| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 16, Number 5, May 2024, pages 208-219
Updated Bivalent COVID-19 Vaccines Reduce Risk of Hospitalization and Severe Outcomes in Adults: An Observational Cohort Study
Nicholas Mielkea, b, Steven Johnsonc, Charlotte O’Sullivana, Mohammad Usama Toseefd, Amit Bahle, f
aOakland University William Beaumont School of Medicine, Rochester, MI, USA
bDepartment of Medicine, Creighton University School of Medicine, Omaha, NE, USA
cDepartment of Anesthesia, Keck Medicine of University of Southern California, Los Angeles, CA, USA
dCorewell Health Research Institute, Royal Oak, MI, USA
eDepartment of Emergency Medicine, Corewell Health William Beaumont University Hospital, Royal Oak, MI, USA
fCorresponding Author: Amit Bahl, Department of Emergency Medicine, Corewell Health William Beaumont University Hospital, Royal Oak, MI 48073, USA
Manuscript submitted March 2, 2024, accepted April 24, 2024, published online May 29, 2024
Short title: Bivalent COVID-19 Vaccines Improve Outcomes
doi: https://doi.org/10.14740/jocmr5145
| Abstract | ▴Top |
Background: This study evaluates the real-world effectiveness of updated bivalent coronavirus disease 2019 (COVID-19) vaccines in adults, as the virus evolves and the need for new vaccinations increases.
Methods: In this observational, retrospective, multi-center, cohort analysis, we examined emergency care encounters with COVID-19 in metro Detroit, Michigan, from January 1, 2022, to March 9, 2023. Patients were categorized by vaccination status: unvaccinated, fully vaccinated, fully vaccinated and boosted (FV&B), or fully vaccinated and bivalent boosted (FV&BB). The primary outcome was to assess the impact of bivalent COVID-19 vaccinations on the risk of composite severe outcomes (intensive care unit (ICU) admission, mechanical ventilation, or death) among patients presenting to a hospital with a primary diagnosis of COVID-19.
Results: A total of 21,439 encounters met inclusion criteria: 9,630 (44.9%) unvaccinated, 9,223 (43.0%) vaccinated, 2,180 (10.2%) FV&B, and 406 (1.9%) FV&BB. The average age was 48.8, with 59.6% female; 61.1% were White, 32.8% Black, and 6.0% other races. Severe disease affected 5.5% overall: 5.0% unvaccinated, 5.7% vaccinated, 7.0% FV&B, and 4.7% FV&BB (P = 0.001). Severe disease rates among admitted patients were 13.3% unvaccinated, 11.9% vaccinated, 12.2% boosted, and 8.1% FV&BB (P = 0.052). The FV&BB group showed a 4.0% (P = 0.0369) lower risk of severe disease compared to FV&B and a 5.1% (P = 0.0203) lower probability of hospitalization.
Conclusions: As the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to mutate and evolve, updated vaccines are necessary to better combat COVID-19. In a real-world hospital-based population, this investigation demonstrates the incremental benefit of the bivalent booster vaccine in reducing the risk of hospitalization and severe outcomes in those diagnosed with COVID-19 compared to all other forms of vaccination.
Keywords: COVID-19; SARS-CoV-2; Booster dose; Vaccination; Hospitalization; Severe illness; Mortality; Death
| Introduction | ▴Top |
The coronavirus disease 2019 (COVID-19) virus has proved to be one of the deadliest viruses to date, as the death toll approaches 7,000,000 people and nearly 800,000,000 confirmed infections in just 3 years [1, 2]. This disease has quickly become one of the top causes of death in the USA, next to heart disease and cancer [3].
Fortunately, a variety of therapeutics have been identified or developed to combat this virulent pathogen. For active infection, anti-inflammatories, monoclonal antibodies, and antivirals have resulted in improved outcomes in some patient populations [4-7]. However, infection control and prevention remain the most effective method of reducing mortality. After the first rollout of vaccines in early 2021, nearly 644.7 million vaccinations were distributed in the USA within 1 year [8]. The original monovalent vaccine was developed using mRNA to produce the spike protein found on the surface of the virus [9]. Both the primary series of two vaccines, plus the rollout of booster shots 1 year later, quickly demonstrated significant reductions in COVID-19-related hospitalizations and in-hospital deaths [10, 11].
Unfortunately, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to evolve, and changes in its genetic code diminish the effectiveness of targeted vaccinations. mRNA viruses like SARS-CoV-2 mutate five times faster than DNA viruses, therefore, it is not surprising to observe significant changes to the virus’s genome over the course of several years [12]. Emerging in India in late 2020, the delta variant was highly contagious and was an early real-world example of how viral variants could reduce vaccine efficacy [12, 13]. Subsequently, the omicron variant, first detected in November 2021, proved to be even more infectious with an even greater ability to infect vaccinated individuals [14, 15]. In order to combat these variants, a new series of bivalent boosters was developed. These boosters targeted a component of the original SARS-CoV-2 strain and a shared component of the BA.4/BA.5 lineages of the omicron variant. These vaccines were disseminated to the American public in September 2022 [16]. Early trials showed additional efficacy in preventing infection when compared to the monovalent vaccines [17]. However, there is still a lack of data regarding the efficacy of these vaccines in a real-world population, particularly in patients requiring hospital-level care. Thus, the primary aim of this study was to investigate the impact of the bivalent SARS-CoV-2 vaccinations compared to their monovalent counterparts in preventing severe outcomes among hospitalized patients.
| Materials and Methods | ▴Top |
Study design
This study was an observational, retrospective, multi-center cohort analysis of patient encounters extracted from electronic health records (EHRs). Eight distinct hospitals, ranging from small community hospitals to a large, academic, tertiary care center in Southeast Michigan, USA, comprised the study setting.
