| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Case Report
Volume 16, Number 4, April 2024, pages 182-188
Intractable Postoperative Pancreatic Fistula: Undefined Type of Pancreatic Fistula Managed by Puestow-Like Procedure
Basel Darawshaa, Subhi Mansoura, Tawfik Fahouma, Naseem Azzama, Hayim Gilshteina, b, Yoram Klugera, Ahmad Assaliaa, c, Safi Khuria, d, e
aDepartment of General Surgery, Rambam Medical Center, Haifa, Israel
bColorectal Surgery Unit, General Surgery Department, Rambam Health Care Campus, Haifa, Israel
cAdvanced Laparoscopic and Bariatric Surgery Unit, Rambam Medical Center, Haifa, Israel
dBilio-Pancreatic Surgery Service, HPB and Surgical Oncology Unit, Rambam Medical Center, Haifa, Israel
eCorresponding Author: Safi Khuri, Department of General Surgery, Rambam Medical Center, Haifa, Israel
Manuscript submitted February 1, 2024, accepted March 23, 2024, published online April 30, 2024
Short title: Intractable POPF Managed by Puestow-Like Procedure
doi: https://doi.org/10.14740/jocmr5123
| Abstract | ▴Top |
Pancreatoduodenectomy (PD) is a very complex and highly challenging operation for surgeons worldwide. It is the surgical procedure of choice for the management of benign and malignant diseases of the periampullary region. Although mortality rate following this complicated surgery has fallen to 1-3%, morbidity rate following PD remains high, with almost 30-40% of patients developing at least one complication. Postoperative pancreatic fistula (POPF) is one of the most common complications following PD. Therefore, Pancreatico-enteric anastomosis has been regarded as the “Achilles heel” of the modern, one-stage PD procedure. According to the International Study Group of Pancreatic Surgery (ISGPS), three types of POPF are recognized nowadays: biochemical leak, previously known as grade A POPF, grade B and grade C, with the latter being the most dangerous. Most POPFs, especially of the biochemical leak and grade B heal with non-operative management to recur later and present as an intra-abdominal abscess or pseudocyst, necessitating management by means of interventional radiology, endoscopy or surgery. These types of fistulas are undefined and occasionally intractable. Herein, we present two patients who presented with the aforementioned type of pancreatic fistula following duct occlusion PD. The first patient, a 53-year-old female patient, suffered from intolerance to oral feeding, severe weight loss and recurrent hospital admission, while the second patient, a 72-year-old patient, suffered from recurrent bouts of abdominal sepsis. Their management involved step-up approach, starting with non-operative management, followed by percutaneous drainage and operative treatment in the form of Puestow-like procedure (longitudinal pancreatojejunostomy), as a recourse due to the inadequacy of preceding therapeutic modalities.
Keywords: Pancreatoduodenectomy; POPF; Poorly defined type of pancreatic fistula; Puestow-like procedure
| Introduction | ▴Top |
Pancreatoduodenectomy (PD), firstly reported by Allen Whipple in 1945, is a very complex and highly challenging operation for surgeons, especially hepato-pancreato-biliary (HPB) surgeons, worldwide [1]. It is the surgical procedure of choice for the management of benign and malignant diseases of the peri-ampullary region (head of the pancreas, ampulla of Vater, distal common bile duct and second part of the duodenum), severe trauma to the pancreatic head and duodenum, and highly selective cases of chronic pancreatitis [2]. Development of highly specialized centers, along with improvement of perioperative care has led to the dramatic decrease in mortality rate to 1-3% [3]. On the contrary, morbidity rates remain high at about 30-40% [4]. Complications following PD are diverse and classified as minor, such as delayed gastric emptying (DGE), intra-abdominal collections and surgical site infection (SSI), or major, including postoperative acute pancreatitis (POAP), post-PD hemorrhage (PPH) and postoperative pancreatic fistula (POPF), with the latter being the most common (30%) and feared one [5]. Being the most feared complication, pancreatico-enteric anastomosis (pancreaticogastrostomy vs. pancreatojejunostomy) has been regarded as the “Achilles heel” of the modern, one-stage PD procedure. Different techniques and adjuncts for safer anastomosis were suggested. No technique is superior, as the rates of POPF have not significantly diminished (5-40%) [6]. According to the International Study Group of Pancreatic Surgery (ISGPS), three types of POPF are well recognized nowadays: biochemical leak, formerly known as grade A POPF, grade B and grade C, with the latter known nowadays as clinically relevant POPF (CR-POPF) [7]. Biochemical leak is not considered as a true pancreatic fistula or actual complication, as it has no clinical impact following operation. Grade B POPF is characterized either by persistent peripancreatic drains for more than 3 weeks, percutaneous or endoscopic intervention for intra-abdominal collection or signs of infection without organ failure. In case reoperation is warranted or organ failure occurs, the fistula is regarded as grade C POPF. In most circumstances, POPFs, especially of the biochemical leak and grade B types, heal with non-operative management. Some of these POPF types recur later and present as an intra-abdominal abscess or pseudocyst necessitating management by different means. Intra-cystic/intra-abscess fluid amylase levels are essential for diagnosis. These types of fistulas are poorly defined and occasionally intractable.
