SMA and the PECS/ESP groups. However, these differences diminish and are not statistically significant by 120 min post-surgery (Fig. 3).
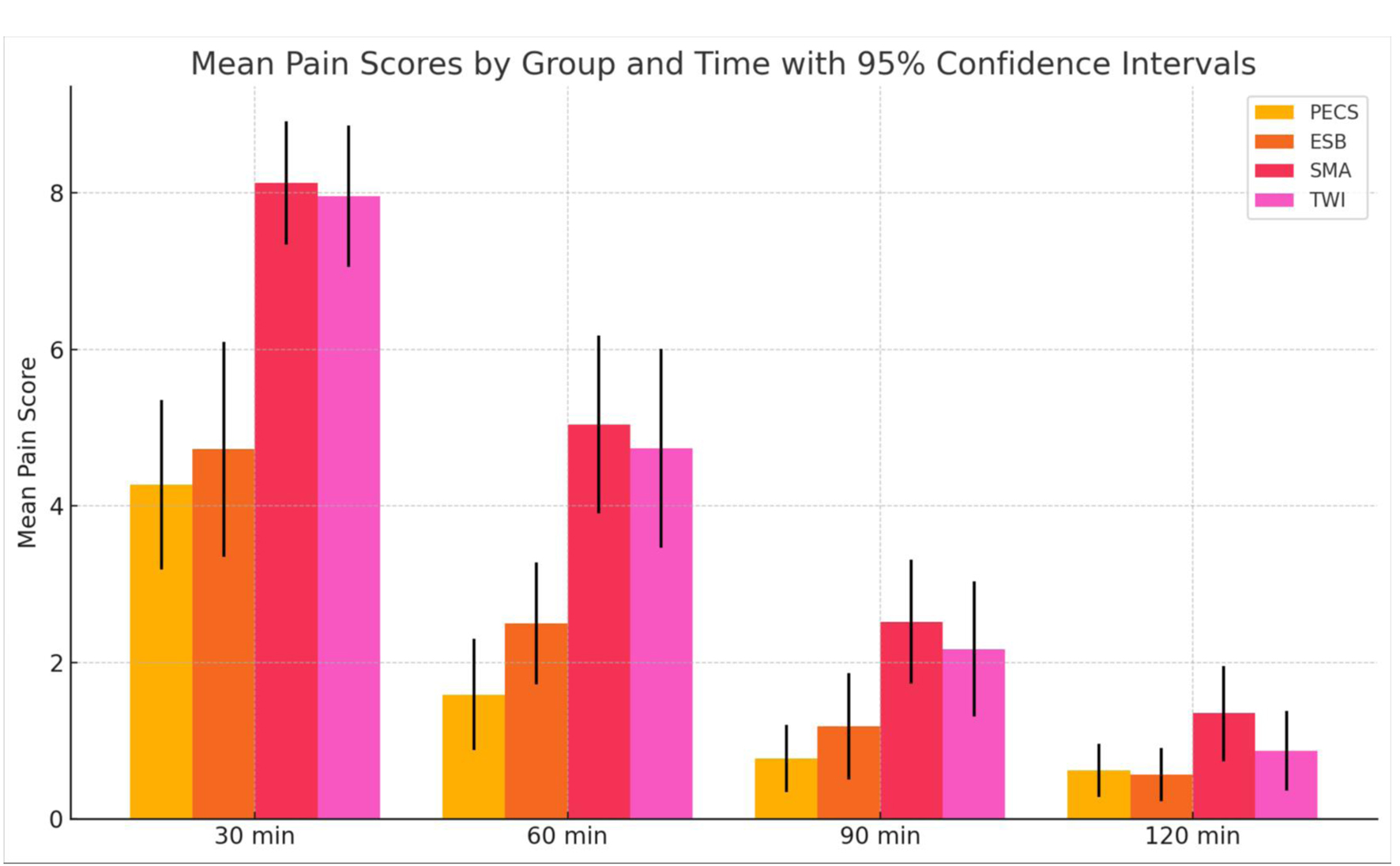 Click for large image | Figure 3. The mean pain scores at different time intervals (30, 60, 90, and 120 min) for each group (PECS, ESP, SMA, TWI) along with their 95% confidence intervals allowing for a direct comparison of how pain scores evolve for each treatment group. PECS: pectoral nerve; ESP: erector spinae plane; TWI: thoracic wall local anesthesia infiltration; SMA: systemic multimodal analgesia. |
The NRS pain scores on postoperative day 2 (POD 2) did not significantly differ across the four groups (PECS 1.95 ± 0.722, ESP 1.82 ± 1.097, SMA 2.70 ± 1.521, and TWI 2.09 ± 1.564, P > 0.05) (Fig. 4). The study found that there was a significant difference in the time to first rescue dose among the groups (in minutes, PECS 20.73 ± 3.712, ESP 11.52 ± 10.939, SMA 13.39 ± 2.388, TWI 13.48 ± 2.410, P < 0.05) (Fig. 5).