Selection of participants
Encounters of patients with a principal diagnosis (laboratory confirmed at time of the emergency department (ED) presentation) of COVID-19 (U07.1), among patients who were 18 or older at the time of the encounter and presented to one of Corewell Health East’s ED between January 1, 2022, and March 9, 2023, met inclusion criteria. Any patients with laboratory-verified positive COVID-19 test within the preceding 28 days were excluded, as this was likely not an acute infection but rather a persistent one (Fig. 1). The Corewell Health Institutional Review Board (IRB) approved this (IRB #2022-266); given the retrospective nature of the study, a waiver for written informed consent was granted. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
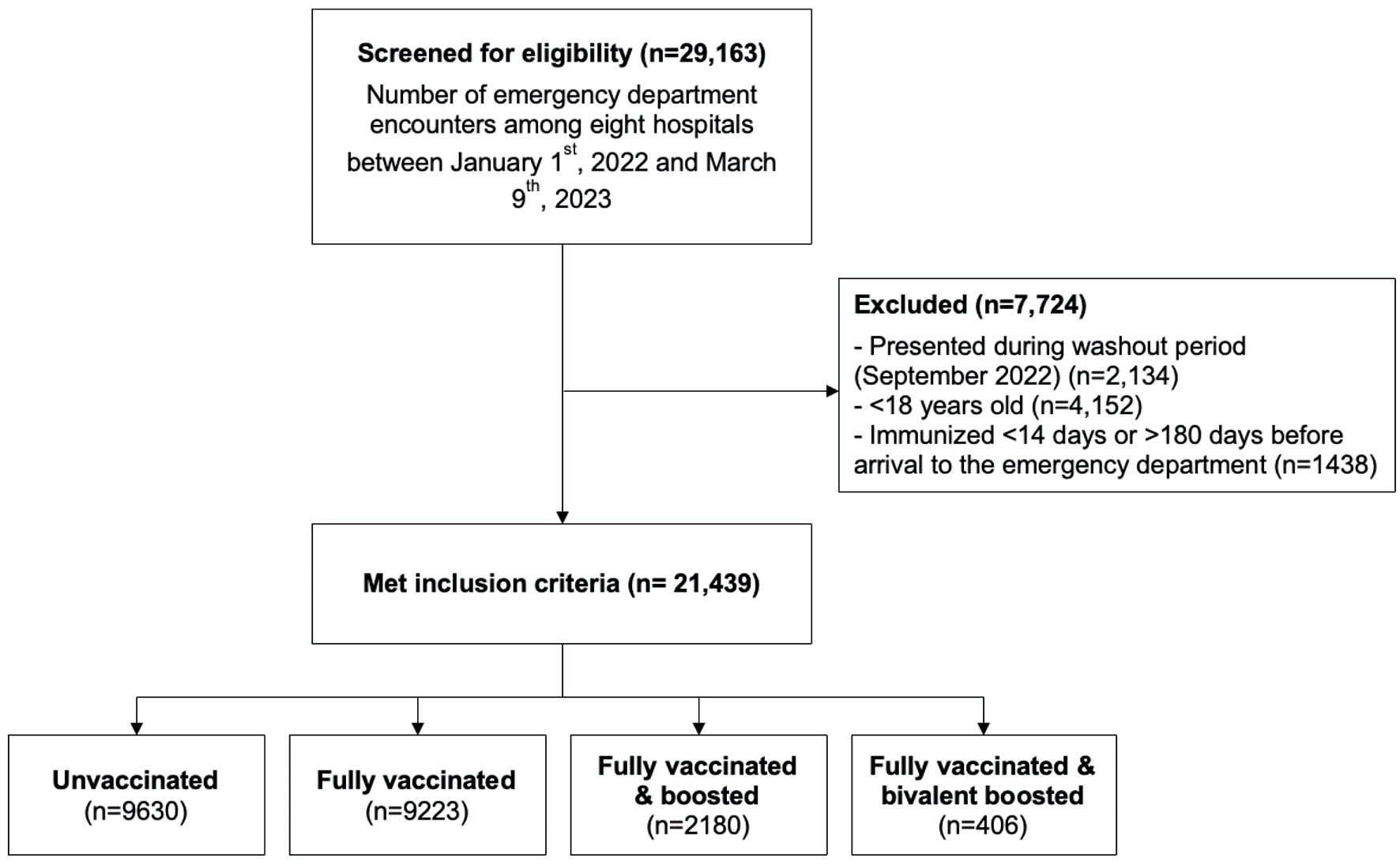 Click for large image | Figure 1. Flow figure of inclusion and exclusion criteria. Flow figure of encounters screened for eligibility, encounters excluded from the study, and vaccination categorization. |
Measurements
All data were extracted from the EHR system (Epic, Verona, Wisconsin). These data included demographic, clinical, laboratory, and outcomes variables, including age, sex, ethnicity, past medical history, vaccination status, initial vital signs, in-hospital therapies, and outcomes of interest, such as the need for care in the intensive care unit (ICU), mechanical ventilation, death, and hospital length of stay. Comorbidities were assessed using the Agency for Healthcare Research and Quality (AHRQ) Elixhauser Comorbidity Index [18]. Immunocompromised individuals were identified based on the International Classification of Diseases, 10th Revision (ICD-10) codes per the AHRQ definition, such as autoimmune diseases, organ transplants, nutritional deficiencies, certain genetic conditions, certain chronic diseases, and human immunodeficiency virus disease [19]. The vaccination status for SARS-CoV-2 of patients was confirmed through the EHR at the institution, connected with the Michigan Care Improvement Registry (MCIR) [20]. This integration ensured that even those who received their vaccinations outside the Corewell Health System had updated vaccination data. The MCIR keeps detailed records of SARS-CoV-2 vaccinations for people immunized in Michigan, including the specific type of vaccine received and the date it was administered.