In these two case reports, we present a dual illustration of patients who confronted the aforementioned type of pancreatic fistula following duct-occlusion PD (PD without pancreato-enteric anastomosis). One patient suffered biochemical leak and the other suffered grade B POPF (drain persisted for more than 3 weeks). Their management involved step-up approach: first, non-operative management including nothing per os (NPO), subcutaneous (SC) octreotide analogue (Sandostatin) and intravenous (IV) antibiotics were initiated. This was followed by percutaneous drainage (ultrasound (US)-guided (with high fluid levels of amylase)). Due to failure of the previously mentioned therapeutic modalities, operative management in the form of Puestow-like procedure (longitudinal pancreatojejunostomy) was contemplated.
To the best of our knowledge, and following a review of the current English literature, this manuscript represents the first attempt to elucidate the poorly defined and unknown entity of POPF, which is regarded as intractable. In addition, Puestow-like procedure for the management of such type of fistula have never been mentioned before.
| Case Reports | ▴Top |
Case 1
Investigations
A 53-year-old, heavy smoker, female patient underwent duct-occlusion PD due to high suspicion of pancreatic ductal adenocarcinoma (PDAC) of the pancreas head. Her preoperative body mass index (BMI) was 22.3, and she denied heavy alcohol drinking. Intraoperatively, due to findings of chronic inflammation in addition to inability to detect the main pancreatic duct (MPD), duct-occlusion PD was contemplated. Her fistula risk score (FRS) for POPF was intermediate (12.9%), mainly due to very small MPD caliber and no pathological evidence of pancreas cancer preoperatively. During her postoperative period, she suffered biochemical leak (high levels of drain amylase on postoperative day (POD) 3 and 5). The intra-abdominal drains were removed 14 days following surgery, when daily excretion was almost 5 - 10 mL. She was prescribed daily pancreas replacement therapy (Creon) with meals. The final histopathological report was consistent with chronic pancreatitis without neoplastic or dysplastic changes.
Two months following the operation, the patient presented to our Emergency Room (ER) Department with a complaint of abdominal pain and weight loss. An abdominopelvic computed tomography (CT) scan showed an intra-abdominal collection of 3.5 × 2 cm at the right upper abdominal cavity in addition to another smaller fluid collection at the left upper abdomen. A conservative management by means of NPO and IV antibiotics alleviated the patient’s symptoms. The patient was discharged home after 2 days.
Diagnosis
After 2 months (4 months since the index operation), the patient was admitted again due to abdominal pain of several weeks, along with weight loss of 15 kg (BMI of 17.5) and intolerance to fluid and solid intake. On physical examination upon her admission, her vital signs were within normal limits. By abdominal exam, the abdomen was distended with local tenderness at the left upper quadrant. Complete blood count (CBC), kidney function tests (KFT) and liver function tests (LFT) were all within normal limits. Serum and urinary amylase levels were very high. A subsequent abdomino-pelvic CT scan demonstrated an intra-abdominal multilocular cystic collection at the left upper quadrant with mass effect on the stomach remnant (Fig. 1). In addition, another multilocular cystic collection around the left lobe of the liver was demonstrated. Of notice, the MPD was dilated to its entire length up to 8 mm (Fig. 2).
