| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 16, Number 4, April 2024, pages 164-169
Significance of Hypoalbuminemia in the Development of Thromboembolic Complications in Severe Cases of SARS-CoV-2 Coronavirus Infection
Aida Tarzimanovaa , Anna Braginaa
, Anna Pokrovskayaa, c
, Alexander Ivannikova
, Ekaterina Sokolovaa
, Igor Cherkesovb
, Tatyana Safronovaa, Tatyana Varginaa
, Liubov Ponomarevaa
, Alyona Isaevaa
, Karine Oganesyana
, Valery Podzolkova
aDepartment of Faculty Therapy No. 2, I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russian Federation
bDepartment of plastic surgery, I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russian Federation
cCorresponding Author: Anna Pokrovskay, Department of Faculty Therapy No. 2, I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow, Russian Federation
Manuscript submitted January 26, 2024, accepted March 9, 2024, published online April 30, 2024
Short title: Hypoalbuminemia and TECs in COVID-19
doi: https://doi.org/10.14740/jocmr5119
| Abstract | ▴Top |
Background: The course of coronavirus disease 2019 (COVID-19) is associated with the progression of a wide range of complications, among which thrombosis and thromboembolism are of particular importance. The significance of hypoalbuminemia in the development of thromboembolic complications (TECs) in patients with a severe course of COVID-19 is currently under active discussion. The objective of our study was to evaluate the significance of hypoalbuminemia in the development of TECs in patients with severe SARS-CoV-2 coronavirus infection.
Methods: In a single-center observational retrospective study, case histories of 1,634 patients with a verified diagnosis of SARS-CoV-2 coronavirus infection were analyzed. Patients were divided into two groups according to the presence of TECs: 127 patients with venous TECs constituted the main group and 1,507 patients, in whom the course of COVID-19 was not complicated by the development of TECs, constituted the comparison group.
Results: The patients with TECs were older, and the prevalence of arterial hypertension, coronary heart disease, chronic heart failure, chronic kidney disease, and diabetes mellitus was higher than that in the comparison group. A single-factor regression analysis showed that a decrease in albumin levels of less than 35 g/L is associated with an eightfold increase in the risk of developing TECs in patients with severe SARS-CoV-2 coronavirus infection (area under the curve (AUC): 0.815, odds ratio (OR): 8.5389, 95% confidence interval (CI): 4.5637 - 15.977, P < 0.001). The sensitivity of the method was 76.34%, and the specificity was 72.58%.
Conclusion: The study revealed that hypoalbuminemia is a predictor of development of TECs in severe cases of SARS-CoV-2 coronavirus infection.
Keywords: COVID-19; Hypoalbuminemia; Thromboembolic complications
| Introduction | ▴Top |
Coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, remains a topical issue for the global community. COVID-19 advancement is fraught with a broad spectrum of complications, notably thrombosis and thromboembolism [1].
Known indicators associated with a high risk of thromboembolic complications (TECs) in COVID-19 include elevated levels of D-dimer, C-reactive protein (CRP), and pro-inflammatory cytokines. The significance of hypoalbuminemia in the development of TECs in patients with high inflammatory response is also being currently discussed [2].
The physiological effects of serum albumin include the maintenance of colloid osmotic pressure and anti-inflammatory, antioxidant, anticoagulant and antiaggregant activity [3].
Some fundamental studies have shown the significance of albumin in variations of vascular wall permeability. Aldecoa et al showed albumin and lipoproteins to play an important role in delivering sphingosine-1-phosphate to endothelial cells’ surface to alter vascular wall permeability [4].
Clinical researches done showed low albumin to be a marker of venous thromboembolism developing in patients with ischemic heart disease, atrial fibrillation, and/or chronic heart failure (CHF) [5, 6]. Hypoalbuminemia may be an independent predictor of the development of infectious endocarditis [7, 8]. However, to date, there have been no clinical studies investigating the significance of hypoalbuminemia in the development of TECs in severe COVID-19 patients.
| Materials and Methods | ▴Top |
Study design and data collection
In a single-institution retrospective observational study, we reviewed the case histories of 1,634 patients, aged 68.5 on average (53.7 - 75.1), with a verified diagnosis of SARS-CoV-2 coronavirus infection, admitted to the University Clinical Hospital No. 4 of the Sechenov University that was then operating as a COVID-19 hospital.