Definitions
Vaccination status was grouped into four categories: unvaccinated, vaccinated, boosted, and bivalent boosted. The unvaccinated group consisted of patients with no records of vaccination. The vaccinated category consisted of patients who received the primary two-dose monovalent mRNA vaccination series (Pfizer, Moderna) or received one viral vector vaccine (Janssen). The fully vaccinated and boosted (FV&B) cohort consisted of patients who met criteria of the vaccinated category and received at least one additional vaccine before September 1, 2022. The fully vaccinated and bivalent boosted (FV&BB) cohort consisted of patients who met criteria of the FV&B category and received one of the bivalent boosted vaccines between October 1, 2022, and March 9, 2023. A washout period (September 2022) was utilized. All data were queried on March 31, 2023. For all vaccination groups, the immunization date must be at least 14 days after vaccination administration, which is consistent with previous studies [21, 22]. Safety information regarding potential side effects is monitored and reported by the Centers for Disease Control and Prevention (CDC) [23].
Outcomes
The primary outcome of this study was to assess the impact of bivalent COVID-19 vaccinations on the risk reduction of composite severe outcomes among patients presenting to a hospital with a primary diagnosis of COVID-19. Severe outcomes included admission to the ICU, mechanical ventilation, or death, as first described by the CDC [24]. The secondary outcome evaluated the need for hospitalization following initial ED evaluation. Of note, mechanically ventilated patients required an ICU level of care per institutional policy. Additional outcome measures included need for oxygen therapy, and hospital length of stay.
Data analyses
First, descriptive statistics were used to summarize patient characteristics based on their COVID-19 vaccination status. Continuous variables were expressed as means, standard deviation of the mean, median, and interquartile range. Categorical variables were reported as frequencies and percentages. Differences in the characteristics based on vaccination status were tested using Pearson’s Chi-square test for categorical variables, and Kruskal-Wallis test for continuous variables.
Next, three logistic regression models were fit to evaluate the effect of vaccination status on the above-mentioned composite outcome: 1) unadjusted model did not control for any additional covariate; 2) partially adjusted model controlled for age and Elixhauser comorbidity index; 3) fully adjusted model, controlled for age, Elixhauser comorbidity index, sex, race pre-existing end-stage renal disease and if the patient was immunocompromised. Following each estimated regression model, average marginal effects of vaccination status on composite outcome were calculated. Using these marginal effects, analysis of variance contrast tests was used to estimate the differences in predicted probabilities (along with 95% confidence intervals) of composite outcome between vaccination status groups using the boosted as the reference category. The same steps were followed for the secondary outcome. The contrasts test results are presented in figures for ease of visual inspection and assessment. All the statistical analysis was performed using Stata 17.0 [25].
| Results | ▴Top |
Between January 1, 2022, and March 9, 2023, 21,439 patient encounters met inclusion criteria: 9,630 (44.9%) were unvaccinated, 9,223 (43.0%) were vaccinated, 2,180 (10.2%) were boosted, and 406 (1.9%) were bivalent boosted. The average age was 48.8 years old, 59.6% (n = 12,777) of the population was female, and 61.1% (n = 13,109) identified as White, 32.8% (7,034) identified as Black, and 6.0% (n = 1,296) identified with a race other than Black or White. Immunocompromised state was present in 12.8% of the population, with the largest proportion among boosted patients (18.5%) and the lowest proportion among the unvaccinated patients (9.5%; P < 0.001). The bivalent boosted group comprised the highest proportion requiring oxygen therapy (37.2%), compared to the boosted, vaccinated, and unvaccinated groups (32.7%, 28.4%, and 22.1%, respectively. P < 0.001). Overall, 5.5% (n = 1,178) of the population experienced composite severe disease; 5.0% (n = 477) unvaccinated, 5.7% (n = 530) vaccinated, 7.0% (n = 152) boosted, and 4.7% (n = 19) bivalent boosted (P = 0.001). The average length of stay was longest in the unvaccinated cohort (183.0 h) and shortest in the bivalent boosted cohort (147.0 h) (Table 1).