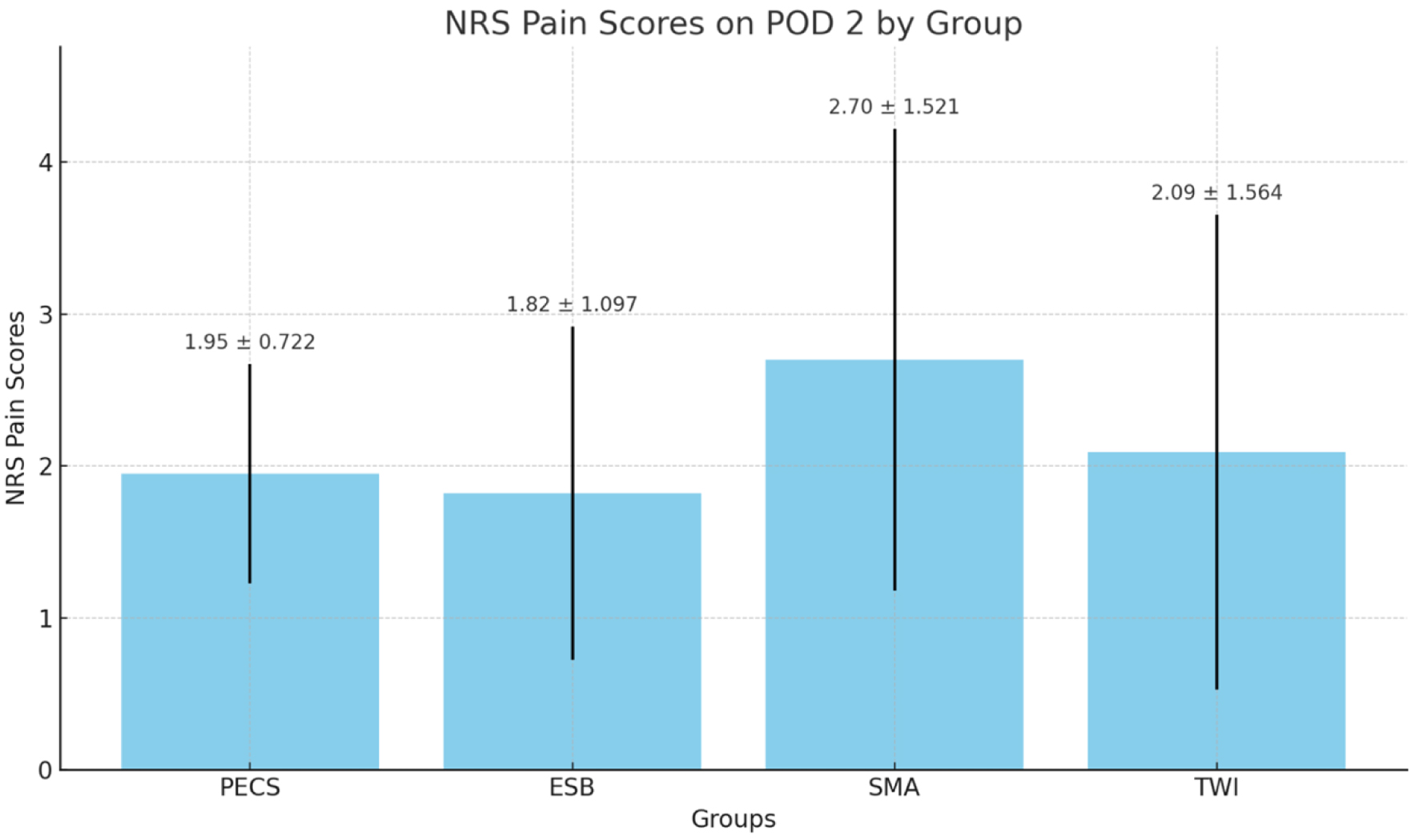 Click for large image | Figure 4. NRS pain scores on postoperative day 2 (POD 2) for the four groups. NRS: numeric rating scale. |
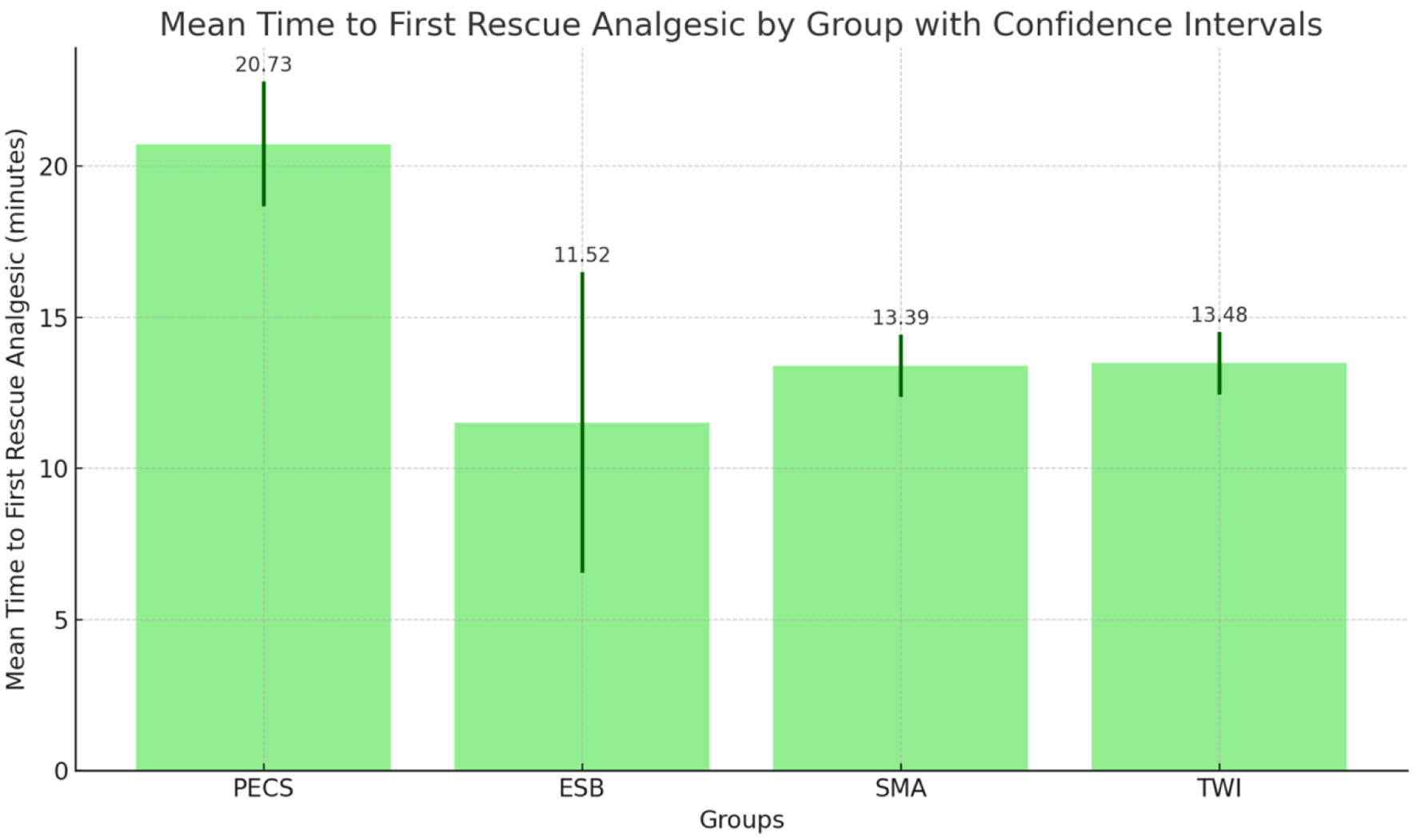 Click for large image | Figure 5. The mean interval between arrival to post-anesthesia care unit (PACU) and administration of first rescue dose, with error bars representing the standard deviation for each group. |
PECS group had the highest average time to rescue dose, and this was significantly different from all the other groups. However, no significant differences in time were observed between the groups ESP, SMA, and TWI, using both parametric (ANOVA) and non-parametric (Kruskal-Wallis) tests. Between the four groups, the time spent in the PACU in minutes was not statistically significant (PECS 135.27 ± 52.294, ESP 175.82 ± 83.649, SMA 151.87 ± 43.262, TWI 144.09 ± 45.130, P > 0.05), with patients who received ESP, spending the most time in PACU (Fig. 6). There was no inter-group significance either. The SMA and TWI groups had similar average times of 151.87 and 144.09 min, respectively. Looking at the data collectively, across all 88 samples, the overall mean time spent in the PACU was 151.68 min. This central tendency closely mirrors the averages of the SMA and TWI groups.