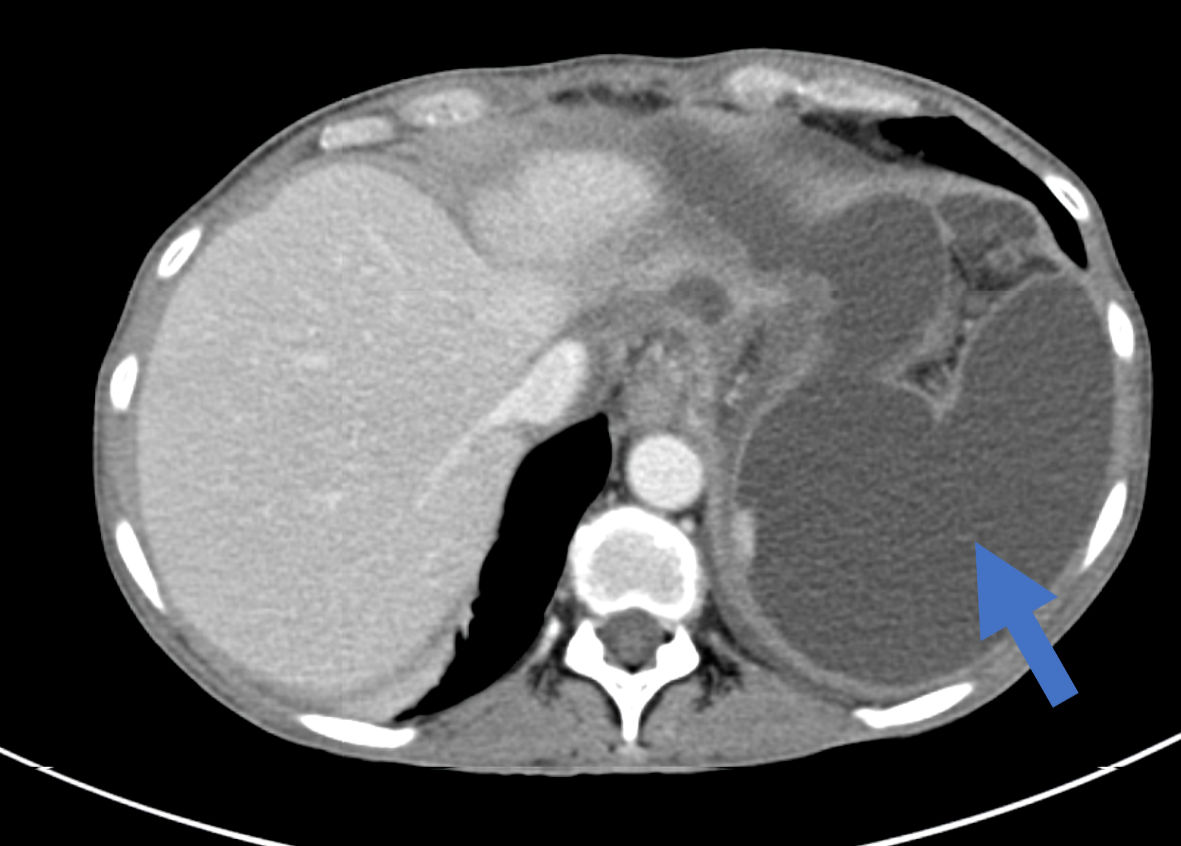 Click for large image | Figure 1. An axial abdominal CT scan showing a multilocular cystic intra-abdominal collection at the left upper abdomen (arrow). CT: computed tomography. |
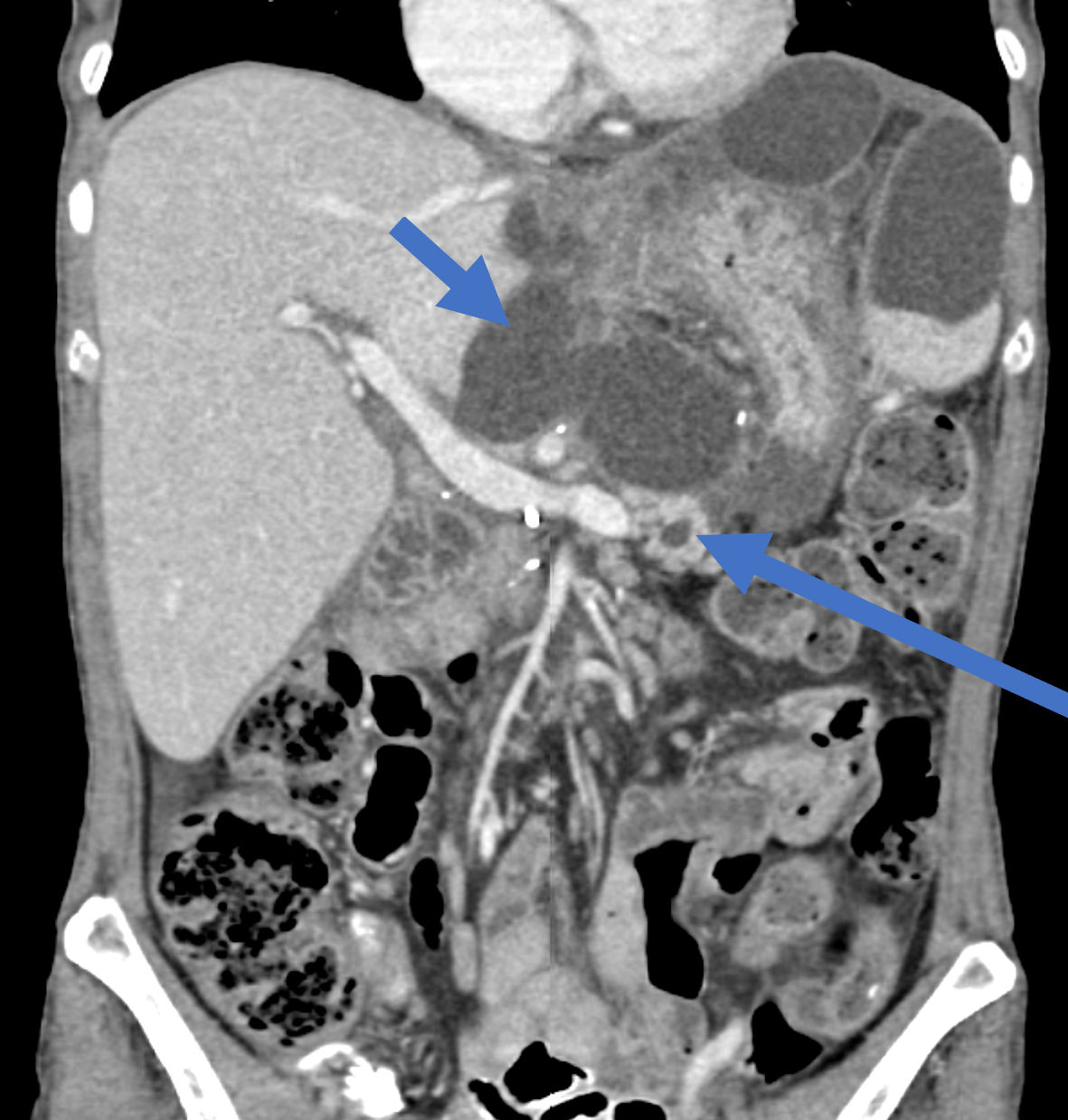 Click for large image | Figure 2. A coronal CT scan of the abdomen demonstrating multilocular cystic mass encompassing the left liver lobe (short arrow) in addition to dilated MPD (long arrow). CT: computed tomography; MPD: main pancreatic duct. |
Treatment
A step-up approach was employed. The initial treatment regimen was conservative, and involved NPO, IV Sandostatin (octreotide analogue) and total parenteral nutrition (TPN). This was followed by an US-guided drainage of both multilocular cyst. The intracystic amylase levels were very high at 17,282 U/L. The diagnosis of intractable POPF was made. A subsequent abdominal magnetic resonance imaging (MRI)/magnetic resonance cholangiopancreatography (MRCP) showed a significant improvement in abdominal collection sizes, pancreas remnant without any abnormality except for dilated MPD up to 7 - 8 mm (Fig. 3). Following the initiation of the initial nonoperative treatment regimen, the patient exhibited significant clinical improvement, and she was subsequently discharged for further outpatient clinic follow-up.