 Click to view | Table 1. Demographics, Comorbidities, Initial Vital Signs, In-Hospital Therapies, and Outcomes of All Patients |
In a subgroup analysis of patients admitted to the hospital (n = 9,390), 54.3% (5,101) of the subgroup was female, with 24.8% (n = 2,325) identifying as Black, 69.9% (6,567) identifying as White, and 5.3% (n = 498) identifying with a race other than Black or White. Immunocompromised state was present in 20.5% (n = 1,922) of the population, and 52.1% (4,888) had an Elixhauser score of ≥ 5. Thirty-seven point seven percent (n = 3,543) were unvaccinated, 46.5% (n = 4,366) were vaccinated, 13.3% (n = 1,246) were boosted, and 2.5% (n = 235) were bivalent boosted. There was no difference in the proportion of the need for oxygen therapy (bivalent boosted = 59.6%, boosted = 55.5%, unvaccinated = 56.9%, and vaccinated = 56.8%; P = 0.645). For the primary outcome, 13.3% (n = 472) of the unvaccinated patients experienced composite severe disease, compared to 11.9% of vaccinated patients, 12.2% of boosted patients, and 8.1% of bivalent boosted patients (P = 0.052) (Table 2). The risk of severe disease among the FV&BB cohort of admitted patients was 4.0% lower compared to the FV&B cohort of admitted patients (P = 0.0369) (Fig. 2a).
 Click to view | Table 2. Demographics, Comorbidities, Initial Vital Signs, In-Hospital Therapies, and Outcomes of Admitted Patients |
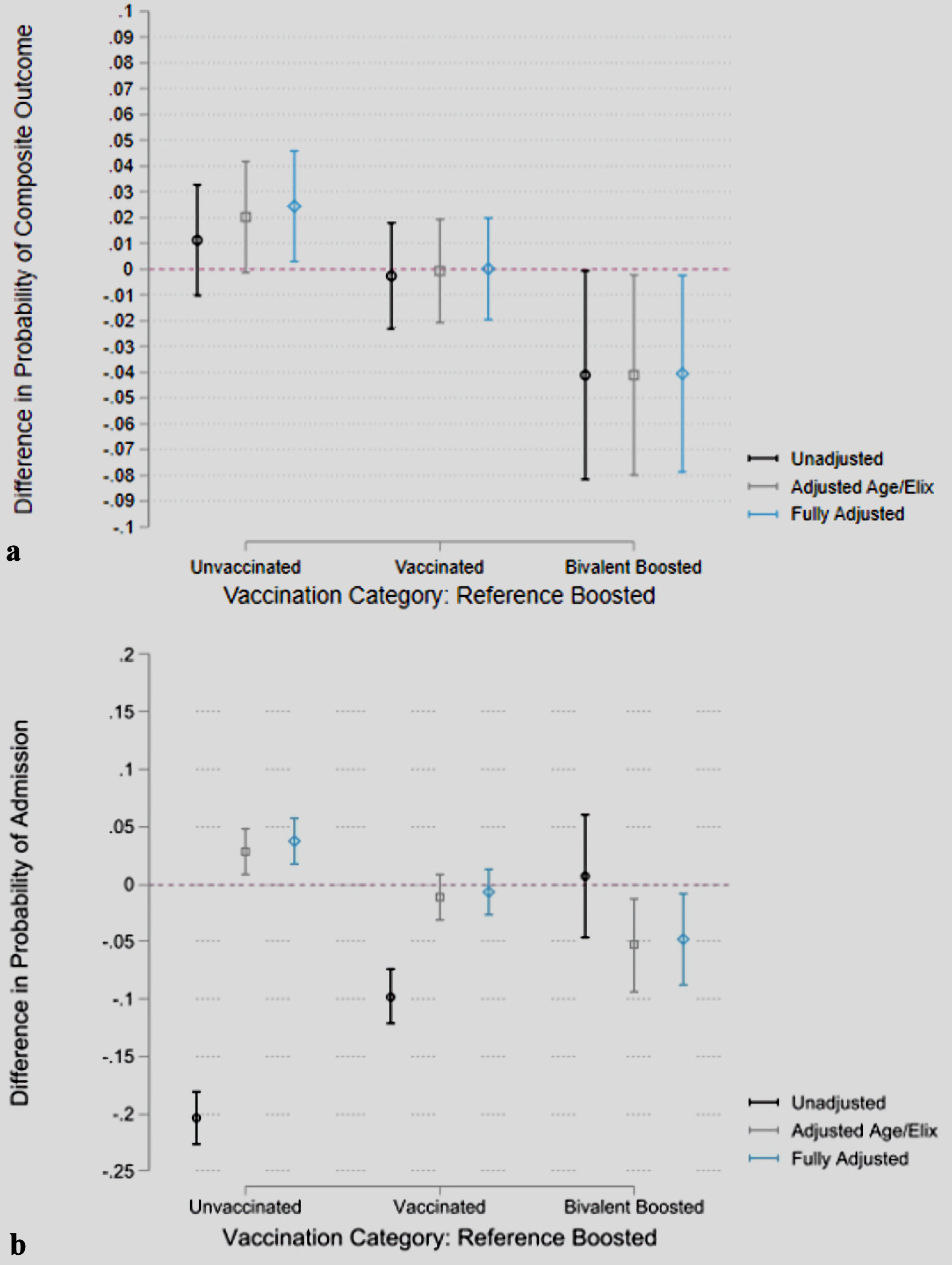 Click for large image | Figure 2. Probability differences in composite outcomes and hospital admission by vaccination status, adjusted for baseline risk factors. Predictions are based on logistic regression models. Unadjusted model does not control for any covariate, adjusted age/Elix controls for age and Elixhauser comorbidity index and fully adjusted further controls for sex, race pre-existing end-stage renal disease and if the patient was immunocompromised. Error bars represent 95% confidence intervals. Point estimates represent the difference in marginal effects of vaccination status on (a) composite outcome with boosted being the reference and (b) probability of hospital admission with boosted being the reference. |
The difference in the probability of requiring hospital admission, after adjusting for age, sex, race, Elixhauser comorbidity index, pre-existing end-stage renal disease, and immunocompromised state was 2.9% (P = 0.0351) greater in the unvaccinated group, compared to the FV&B group. There was no difference in the contrast of predictions for the vaccinated versus the boosted groups (P > 0.05). The probability of requiring hospitalization was 5.1% higher in the FV&B group compared to the FV&BB group (P = 0.0203) (Fig. 2b) (Supplementary Material 1, www.jocmr.org).
| Discussion | ▴Top |