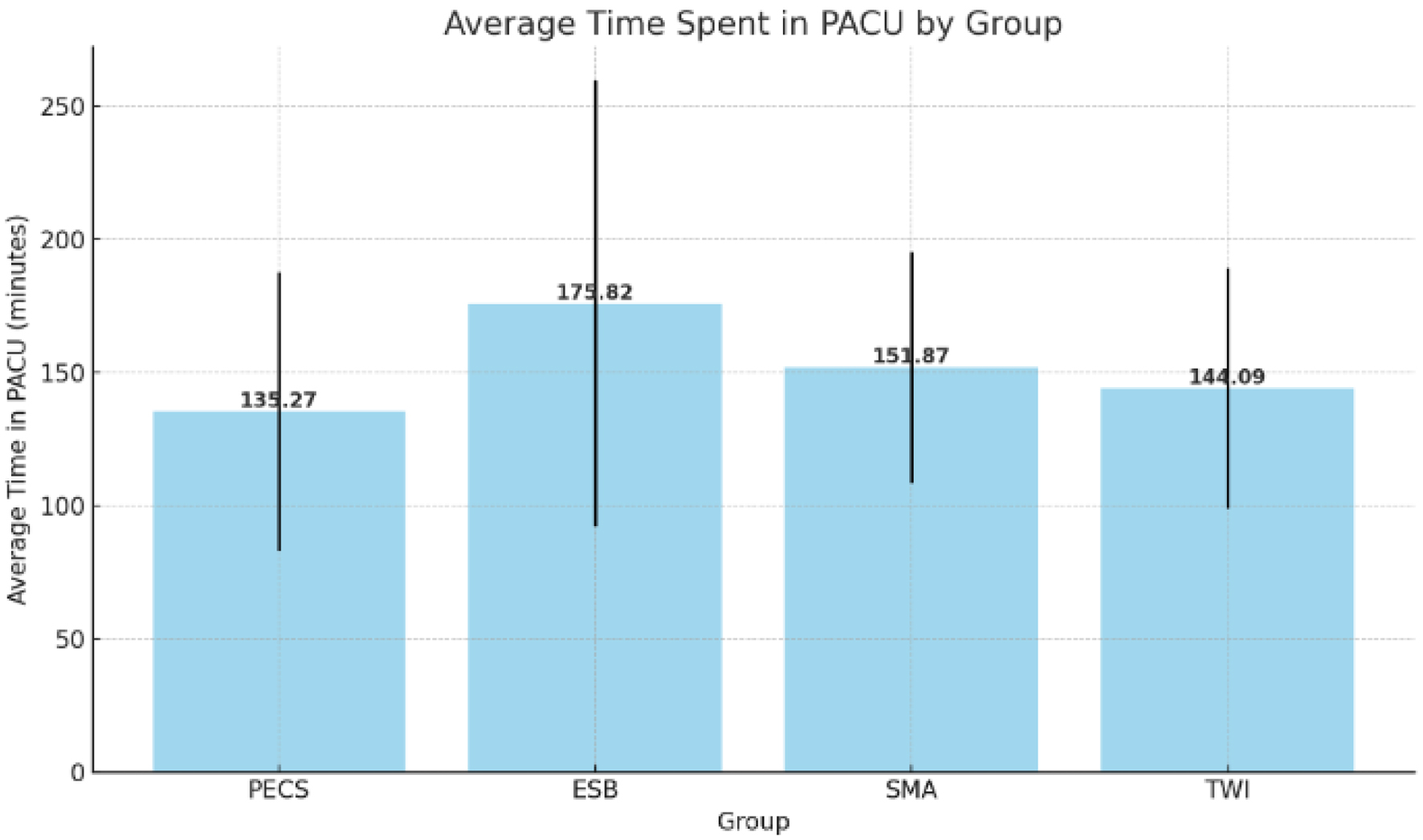 Click for large image | Figure 6. The average time spent in the post-anesthesia care unit (PACU) by group, with error bars representing the standard deviation for each group. |
The same 88 patients were analyzed for pain scores based on their chronic testosterone therapy. Table 2 delineates two cohorts of patients differentiated by their chronic testosterone therapy status, where group A, constituting 54 patients, was on such therapy, and group B, consisting of 34 patients, was not. Group A’s mean intraoperative MME was recorded at 18.45 mg, with a confidence interval (CI) spanning 16.0 to 20.9 mg. Group B demonstrated a higher mean MME of 21.54 mg, with a CI extending from 19.0 to 24.1 mg. In assessing the difference in intraoperative MME between the two groups, we applied Welch’s t-test. This statistical method does not presume equal variances or sample sizes between the groups. This approach is particularly pertinent in our study, as group A (T therapy) comprised 54 patients while group B (no T therapy) had 34 patients. The Welch’s t-test yielded a t-statistic of -1.76 and a P-value of 0.082. Although this P-value suggests a trend toward lower MME usage in group A, it did not reach the conventional threshold for statistical significance (P = 0.082). This finding indicates that, while there is a numerical difference in mean MME between the groups, the evidence is not strong enough to rule out the possibility that this difference is due to random variation rather than the effect of T therapy (Fig. 6).