 Click for large image | Figure 3. An abdominal MRI/MRCP shows significant intrabdominal collection improvement with dilated MPD (arrow). MRI: magnetic resonance imaging; MRCP: magnetic resonance cholangiopancreatography; MPD: main pancreatic duct. |
On follow-up at the outpatient clinic, there was no significant improvement in drain output (daily drain output of 500 mL pancreatic secretion fluid).
On multidisciplinary team meeting (pancreas surgeon, interventional gastroenterologists and abdominal radiologist), several therapeutic options were raised. An endoscopic approach in the form of endoscopic ultrasound (EUS)-guided intra-MPD glue injection was offered, but due to absence of a suitable endoscopic window, this option was ruled out. Thus, the decision was made to proceed with a surgical intervention in the form of Puestow-like procedure consisting of a longitudinal pancreatojejunostomy.
Seven months following the initial operation, the patient underwent the aforementioned surgical procedure with intraoperative findings consistent with significant adhesions in the upper abdomen. An inflammatory process in the left upper quadrant was attributed to the leakage of pancreatic enzymes from the collection, which affected the posterior gastric wall, the pancreatic remnant and the transverse colon. Adhesiolysis was performed. The pancreatic stump was sutured by non-absorbable suture. The MPD was identified through cautious needle puncture, affirmative by the presence of pancreas secretion. A longitudinal incision along the MPD extending to the tail of the pancreas was executed. The jejunal loop of the alimentary limb was deployed and anastomosed to the jejunum in a double layer continuous suture, completing a Puestow-like procedure. Her postoperative period was uneventful, and drain amylase levels on POD 3 and 5 were within normal limits. She was discharged home on POD 5, following drains removal.
Follow-up and outcomes
On an outpatient follow-up clinic at 2, 6 and 12 weeks, patient was doing well without any complaints. Six months after surgery, the patient regained her previous weight without treatment by oral pancreatic enzyme replacement therapy.
Case 2
Investigations
A 72-year-old, healthy male patient underwent duct-occlusion PD due to clinical suspicion of a PDAC of the pancreatic head. His preoperative BMI was 25.7, and he denied any alcohol drinking or cigarette smoking. Intraoperatively, due to technical difficulties encountered in identifying the MPD, along with soft pancreas gland, a pancreatico-jejunal anastomosis was not done, and the pancreatic stump remnant was sutured primarily by non-absorbable sutures. His FRS for POPF was high (28.1%), mainly due to the previously mentioned risk factors (MPD diameter less than 1 mm, soft texture of the pancreas and absence of preoperative histopathological PDAC diagnosis). Due to POPF following operation, the patient was discharged home on POD 8 with the drains. Later on, drains were removed on an outpatient clinic follow-up, due to lack of secretions, on POD 22. The final histopathological report was consistent with low-grade intraductal papillary mucinous neoplasm (IPMN), without dysplasia or carcinoma.
Diagnosis
Two months following the index surgery, the patient presented to the ER Department with complaints of fever and abdominal discomfort in the last 2 days before admission. On physical examination upon his admission, his vital signs were within normal limits except for a temperature of 38.4 °C. The abdomen was soft, non-distended with local tenderness at the right upper quadrant. CBC showed a marked leukocytosis of 20 × 103, KFT and LFT were all within normal limits. A comprehensive evaluation encompassing an abdominal CT scan revealed a multilocular collection measuring 8 × 11 × 5 cm near the stump of the pancreatic remnant (Fig. 4). A conservative management was initiated, and the patient was admitted with a diagnosis of intra-abdominal collection/abscess for further investigation and management.
 Click for large image | Figure 4. An axial abdominal CT scan showing a multilocular cystic intra-abdominal collection near the pancreas stump remnant (arrow). CT: computed tomography. |
Treatment
In alignment with the previous patient’s management, a step-up approach was implemented. Following the initial conservative treatment regimen that included dietary restrictions (NPO), IV Sandostatin, and IV antibiotics, it became apparent that an intervention in the form of US-guided drainage is necessary.