This study serves as one of the first to investigate the real-world effectiveness of the bivalent formulations for the mRNA vaccines. After adjusting for baseline risk factors, we observed a reduced rate of hospital admission as well as a reduction in the rate of severe outcomes amongst patients who had received the bivalent booster as compared to all other groups. While fully vaccinated and FV&B demonstrated a significant reduction in these risks when compared to unvaccinated individuals, bivalent boosted patients demonstrated an even greater reduction in these risks. Additionally, despite the bivalent boosted population being the oldest and highest risk for inpatient mortality, as predicted by the Elixhauser index, we observed the lowest absolute mortality and composite severe outcome rate in this group. Further, after adjusting for age, sex, race, and comorbidities, the bivalent mRNA booster patients had the lowest rates of composite severe disease, shortest hospital length of stay, and lowest probability of requiring hospitalization when compared to all other groups. These findings align with other similar previous studies [26-30]. These results not only re-enforce the known efficacy of the bivalent boosters but demonstrate a significant reduction in morbidity and mortality among the highest-risk population in our cohort.
Unfortunately, reception and distribution of the vaccines have decreased over the past year despite COVID-19 remaining one of the highest causes of morbidity and mortality in the USA [31]. It is notable that the unvaccinated cohort still comprises the highest burden to hospitals. In Oakland County, Michigan, 25.4% of residents were unvaccinated during the study period, however they comprised 44.9% of all patients hospitalized for COVID-19 [32]. This represents an ongoing and significant burden to the hospital systems across the country which is potentially avoidable with wider adoption of vaccines. Looking specifically at the most vulnerable individuals, while we observed a trend towards older individuals with higher levels of comorbidities being higher on the vaccination status ladder, our overall population of bivalent boosted individuals was still small. Current data report that in Oakland County, 18% of residents are older than 65. However, only 8% of Oakland County residents have received the bivalent booster [2]. These numbers suggest that even in a best-case scenario, we would still expect that at least 10% of individuals older than 65 have not yet received their bivalent booster.
It is important for individuals with elevated baseline risk to understand that there is an added layer of protection with the bivalent vaccine formulation, even compared to the very effective monovalent boosted cohort. Furthermore, when compared to other common upper respiratory viruses like influenza and respiratory syncytial virus, COVID-19 infection comprises higher rates of hospitalization and death overall [33]. Given the significant added protection from the bivalent boosters we observed in our study, it is critical that older, higher-risk individuals receive these vaccinations prior to SARS-CoV-2 exposure to reduce the risk of severe outcomes. Overall, our findings demonstrate the need for individuals to stay up to date on their COVID-19 vaccinations according to current guidelines per the CDC.