We divided our patients into two cohorts depending on whether they had TECs. The group with TECs (group I) included 127 patients with venous TECs (pulmonary artery thromboembolism (PATE) or lower extremity deep vein thrombosis, developed during hospitalization). One thousand five hundred and seven (1,507) patients with COVID-19 not complicated by any thromboembolic events constituted our comparison group (TEC-free, group II).
Their COVID-19 diagnosis was verified as SARS-CoV-2 RNA was found by PCR method in their nasopharyngeal swаbs, or using chest computed tomography (CT) scans. The diagnosis of PATE was verified using contrast-enhanced chest CT scans.
All the patients’ blood plasma albumin was measured on their first day of hospitalization before infusion therapy was prescribed.
Ethics statement
The study was conducted with the approval of the Institutional Review Board of the Sechenov University (No. 07-22, April 7, 2022). This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Statistical analysis
We used the jamovi project (2023, version 2.3, retrieved from https://www.jamovi.org). The quantitative data are presented as the median and interquartile range (Me (Q1 - Q3)). Binomial and categorical variables are presented in terms of both absolute number and percentage. Comparative analysis used the Mann-Whitney U test to compare the quantitative variables. The predictors were established using univariate logistic regression with odds ratio (OR) calculation and a 95% confidence interval (CI). The classification model’s cut-off point threshold value was established using receiver-operating characteristic (ROC) analysis with area under the curve (AUC) calculation. Variations with P < 0.05 were considered statistically significant.
| Results | ▴Top |
Basic characteristics of the study group
Our TEC patients were generally older than patients in the TEC-free group. The incidence of arterial hypertension, ischemic heart disease, CHF, chronic kidney disease, and diabetes mellitus was higher in group I than in group II.
CHF with reduced left ventricular ejection fraction was observed in 18 (19.35%) TECs group patients and 35 (9.09%) TEC-free group patients (P = 0.0047). TEC patients’ glomerular filtration rate was lower than in the TEC-free group (50.9 (37.6 - 71.9) vs. 69.8 (56.3 - 82.0) mL/min/1.73 m2) (P < 0.001). The study excluded patients with glomerular filtration rate below 15 mL/min/1.73 m2. Decompensated diabetes mellitus was found in 14 (11%) and 52 (3.5%) patients, respectively. Body mass index was 30.9 (25.0 - 35.4) kg/m2 in the TECs group and 29.2 (25.4 - 33.3) in the TEC-free group (P = 0.388).
The percentage of lung tissue involvement was veritably higher in the TECs group than in the TEC-free group (55% (37.5 - 67.5) on average vs. 37.5% (25.0 - 47.5)) (P < 0.001). TEC patients’ oxygen saturation (SpO2) on room air was veritably lower than in the TEC-free group (92.0% (88.0 - 95.0) vs. 95.0% (93.0 - 97.0)) (P < 0.001) (Table 1).
 Click to view | Table 1. Patients’ Clinical Profile |
The treatment of comorbid diseases, which patients received before hospitalization, corresponded to modern recommendations. During hospitalization, intravenous glucocorticoids were prescribed to 62 (48.8%) TEC patients and 502 (33.3%) TEC-free patients, tocilizumab or netakimab to 36 (28.3%) and 409 (27.1%) of the patients, respectively, and all the patients received non-fractionated heparins.
All the 127 (100%) TECs group patients were diagnosed with PATE, and 100 cases (78.74%) were fatal. All the lethal outcomes were caused by massive PATE confirmed by autopsy.
Changes of laboratory findings in the groups under scrutiny
Group I patients had lower hemoglobin levels (126 (109 - 139) g/L) than group II patients (135 (125 - 146) g/L) (P < 0.001), and their average thrombocyte and leukocyte counts were within the normal range.