 Click to view | Table 2. Mean Intraoperative MME Scores for Both Group A (T Therapy) and Group B (No T Therapy) With 95% CIs |
| Discussion | ▴Top |
Opioid sparing anesthesia and opioid free anesthesia (OFA) favors multimodal analgesia to reduce or avoid opioids during perioperative care [9]. Benefits include less risk of side effects like nausea, vomiting, and respiratory depression, leading to higher patient satisfaction and possibly faster recovery. This approach is crucial amid the opioid crisis [9]. This study finds that the PECS 2 block is superior in reducing overall opioid consumption and providing effective immediate postoperative pain control, which aligns with the philosophy of opioid sparing anesthesia. The lower PACU MME requirement and shorter PACU stays observed in patients who received PECS 2 block further support their potential role in an OFA protocol.
The PECS 2 block, due to its anatomical specificity, provides targeted analgesia to the pectoral nerves, intercostobrachial nerve, long thoracic, and thoracodorsal nerves, which are essential in the innervation of the breast and axillary regions [10, 11]. This specificity starkly contrasts the ESP block, where the local anesthetic is deposited deep in the erector spinae muscle, necessitating a greater diffusion distance through muscle and fascia to reach the ventral and dorsal rami of the spinal nerves [12, 13]. The reduced diffusion distance required for the PECS 2 block means that the anesthetic can more rapidly and directly affect the intended nerves, leading to a faster onset of action and a reduction in the overall need for opioids. The anatomically targeted analgesic precision provided by the PECS block might be the underlying factor contributing to the observed superiority of the PECS 2 block in providing intraoperative analgesia, as indicated by the reduced requirements for opioids both during the surgery and in the PACU as measured by MME consumption. Conversely, the trans-fascial anesthetic diffusion effect associated with the ESP block could explain the increased intraoperative opioid consumption observed in this group. However, it is noteworthy that despite the higher intraoperative opioid use, this group demonstrated better analgesia in the PACU, which is evidenced by a lesser need for opioids in terms of PACU MME requirements.
Our research demonstrated a notable reduction in intraoperative opioid use, with nerve blocks showing significantly lower MME consumption in the PACU compared to the SMA and TWI groups. Local anesthetic infiltration, which offers limited and localized pain relief, outperforms the PECS block’s anatomically targeted analgesic precision. This technique achieves a more comprehensive blockade of superficial and deeper chest wall structures, leading to superior pain control [10]. Moreover, the PECS block’s ability to employ higher volumes and concentrations of anesthetics extends the analgesic effect, enhancing the duration and quality of pain management. This extended analgesia likely contributes to the improved POD 2 pain scores observed with the PECS and ESP blocks relative to SMA and TWI, minimizing the need for additional analgesics in the critical initial recovery period.
Our study found that, at 30, 60, and 90 min post-surgery, the SMA group experienced significantly higher pain levels than the PECS and ESP groups. However, by 120 min, this difference had disappeared. These early benefits are crucial, as adequate pain control in this phase is vital for patient comfort and early mobilization. Interestingly, the significance of these differences diminished over time, possibly due to titration of opioids to pain levels. Higher pain scores and higher opioid consumption in the PACU suggest that SMA might not be the most effective method for managing immediate postoperative pain. All pain management techniques converge in efficacy which is evident from less pain scores at 120 min after reaching PACU but at the cost of higher opioid consumption in SMA and TWI. Several studies [14-21] have indicated that PECS blocks are particularly effective for chest surgeries. Our results contribute to this body of work by suggesting that both PECS and ESP blocks may be effective in the immediate postoperative period compared to SMA or TWI, which is invaluable for gender-affirming mastectomies. Another noteworthy observation was the reduced PACU MME requirement and shorter PACU duration for patients in the PECS group. This is consistent with studies that also observed reduced opioid requirements for PECS and ESP blocks. Lower opioid consumption in the PACU is laudable due to the associated risks like respiratory depression, nausea, and pruritus. Additionally, shorter PACU stays indicate faster patient recovery and more efficient utilization of healthcare resources.