An US-guided drainage was contemplated, which revealed a purulent exudative fluid. Intra-abdominal collection amylase levels were very high, consistent with pancreatic fluid. Cultures were negative for any bacteria. Following the previously mentioned therapeutic approach, the patient’s symptoms were relieved. He regained oral intake and was discharged home for an outpatient clinic follow-up.
On an outpatient clinic follow-up after 3 weeks, the patient was doing well, without any complaint. Daily secretions of intra-abdominal drain were about 100 - 200 mL.
Four weeks afterward, the patient experienced a recurrence of abdominal pain, fever and vomiting. Subsequent imaging via CT abdomen confirmed the existence of a pre-existing collection, measuring 2 × 3.8 × 2.4 cm at the surgical site. Blood cultures confirmed bacteremia marked by the presence of Escherichia coli. Aggressive management with antibiotic regimen complemented by Sandostatin for a duration of 1 week, resulted in clinical and laboratory improvement, leading to his discharge.
Three weeks following the last admission, the patient was admitted to the Surgical Department with recurrence of fever and abdominal pain. A subsequent CT abdomen unveiled a persistent collection, measuring 2.4 × 2.5 × 4.2 cm, at the site of surgical intervention. The MPD has become dilated from 5 mm up to 13 mm throughout the last repeated imaging tests during the last several weeks.
Due to inadequate efficacy of the preceding drainage approach, a re-drainage procedure was carried out, guided by CT imaging. The drained fluid exhibited elevated amylase levels, compatible with a diagnosis of intractable POPF.
Due to the increased secretions via the drains, diminished quality of life, and the recurrent hospitalizations with life-threatening sepsis, the patient’s case was discussed by a multidisciplinary team meeting. The multidisciplinary team decided on a definitive surgical intervention aimed at treating the POPF.
Six months following the index admission, a semi-elective surgery was performed, characterized by a longitudinal incision across the pancreatic duct, extending from the pancreatic neck to the tail. Given the anatomical constraints related to the biliary limb, the alimentary limb was deployed and anastomosed approximately 20 cm distal to the gastrojejunal anastomosis, thereby completing a longitudinal pancreaticojejunostomy (Puestow-like procedure).
His postoperative period was uneventful. Drains amylase levels on POD 3 and 5 were within normal limits. The patient was discharged home on POD 6 after drains removal.
Follow-up and outcomes
During follow-up appointments at outpatient clinic over several weeks, regular postoperative assessments by the attending surgeon indicated significant advancement in the patient’s overall well-being, marked by weight regain (without oral replacement therapy) and absence of abdominal pain or fever.
| Discussion | ▴Top |
Being the most common post-PD complications, POPF has become one of the most common hot topics in the field of pancreas surgery during the last decades. Although several adjuncts and variable techniques for a better pancreato-enterostomy anastomosis were suggested, rate of POPF did not change, as it is about 30% [6]. The definitions and types of POPF established by the ISGPS in 2016 were very clear and applied worldwide [7]. However, some types of POPF, such as those in our cases, do not fit for any of the above classifications.
In the absence of an international consensus to perform an imaging test before intra-abdominal drains removal after PD operations, drains are removed, as a routine, at our surgical ward when daily secretions are less than 20 mL (when patients suffer POPF) or less than 50 mL (without high amylase levels), and the patients are otherwise asymptomatic. In our two presented cases, the first patient had a biochemical leak following PD, and the second patient suffered grade B1 POPF. Drains were removed within 2/3 weeks, respectively, as daily discharges were about 5 - 10 mL. Both patients had a later presentation of an intra-abdominal collection due to intractable POPF. The first patient experienced severe weight loss and dysphagia due to mass effect exerted by the intra-abdominal collection. The latter presented with recurrent episodes of sepsis and bacteremia, necessitating immediate antibiotic treatment and semi-elective source control surgery.
With this undescribed and rare type of POPF, the gold standard of management is not established yet. We treated both patients by a multidisciplinary team approach. A step-up approach was initiated, starting with conservative management (NPO, IV octreotide and TPN). Due to its failure, endoscopic treatments by EUS-guided intra MPD glue injection or endoscopic cyst-jejunostomy were suggested. However, it became apparent that due to anatomical difficulties post-PD and specifically a challenge in identifying the MPD, it was withdrawn.