The TEC patients’ chemistry panel showed significantly higher levels of urea (8.40 (6.20 - 12.9) mmol/L) and creatinine (109 (87.5 - 133) µmol/L) than the TEC-free group (5.50 (4.30 - 7.10) mmol/L and 92.5 (80.9 - 106) µmol/L, respectively). Group I patients presented with higher glucose levels (6.08 (4.73 - 8.18) mmol/L) than group II (5.10 (4.60 - 5.90) mmol/L) (P < 0.001).
The plasma concentration of D-dimer was 3.37 (1.87 - 5.83) mg/mL in group I, veritably higher than that in group II patients (0.831 (0.4 - 1.87) mg/mL). TEC patients presented with significantly higher levels of CRP and interleukin-6 (IL-6) than patients in the TEC-free group (Table 2).
 Click to view | Table 2. Plasma Concentrations of Inflammation Markers in the Groups Under Scrutiny |
Significance of hypoalbuminemia in TEC development
Hypoalbuminemia was observed in 45 (35.43%) group I patients and 62 (4.11%) group II patients (P < 0.001). The TEC patients’ plasma albumin level was veritably higher (30.9 (26.2 - 35.3) mg/L) than in the TEC-free group (39.0 (35.1 - 41.7) mg/L) (Fig. 1).