This investigation had some limitations. One limitation was the inability to identify the precise viral variant causing each infection. While specific variant data could further eliminate confounding variables, our study has the benefit of observing a real-world population for patient-centered outcomes. Therefore, we feel that our results demonstrate real-world efficacy despite our lack of specific variant data. In addition, Figure 3 visually demonstrates the number of cases meeting inclusion criteria group by predominant COVID-19 variant in the region per CDC genomic surveillance [34]. Another factor that limits the strength of our results is the small sample size of the bivalent boosted cohort. While this is a limitation, we still observed statistically significant results for our outcomes of interest, suggesting that the impact of bivalent vaccines is large enough to overcome this limitation. Next, there is a small chance that who tested positive for COVID-19 on admission to the hospital did not remain positive during the entirety of their hospitalization. Additionally, we did not have data on whether patients had previously tested positive for SARS-CoV-2 prior to vaccination, and therefore were unable to evaluate for the effects of innate immunity within our cohort. There is also a possibility that varying time between initial vaccinations and booster doses may have an effect on immunity, and this is not something that we evaluated. Finally, the retrospective nature of our study design may have caused issues with data collection and review. However, we have spent a significant amount of time validating our dataset over the course of the COVID-19 pandemic and believe that our collection methods retain the highest level of accuracy and integrity possible. Additionally, the retrospective nature did not allow us to standardize the rationale for intubating patients, potentially limiting our ability to use this as a surrogate outcome. However, given that all patients were seen within the same hospital system, we feel that protocols and rationale for intubation were very similar among sites. We were also unable to account for reinfection given the lack of serological information and, therefore, were unable to evaluate this outcome. Several additional manuscripts with similar data acquisition methods have been published by the core investigative team [25, 26, 35].
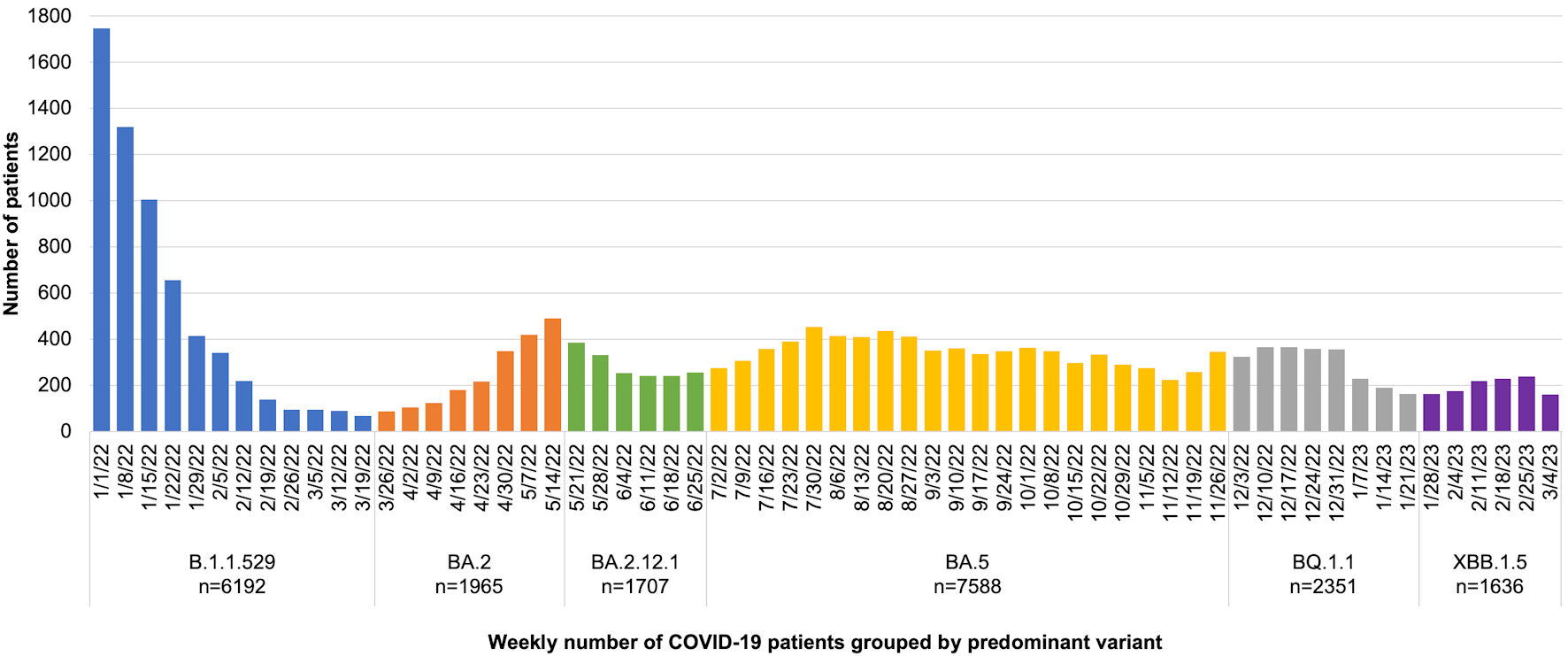 Click for large image | Figure 3. The number of cases meeting inclusion criteria group by predominant coronavirus disease 2019 (COVID-19) variant. |
Conclusions
Immunization with the updated bivalent mRNA formulations for the COVID-19 vaccines confer reduced risk of hospitalization and severe outcomes compared to all other groups. Further large-scale real-world research is needed to confirm our findings, particularly with newer variants.
| Supplementary Material | ▴Top |
Suppl 1. Regression analysis of composite outcome for fully adjusted, partially adjusted, and unadjusted cohorts.
Acknowledgments
Special thanks to Dr. Shirley Qu for her support with data acquisition.
Financial Disclosure
None to declare.