Therefore, surgery was decided upon and performed. Our intervention was a Puestow-like procedure, a variant of the classical Puestow surgery, in order to reconstruct the pancreas remnant to the gastrointestinal tract (Fig. 5). Since the MPD became dilated on subsequent imaging tests, we decided to apply surgical treatment of another pathology (chronic pancreatitis) to the current pathology (intractable POPF). The conventional Puestow surgery is traditionally reserved for chronic pancreatitis patients [8]. In our cases, the deviation from the classical procedure was in two aspects. Firstly, considering the unique patient population being post-PD surgery, the anastomosis extended from the body to the tail of the pancreas. Secondly, due to technical challenges, the anastomosis was directed to the jejunal loop of the alimentary limb rather than the biliary limb. To the best of our knowledge, and following review of the current English literature, Puestow-like procedure has been used as an alternative pancreatic anastomosis following PD-during the index operation [9] but has never been mentioned as a surgical technique for the management of such type of pancreatic fistula.
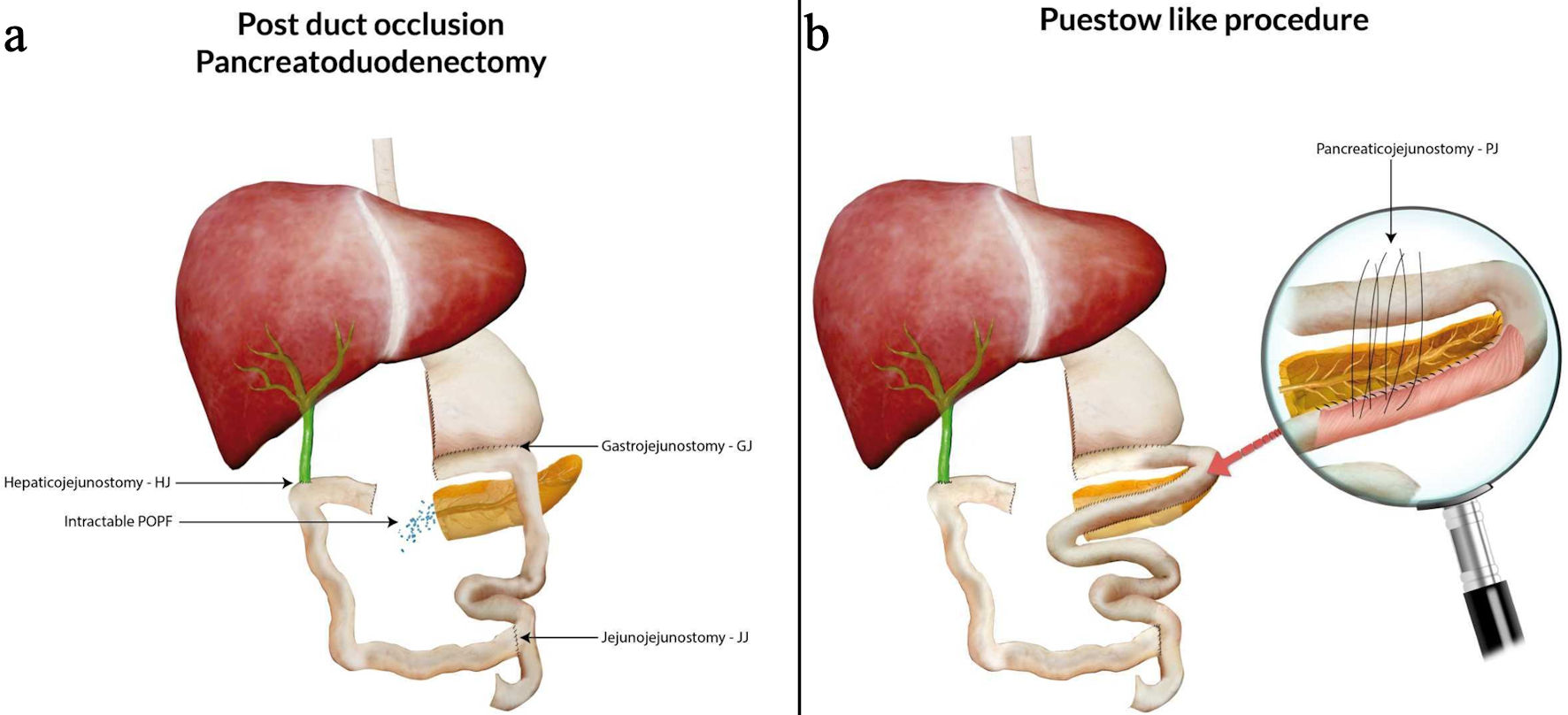 Click for large image | Figure 5. Duct-occlusion PD (a) and the Puestow-like procedure (b) following reconstructing the pancreatic duct to the gastrointestinal tract. PD: pancreatoduodenectomy; POPF: postoperative pancreatic fistula. |
In the follow-up period, both patients exhibited significant improvement in overall well-being, marked by weight regain and improved quality of life. Most importantly, there were no documented recurrences of the initial complaints in 12 months’ follow-up. These promising outcomes underscore the potential efficacy of the Puestow-like procedure in managing this rare type of POPF.