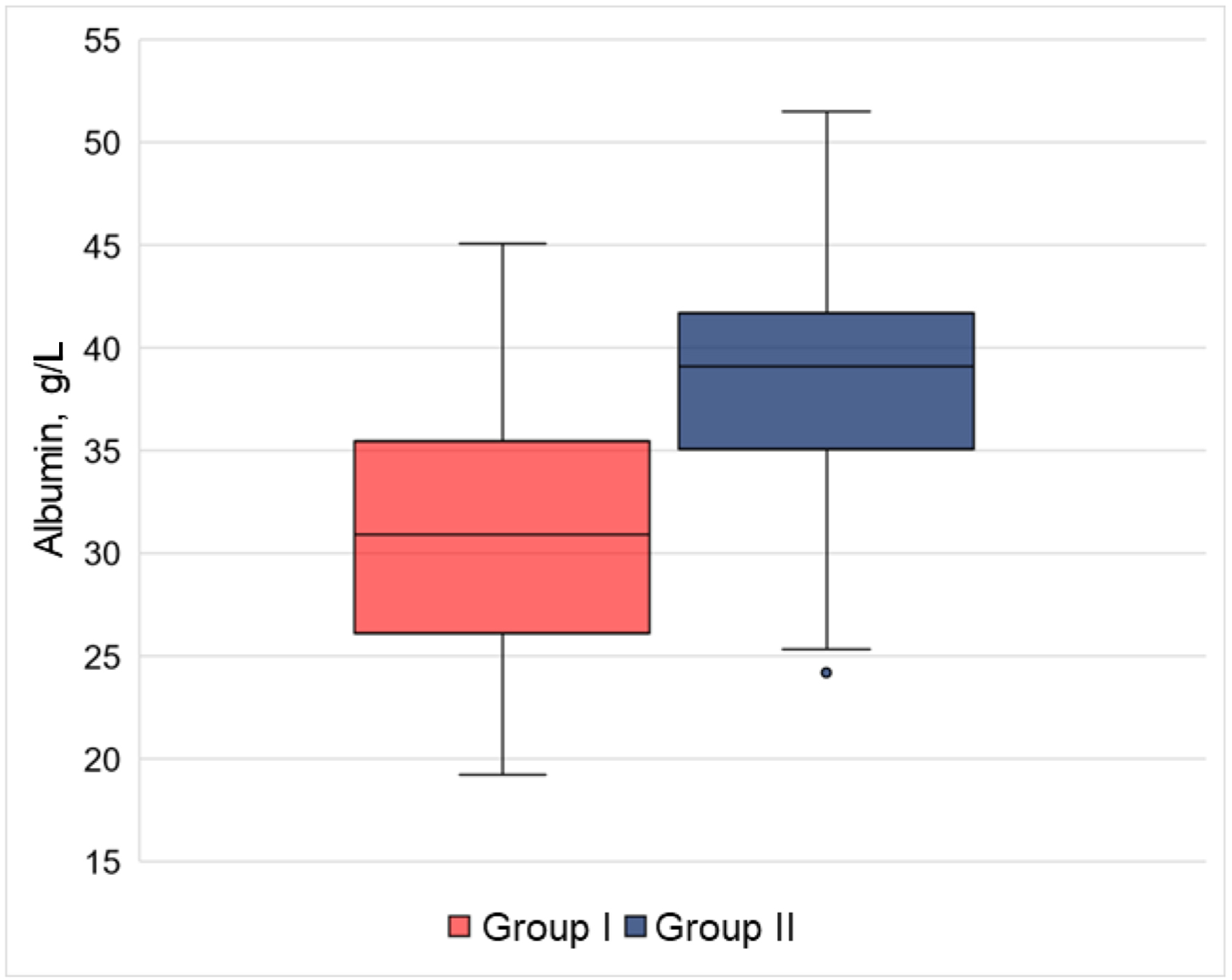 Click for large image | Figure 1. Significance of blood plasma albumin in the groups under scrutiny. The TEC patients’ plasma albumin level was veritably higher than in the comparison group. Group I: with TECs, Group II: TEC-free. TECs: thromboembolic complications. |
Correlation analysis showed negative correlations between albumin level and inflammation markers. Reduced blood plasma albumin concentrations were correlated with heightened levels of IL-6 (r = -0.359, P < 0.001), CRP (r = -0.438, P < 0.001), and D-dimer (r = -0.413, P < 0.001) (Fig. 2).
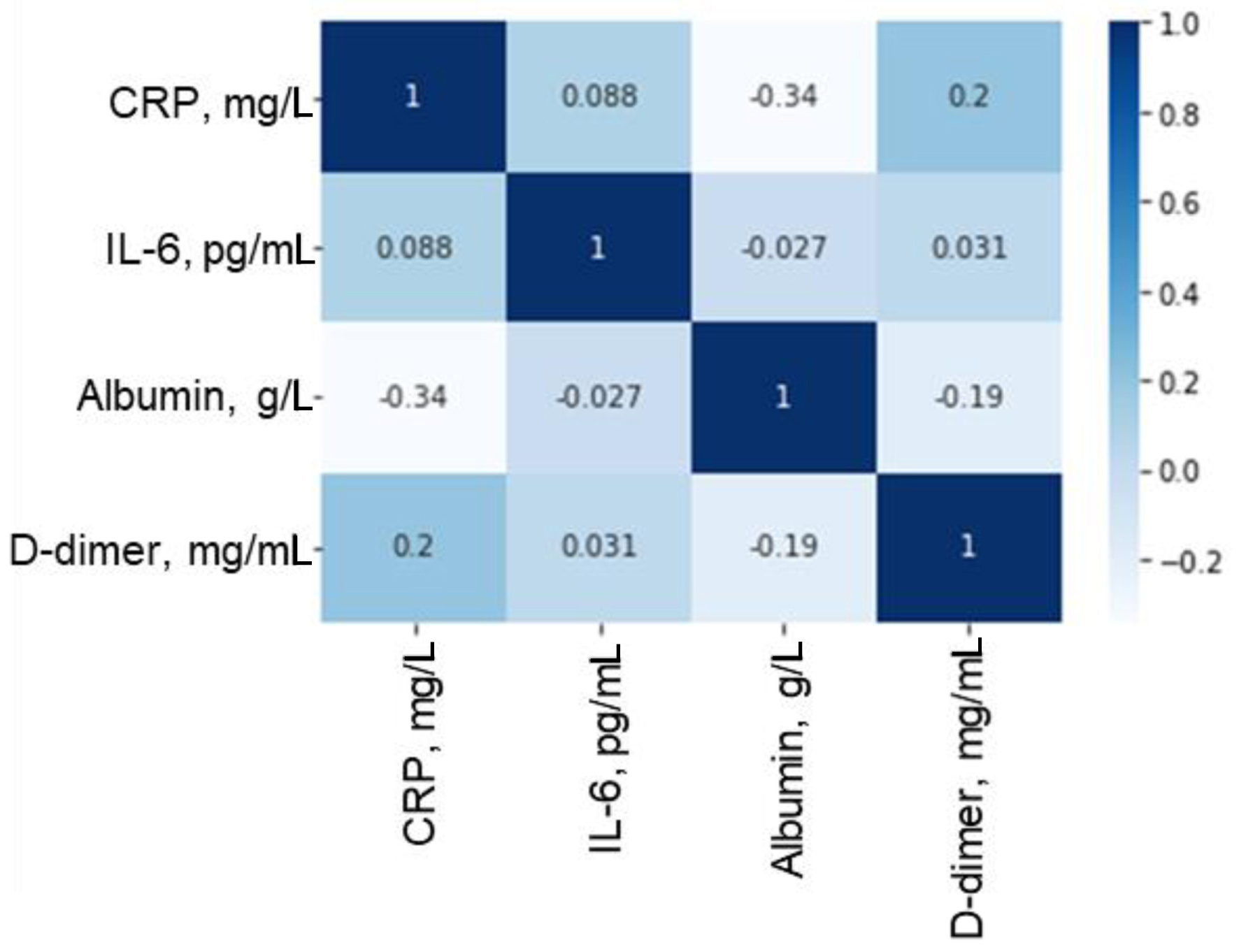 Click for large image | Figure 2. Correlation matrix of relationships between plasma concentrations of albumin, inflammatory markers and D-dimer. CRP: C-reactive protein; IL-6: interleukin-6. |
In our study, we evaluated the prognostic significance of hypoalbuminemia in the development of TECs in patients with severe COVID-19. By performing single-factor regression analysis, it was shown that a decrease in plasma albumin concentration has a statistically significant effect on the development of TECs in patients with severe course of SARS-CoV-2 infection. It was found that each subsequent 1 g/L decrease in plasma albumin concentration increased the odds of developing TECs by a factor of 1.16 (OR: 0.858, 95% CI: 0.817 - 0.902, P < 0.001). This pattern was described by the logistic regression equation as follows:
In this study, we assessed the prognostic significance of hypoalbuminemia in TECs development in severe COVID-19 patients. Univariate regression analysis showed a reduced plasma concentration of albumin to have a statistically significant effect on the development of TECs by grave SARS-CoV-2 patients. ROC analysis established the breakpoint values of blood plasma albumin. Albumin below 35 g/L increases the chance of TEC development eightfold (AUC: 0.815, OR: 8.5389, 95% CI: 4.5637 - 15.977, P < 0.001). The method’s sensitivity was 76.34%, and specificity was 72.58% (Fig. 3).