In the study, postoperative nausea and vomiting were experienced by six patients in the SMA group and three patients in the TWI group. This phenomenon can be linked to increased consumption of opioids. Meanwhile, four patients who received ESP blocks presented with hypotension in the PACU, a condition that was effectively managed with fluid boluses. The duration of observation for these four patients reached up to 4 h, significantly influencing the average recovery time calculated for the ESP group. The ESP block, when correctly performed, has a low incidence of complications due to the injection site being away from the pleura, major blood vessels, and the spinal cord. However, complications such as hypotension can occur, although it is not a commonly reported adverse effect. The ESP block can potentially lead to a sympathetic blockade which may cause vasodilation and subsequently hypotension. It is important to note that comprehensive data on complications like hypotension are still limited, and more studies, such as randomized controlled trials, are needed to better understand the safety and complication rates of ESP blocks. The mechanism of action is likely related to the spread of local anesthetic to the nerve roots that affect sympathetic tone, but the exact pathways and extent of spread require further research to fully elucidate [21].
The safety profile of the PECS block, which is typically utilized in breast surgeries, may be favored due to its targeted approach that minimally affects systemic physiological responses such as blood pressure. This could contrast with the ESP block that, although rare, may cause hypotension through a sympathetic blockade leading to vasodilation. The use of PECS blocks could therefore offer a dual benefit of reducing the risk of certain complications while delivering effective intraoperative analgesia specific to the surgical site. Results of our research align with studies [11, 22] which also favor PECS block over ESP block. Further research, like randomized controlled trials, could help clarify these advantages, enhancing our understanding of the relative safety and efficacy of these anesthetic techniques.
Chronic testosterone therapy has been associated with decreased pain levels through a confluence of physiological and neurobiological mechanisms [8]. It potentially enhances the analgesic effects of opioids by modulating opioid receptors and the endogenous opioid system, thereby increasing pain tolerance [23]. Additionally, testosterone exhibits anti-inflammatory properties, reducing pro-inflammatory cytokine production and directly contributing to pain alleviation in inflammatory conditions [24]. Its role in neuroprotection and neural growth further supports the healing of nerve tissues and maintenance of neural pathways critical for pain transmission, suggesting a direct impact on reducing pain perception [25]. Moreover, testosterone therapy may improve mood and psychological well-being, indirectly affecting pain perception by reducing anxiety and depression, which are common in chronic pain sufferers [26, 27]. Lastly, its involvement in regulating vascular tone and endothelial function might also contribute to pain reduction, particularly in conditions where vascular dysfunction plays a role [28]. This multifaceted interaction underscores the complex relationship between testosterone and pain, highlighting the need for a nuanced understanding of hormonal therapy’s role in pain management. In our study, a total of 88 patients were divided into two groups (groups A and B) according to chronic testosterone therapy status. Despite the substantial theoretical explanations supporting the notion that testosterone modulates pain, our study did not reveal any statistical significance between the groups, suggesting that the relationship between testosterone therapy and pain levels may not be straightforward and is influenced by a multitude of factors.
Our research is subject to several limitations that warrant acknowledgment. The inherent nature of its retrospective design lacks the control and randomization afforded by a prospective study, which may introduce biases such as recall or selection biases. Although the sample size was statistically adequate, it may limit the broader applicability of the findings. Variability in the administration techniques of regional anesthetic blocks by different providers might have led to inconsistent pain management efficacy. The absence of patient-reported outcomes in the study design needs to include critical data regarding the subjective experience of pain and treatment satisfaction. Moreover, the study did not capture long-term pain outcomes or the development of persistent postsurgical pain, which are significant for assessing surgical recovery. This study also did not consider psychosocial factors that can substantially affect pain perception and recovery. In addition, the division of the entire cohort into groups A and B resulted in an unbalanced sample size, which may undermine the reliability of significant difference levels ascertained, notwithstanding the use of Welch’s t-test to adjust for unequal sample sizes and variances [29]. Furthermore, as our study exclusively involved individuals assigned female at birth who underwent gender-affirming mastectomy as part of their transition to male, it does not address sex-related differences in outcomes. This limitation is critical to note, as it confines the generalizability of our findings specifically to this demographic without broader implications for sex-based comparative analysis.