In summary, intractable POPF is a newly introduced and undefined type of POPF. The Puestow-like procedure emerges as a promising intervention for patients diagnosed with intractable POPF. However, careful patient selection is important, considering factors such as previous abdominal interventions, the specific subtype of pancreatic fistula, MPD diameter, and the patient’s current nutritional status. This novel approach offers a tailored solution, showcasing its potential as an effective therapeutic option in these challenging clinical scenarios.
Acknowledgments
None to declare.
Financial Disclosure
There was no specific funding source to be mentioned.
Conflict of Interest
The authors have no conflict of interest to declare.
Informed Consent
Written informed consent was obtained from the patients for publication of this case report and accompanying images.
Author Contributions
Basel Darawsha and Subhi Mansour contributed to the writing of the manuscript. Tawfik Fahoum and Naseem Azzam contributed to the design. Hayim Gilshtein, Yoram Kluger and Ahmad Assalia contributed to literature research and editing of the manuscript. Safi Khuri was the mentor and contributed to critical revision of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
PD: pancreatoduodenectomy; POPF: postoperative pancreatic fistula; CR POPF: clinically relevant postoperative pancreatic fistula; ISGPS: International Study Group of Pancreas Surgery; POD: postoperative day
| References | ▴Top |
- Whipple AO. Pancreaticoduodenectomy for islet carcinoma: a five-year follow-up. Ann Surg. 1945;121(6):847-852.
doi pubmed pmc - Scaife CL, Hewitt KC, Mone MC, Hansen HJ, Nelson ET, Mulvihill SJ. Comparison of intraoperative versus delayed enteral feeding tube placement in patients undergoing a Whipple procedure. HPB (Oxford). 2014;16(1):62-69.
doi pubmed pmc - Neoptolemos JP, Russell RC, Bramhall S, Theis B. Low mortality following resection for pancreatic and periampullary tumours in 1026 patients: UK survey of specialist pancreatic units. UK Pancreatic Cancer Group. Br J Surg. 1997;84(10):1370-1376.
pubmed - Gouma DJ, van Geenen RC, van Gulik TM, de Haan RJ, de Wit LT, Busch OR, Obertop H. Rates of complications and death after pancreaticoduodenectomy: risk factors and the impact of hospital volume. Ann Surg. 2000;232(6):786-795.
doi pubmed pmc - Bassi C, Falconi M, Salvia R, Mascetta G, Molinari E, Pederzoli P. Management of complications after pancreaticoduodenectomy in a high volume centre: results on 150 consecutive patients. Dig Surg. 2001;18(6):453-457; discussion 458.
doi pubmed - Khuri S, Ben-Ishay O, Mansour S, Kluger Y. Seldinger technique for duct-to-mucosa pancreaticoenterostomy. Scholarly J Surg. 2019;2(1):13-15.
- Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, Allen P, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161(3):584-591.
doi pubmed - Ceppa EP, Pappas TN. Modified puestow lateral pancreaticojejunostomy. J Gastrointest Surg. 2009;13(5):1004-1008.
doi pubmed - Bitsakou G, Frampton AE, Pai M, Jiao LR. An alternative pancreatic anastomosis following pancreaticoduodenectomy. Arch Surg. 2011;146(6):752-754.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