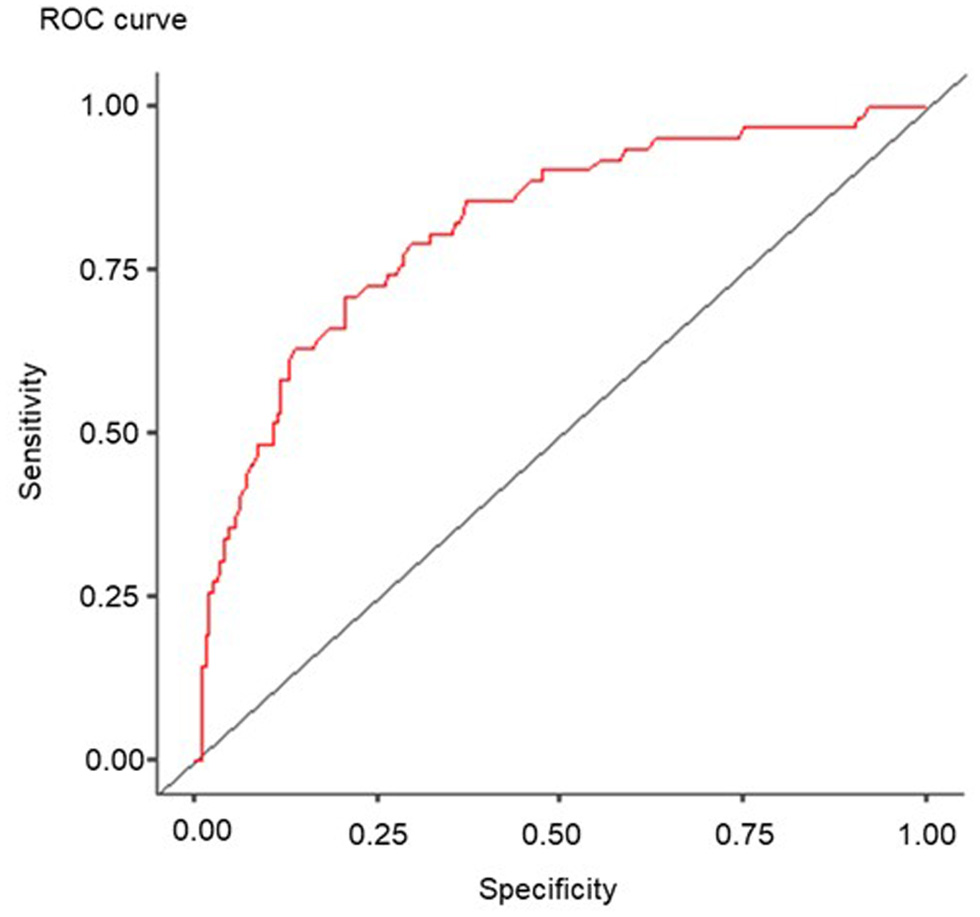 Click for large image | Figure 3. ROC curve of the significance of albumin concentration in severe cases of SARS-CoV-2 coronavirus infection. ROC: receiver-operating characteristic; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2. |
| Discussion | ▴Top |
It is now proven that cardiovascular diseases are significant contributors to the development of TECs in COVID-19 [9]. TECs are the most frequent in patients with arterial hypertension and atrial fibrillation [10, 11], and the significance of inflammation for TEC development in COVID-19 is also being discussed.
Our study shows an adverse prognostic significance of hypoalbuminemia for the development of TECs in severe cases of SARS-CoV-2 coronavirus infection. Albumin is known to possess anti-inflammatory, antioxidant and antithrombotic properties [12].
A number of major studies demonstrated the significance of hypoalbuminemia as a prognostic marker of TECs across the general population. A meta-analysis by Seidu et al that included 54 studies on a total of 1,492,237 patients found reduced albumin levels to be accompanied by a heightened risk of TEC development (OR: 0.71, 95% CI: 0.61 - 0.83) [13]. An APEX study showed patients with hypoalbuminemia to face a double risk of TECs (OR: 2.119, 95% CI: 1.592 - 2.820) [14].
A number of papers show the significance of hypoalbuminemia as a predictive marker of an unfavorable course of SARS-CoV-2 coronavirus infection [15]. Li et al show the degree of lung involvement in COVID-19 to be related to a lower level of albumin. The authors conclude that hypoalbuminemia may be used as an independent predictor of lethal outcomes for grave COVID-19 patients [16].
Paliogiannis et al explored the link between serum albumin level and COVID-19 outcome. Their meta-analysis included 67 studies involving a total of more than 19,000 patients. The authors showed serum albumin concentrations to have been significantly lower in fatal cases. Low serum albumin was significantly associated to the gravity of COVID-19 [17].
The most important mechanisms of the development of hypoalbuminemia in severe cases of SARS-CoV-2 include a marked inflammatory syndrome resulting in a considerable increase in capillary permeability and serum albumin loss into interstitial space [18]. Wu et al suggest that reduced blood plasma albumin in grave COVID-19 patients may be associated with high pulmonary capillary permeability [19].