Conflict of Interest
Amit Bahl, MD, and Steven Johnson, DO, have received grant funding from Moderna. All other authors declare no relevant conflict of interest relevant to this work.
Informed Consent
A Research Waiver of Authorization was granted only for the stipulation of identification/data collection of the specific data variables for this study, given it is not practical or feasible to obtain consent/assent for 10,000+ participants for this chart review. The use of protected health information is necessary to collect study variables/data points in order to achieve the study objectives.
Author Contributions
NM, SJ, CO, and AB designed the study, had full access to the data, and took responsibility for the integrity and accuracy of the data analysis. MT contributed to data and statistical analysis. All authors contributed to the writing and editing of the manuscript. All authors contributed to data acquisition, analysis, and interpretation, and all reviewed and approved the final version of the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Data Availability
Raw data cannot be shared publicly because protected health information is used in the study. Certain data may be available from the Corewell Health Research Institute (contact via amit.bahl@corewellhealth.org) for researchers who meet the criteria for access to confidential data. Data in tables and supplementary material will be available.
Abbreviations
COVID-19: coronavirus disease 2019; FV&B: fully vaccinated and boosted; FV&BB: fully vaccinated and bivalent boosted; ICU: intensive care unit; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; ED: emergency department; EHR: electronic health record; AHRQ: Agency for Healthcare Research and Quality; ICD-10: International Classification of Diseases, 10th Revision; MCIR: Michigan Care Improvement Registry; CDC: Centers for Disease Control and Prevention; RNA: ribonucleic acid; DNA: deoxyribonucleic acid
| References | ▴Top |
- WHO Coronavirus (COVID-19) Dashboard. Accessed December 12, 2023. https://covid19.who.int.
- CDC. COVID Data Tracker. Centers for Disease Control and Prevention. Published March 28, 2020. Accessed December 12, 2023. https://covid.cdc.gov/covid-data-tracker.
- Xu J, Murphy SL, Kochanek KD, Arias E. Mortality in the United States, 2021. NCHS Data Brief. 2022;456:1-8.
pubmed - Lin DY, Abi Fadel F, Huang S, Milinovich AT, Sacha GL, Bartley P, Duggal A, et al. Nirmatrelvir or molnupiravir use and severe outcomes from omicron infections. JAMA Netw Open. 2023;6(9):e2335077.
doi pubmed pmc - Wan EYF, Yan VKC, Wong ZCT, Chui CSL, Lai FTT, Li X, Wong CKH, et al. Effectiveness of molnupiravir vs nirmatrelvir-ritonavir in non-hospitalised and hospitalised patients with COVID-19: a target trial emulation study. EClinicalMedicine. 2023;64:102225.
doi pubmed pmc - Saravolatz LD, Depcinski S, Sharma M. Molnupiravir and nirmatrelvir-ritonavir: oral coronavirus disease 2019 antiviral drugs. Clin Infect Dis. 2023;76(1):165-171.
doi pubmed pmc - Lee TC, Murthy S, Del Corpo O, Senecal J, Butler-Laporte G, Sohani ZN, Brophy JM, et al. Remdesivir for the treatment of COVID-19: a systematic review and meta-analysis. Clin Microbiol Infect. 2022;28(9):1203-1210.
doi pubmed pmc - State-by-state data on COVID-19 vaccinations in the United States. Our World in Data. Accessed December 12, 2023. https://ourworldindata.org/us-states-vaccinations.
- Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, Perez JL, et al. Safety and efficacy of the BNT162b2 mRNA COVID-19 vaccine. N Engl J Med. 2020;383(27):2603-2615.
doi pubmed pmc - Mielke N, Johnson S, Bahl A. Boosters reduce in-hospital mortality in patients with COVID-19: An observational cohort analysis. Lancet Reg Health Am. 2022;8:100227.
doi pubmed pmc - Johnson AG, Amin AB, Ali AR, Hoots B, Cadwell BL, Arora S, Avoundjian T, et al. COVID-19 incidence and death rates among unvaccinated and fully vaccinated adults with and without booster doses during periods of delta and omicron variant emergence - 25 U.S. jurisdictions, April 4-December 25, 2021. MMWR Morb Mortal Wkly Rep. 2022;71(4):132-138.
doi pubmed pmc - Samieefar N, Rashedi R, Akhlaghdoust M, Mashhadi M, Darzi P, Rezaei N. Delta variant: the new challenge of COVID-19 pandemic, an overview of epidemiological, clinical, and immune characteristics. Acta Biomed. 2022;93(1):e2022179.
doi pubmed pmc - Bian L, Gao Q, Gao F, Wang Q, He Q, Wu X, Mao Q, et al. Impact of the Delta variant on vaccine efficacy and response strategies. Expert Rev Vaccines. 2021;20(10):1201-1209.
doi pubmed pmc - Zhou Z, Zhu Y, Chu M. Role of COVID-19 vaccines in SARS-CoV-2 variants. Front Immunol. 2022;13:898192.
doi pubmed pmc - Wang J, Choy KW, Lim HY, Ho P. Laboratory markers of severity across three COVID-19 outbreaks in Australia: has Omicron and vaccinations changed disease presentation? Intern Emerg Med. 2023;18(1):43-52.
doi pubmed pmc - Coronavirus (COVID-19) update: FDA Authorizes Moderna, Pfizer-BioNTech bivalent COVID-19 vaccines for use as a booster dose. FDA. Published August 31, 2022. Accessed December 12, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-pfizer-biontech-bivalent-covid-19-vaccines-use.