Conclusion
Despite the outlined limitations, our study offers meaningful insights into the comparative efficacy of various perioperative pain management strategies for gender-affirming bilateral mastectomies. The findings suggest that the PECS 2 block is a superior analgesic approach with reduced opioid consumption in the intraoperative and immediate postoperative phase. Additionally, our results indicate that chronic testosterone therapy does not exert a significant influence on intraoperative opioid requirements. This research enhances the current literature [11, 12, 14-21] by providing evidence to improve pain management protocols tailored to the transgender and non-binary community, with consideration for their distinct physiological responses to chronic testosterone therapy. To build on the findings of this study, future research should employ a prospective design with standardized regional block techniques. It should include long-term outcomes and patient-reported satisfaction measures. Delving deeper into the complex relationship between hormonal therapy and pain perception remains an intriguing and essential area for further exploration.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
For this retrospective study, formal consent is not required. This research involves the use of existing data, documents, and records that have been collected for non-research purposes. All patient data accessed comply with relevant data protection and privacy regulations. Identifiable information has been removed to ensure the anonymity of individuals. The study protocols were reviewed and approved by the Institutional Review Board, which waived the requirement for informed consent.
Author Contributions
Sengottaian Sivakumar, MD: conceived and designed the analysis; collected the data; contributed data or analysis tools; performed the analysis; wrote the paper. Aron Kressel: collected the data, contributed data and analysis tools. Michael Girshin, MD: conceived and designed the analysis; contributed data or analysis tools. Roni Mendonca, MD: collected the data; contributed data or analysis tools.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Alcon A, Kennedy A, Wang E, Piper M, Loeliger K, Admassu N, Lentz R, et al. Quantifying the psychosocial benefits of masculinizing mastectomy in trans male patients with patient-reported outcomes: the University of California, San Francisco, gender quality of life survey. Plast Reconstr Surg. 2021;147(5):731e-740e.
doi pubmed - Lane M, Kirsch MJ, Sluiter EC, Svientek SR, Hamill JB, Morrison SD, Ives GC, et al. Gender affirming mastectomy improves quality of life in transmasculine patients: a single-center prospective study. Ann Surg. 2023;277(3):e725-e729.
doi pubmed - Wong HY, Pilling R, Young BWM, Owolabi AA, Onwochei DN, Desai N. Comparison of local and regional anesthesia modalities in breast surgery: A systematic review and network meta-analysis. J Clin Anesth. 2021;72:110274.
doi pubmed - Wick EC, Grant MC, Wu CL. Postoperative multimodal analgesia pain management with nonopioid analgesics and techniques: a review. JAMA Surg. 2017;152(7):691-697.
doi pubmed - Rokhtabnak F, Sayad S, Izadi M, Djalali Motlagh S, Rahimzadeh P. Pain control after mastectomy in transgender patients: ultrasound-guided pectoral nerve block II versus conventional intercostal nerve block: a randomized clinical trial. Anesth Pain Med. 2021;11(5):e119440.
doi pubmed pmc - Rahimzadeh P, Imani F, Faiz SHR, Boroujeni BV. Impact of the ultrasound-guided serratus anterior plane block on post-mastectomy pain: a randomised clinical study. Turk J Anaesthesiol Reanim. 2018;46(5):388-392.
doi pubmed pmc - Huang G, Travison TG, Edwards RR, Basaria S. Effects of testosterone replacement on pain catastrophizing and sleep quality in men with opioid-induced androgen deficiency. Pain Med. 2017;18(6):1070-1076.
doi pubmed - Athnaiel O, Cantillo S, Paredes S, Knezevic NN. The role of sex hormones in pain-related conditions. Int J Mol Sci. 2023;24(3):1866.
doi pubmed pmc - Carcamo-Cavazos V, Cannesson M. Opioid-free anesthesia: the pros and cons. Adv Anesth. 2022;40(1):149-166.
doi pubmed - Versyck B, van Geffen GJ, Chin KJ. Analgesic efficacy of the Pecs II block: a systematic review and meta-analysis. Anaesthesia. 2019;74(5):663-673.
doi pubmed - Hong B, Bang S, Oh C, Park E, Park S. Comparison of PECS II and erector spinae plane block for postoperative analgesia following modified radical mastectomy: Bayesian network meta-analysis using a control group. J Anesth. 2021;35(5):723-733.
doi pubmed - Kelava M, Alfirevic A, Bustamante S, Hargrave J, Marciniak D. Regional anesthesia in cardiac surgery: an overview of fascial plane chest wall blocks. Anesth Analg. 2020;131(1):127-135.
doi pubmed - Chin KJ, El-Boghdadly K. Mechanisms of action of the erector spinae plane (ESP) block: a narrative review. Can J Anaesth. 2021;68(3):387-408.
doi pubmed - Ueshima H, Otake H, Hara E, Blanco R. How to use pectoral nerve blocks effectively-an evidence-based update. Asian J Anesthesiol. 2019;57(2):28-36.
doi pubmed - Ardon AE, George JE, 3rd, Gupta K, O'Rourke MJ, Seering MS, Tokita HK, Wilson SH, et al. The use of pectoralis blocks in breast surgery: a practice advisory and narrative review from the society for ambulatory anesthesia (SAMBA). Ann Surg Oncol. 2022;29(8):4777-4786.
doi pubmed - Barrington MJ, Seah GJ, Gotmaker R, Lim D, Byrne K. Quality of recovery after breast surgery: a multicenter randomized clinical trial comparing pectoral nerves interfascial plane (Pectoral Nerves II) block with surgical infiltration. Anesth Analg. 2020;130(6):1559-1567.
doi pubmed - Kamiya Y, Hasegawa M, Yoshida T, Takamatsu M, Koyama Y. Impact of pectoral nerve block on postoperative pain and quality of recovery in patients undergoing breast cancer surgery: A randomised controlled trial. Eur J Anaesthesiol. 2018;35(3):215-223.
doi pubmed - Pirsaharkhiz N, Comolli K, Fujiwara W, Stasiewicz S, Boyer JM, Begin EV, Rubinstein AJ, et al. Utility of erector spinae plane block in thoracic surgery. J Cardiothorac Surg. 2020;15(1):91.
doi pubmed pmc - Nagaraja PS, Ragavendran S, Singh NG, Asai O, Bhavya G, Manjunath N, Rajesh K. Comparison of continuous thoracic epidural analgesia with bilateral erector spinae plane block for perioperative pain management in cardiac surgery. Ann Card Anaesth. 2018;21(3):323-327.
doi pubmed pmc - Guven BB, Erturk T, Ersoy A. Postoperative analgesic effectiveness of bilateral erector spinae plane block for adult cardiac surgery: a randomized controlled trial. J Health Sci Med / JHSM. 2022;5(1):150-155.
doi - De Cassai A, Bonvicini D, Correale C, Sandei L, Tulgar S, Tonetti T. Erector spinae plane block: a systematic qualitative review. Minerva Anestesiol. 2019;85(3):308-319.
doi pubmed - Rashad MM, Abdelhay AA. Ultrasound-guided modified pectoral plane (PECS II) block versus erector spinae plane (ESP) block for perioperative analgesia of surgical treatment of gynecomastia. Ain-Shams J Anesthesiol. 2022;14:97.
doi - Khalil R, Humann J. Testosterone modulation of ethanol effects on the micro-opioid receptor kinetics in castrated rats. Biomed Rep. 2019;11(3):103-109.
doi pubmed pmc - Craft RM, Mogil JS, Aloisi AM. Sex differences in pain and analgesia: the role of gonadal hormones. Eur J Pain. 2004;8(5):397-411.
doi pubmed - Lenert ME, Avona A, Garner KM, Barron LR, Burton MD. Sensory Neurons, Neuroimmunity, and Pain Modulation by Sex Hormones. Endocrinology. 2021;162(8):bqab109.
doi pubmed pmc - Kato Y, Shigehara K, Kawaguchi S, Izumi K, Kadono Y, Mizokami A. Efficacy of testosterone replacement therapy on pain in hypogonadal men with chronic pain syndrome: A subanalysis of a prospective randomised controlled study in Japan (EARTH study). Andrologia. 2020;52(9):e13768.
doi pubmed - Archey M, Goldey K, Crockett E, Boyette-Davis J. An investigation of the effects of testosterone and behavioral expressions of pain on sex/gender differences in pain perception. Psychol Rep. 2019;122(3):826-840.
doi pubmed - Kelly DM, Jones TH. Testosterone: a vascular hormone in health and disease. J Endocrinol. 2013;217(3):R47-71.
doi pubmed - Delacre M, Lakens D, Leys C. Why psychologists should by default use Welch’s T-test instead of student’s T-test. International Review of Social Psychology. 2017;30(1):92-101.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