- Chen SY, Lin CY, Chi H, Weng SL, Li ST, Tai YL, Huang YN, et al. The effectiveness of bivalent COVID-19 vaccination: a preliminary report. Life (Basel). 2023;13(10):2094.
doi pubmed pmc - Moore BJ, White S, Washington R, Coenen N, Elixhauser A. Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: the AHRQ Elixhauser Comorbidity Index. Med Care. 2017;55(7):698-705.
doi pubmed - Agency for Healthcare Research and Quality. APPENDIX I: immunocompromised state diagnosis and procedure codes. Accessed January 4, 2024. https://qualityindicators.ahrq.gov/Downloads/Modules/PSI/V2019/TechSpecs/PSI_Appendix_I.pdf.
- Michigan Care Improvement Registry. Michigan immunization portal for citizens 18 years and older. Published online 2022. Accessed January 4, 2024. https://mdhhsmiimmsportal.state.mi.us/.
- Durability of Bivalent Boosters against Omicron Subvariants | NEJM. Accessed December 27, 2023. https://www.nejm.org/doi/full/10.1056/NEJMc2302462.
- Link-Gelles R, Ciesla AA, Roper LE, Scobie HM, Ali AR, Miller JD, Wiegand RE, et al. Early estimates of bivalent mRNA booster dose vaccine effectiveness in preventing symptomatic SARS-CoV-2 infection attributable to omicron BA.5- and XBB/XBB.1.5-related sublineages among immunocompetent adults - increasing community access to testing program, United States, December 2022-January 2023. MMWR Morb Mortal Wkly Rep. 2023;72(5):119-124.
doi pubmed pmc - Coronavirus Disease 2019 (COVID-19) Vaccines. Accessed April 11, 2024. https://www.cdc.gov/vaccinesafety/vaccines/Covid-19-vaccines.html.
- Team CC-R. Severe outcomes among patients with coronavirus disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-346.
doi pubmed pmc - StataCorp. 2021. Stata statistical software: release 17. College Station, TX: StataCorp LLC.
- Johnson S, Mielke N, Mathew T, Maine GN, Chen NW, Bahl A. Predictors of hospitalization and severe disease due to breakthrough SARS-CoV-2 infection in fully vaccinated individuals. J Am Coll Emerg Physicians Open. 2022;3(4):e12793.
doi pubmed pmc - Bahl A, Van Baalen MN, Ortiz L, Chen NW, Todd C, Milad M, Yang A, et al. Early predictors of in-hospital mortality in patients with COVID-19 in a large American cohort. Intern Emerg Med. 2020;15(8):1485-1499.
doi pubmed pmc - Taylor CA, Patel K, Patton ME, Reingold A, Kawasaki B, Meek J, Openo K, et al. COVID-19-associated hospitalizations among U.S. adults aged >/=65 years - COVID-NET, 13 States, January-August 2023. MMWR Morb Mortal Wkly Rep. 2023;72(40):1089-1094.
doi pubmed pmc - Butt AA, Yan P, Shaikh OS, Mayr FB, Omer SB. Rate and risk factors for severe/critical disease among fully vaccinated persons with breakthrough severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection in a high-risk national population. Clin Infect Dis. 2022;75(1):e849-e856.
doi pubmed pmc - Nagy E, Cseh V, Barcs I, Ludwig E. The impact of comorbidities and obesity on the severity and outcome of COVID-19 in hospitalized patients-a retrospective study in a Hungarian hospital. Int J Environ Res Public Health. 2023;20(2):1372.
doi pubmed pmc - COVID-19 vaccine uptake and CDC’s commitment to vaccine equity. CDC. Published December 28, 2023. Accessed January 4, 2024. https://www.cdc.gov/respiratory-viruses/whats-new/vaccine-equity.html.
- Michigan COVID-19 vaccine dashboard. Accessed January 17, 2024. https://www.michigan.gov/coronavirus/resources/covid-19-vaccine/covid-19-dashboard.
- CDC severe viral respiratory illness. Published January 12, 2024. Accessed January 17, 2024. https://www.cdc.gov/respiratory-viruses/data-research/dashboard/illness-severity.html.
- Ma KC, Shirk P, Lambrou AS, Hassell N, Zheng XY, Payne AB, Ali AR, et al. Genomic surveillance for SARS-CoV-2 variants: circulation of omicron lineages - United States, January 2022-May 2023. MMWR Morb Mortal Wkly Rep. 2023;72(24):651-656.
doi pubmed pmc - Bahl A, Mielke N, Johnson S, Desai A, Qu L. Severe COVID-19 outcomes in pediatrics: An observational cohort analysis comparing Alpha, Delta, and Omicron variants. Lancet Reg Health Am. 2023;18:100405.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