In our study, we showed TEC patients’ pulmonary parenchyma involvement and level of inflammation markers to have been veritably higher than in the TEC-free group. The correlations found demonstrate that a considerable increase in inflammation markers (IL-6 and CRP) is accompanied by a reduced level of blood plasma albumin and development of hypoalbuminemia.
Violi et al arrived at similar findings as they showed plasma albumin level to be inversely related to the levels of D-dimer (r = -0.385, P < 0.001) and CRP (r = -0.418, P < 0.001) [20].
Albumin is known to have heparin-like activity [21]. A considerable decrease in blood plasma albumin serves to activate oxidative stress processes and increase thrombocyte aggregation, which are the primary components in the pathogenesis of thrombotic complications [22]. Reduced albumin levels can be observed in a broad range of urgent clinical situations involving a high risk of TECs [23].
Thus, in addition to such well-known predictors of cardiovascular complications as advanced age, comorbidities and high inflammation activity, albumin level can be factored into a comprehensive assessment of TEC risk in hospitalized patients with SARS-CoV-2 coronavirus infection.
Conclusions
Hypoalbuminemia aggravates the risk of TEC development in severe cases of SARS-CoV-2 coronavirus infection.
Acknowledgments
None to declare.
Financial Disclosure
This research received no external funding.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
The consent was not obtained from patients as data were analyzed anonymously.
Author Contributions
Conceptualization: AT, AB, VP and AI; methodology: AT, AB, VP and AI; software: AI; validation: AT, AB and AI; formal analysis: AI; investigation: AI and ES; resources: KO; data curation: ES, LP, AT, ND, TV, AP, TS, IC and AI; writing - original draft preparation: AI and AT; writing - review and editing: AT, VP and AB; supervision: VP; project administration: VP. All authors have read and agreed to the published version of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
AUC: area under the curve; CI: confidence interval; CRP: C-reactive protein; CT: computed tomography; IL-6: interleukin-6; OR: odds ratio; SpO2: oxygen saturation; TECs: thromboembolic complications
| References | ▴Top |
- Iba T, Connors JM, Levy JH. The coagulopathy, endotheliopathy, and vasculitis of COVID-19. Inflamm Res. 2020;69(12):1181-1189.
doi pubmed pmc - Arques S. Human serum albumin in cardiovascular diseases. Eur J Intern Med. 2018;52:8-12.
doi pubmed - Gremese E, Bruno D, Varriano V, Perniola S, Petricca L, Ferraccioli G. Serum albumin levels: a biomarker to be repurposed in different disease settings in clinical practice. J Clin Med. 2023;12(18):6017.
doi pubmed pmc - Aldecoa C, Llau JV, Nuvials X, Artigas A. Role of albumin in the preservation of endothelial glycocalyx integrity and the microcirculation: a review. Ann Intensive Care. 2020;10(1):85.
doi pubmed pmc - Omar HR, Mirsaeidi M, Rashad R, Hassaballa H, Enten G, Helal E, Mangar D, et al. Association of serum albumin and severity of pulmonary embolism. Medicina (Kaunas). 2020;56(1):26.
doi pubmed pmc - Gyamlani G, Molnar MZ, Lu JL, Sumida K, Kalantar-Zadeh K, Kovesdy CP. Association of serum albumin level and venous thromboembolic events in a large cohort of patients with nephrotic syndrome. Nephrol Dial Transplant. 2017;32(1):157-164.
doi pubmed pmc - Scheggi V, Merilli I, Marcucci R, Del Pace S, Olivotto I, Zoppetti N, Ceschia N, et al. Predictors of mortality and adverse events in patients with infective endocarditis: a retrospective real world study in a surgical centre. BMC Cardiovasc Disord. 2021;21(1):28.
doi pubmed pmc - Cornelissen CG, Frechen DA, Schreiner K, Marx N, Kruger S. Inflammatory parameters and prediction of prognosis in infective endocarditis. BMC Infect Dis. 2013;13:72.
doi pubmed pmc - Hessami A, Shamshirian A, Heydari K, Pourali F, Alizadeh-Navaei R, Moosazadeh M, Abrotan S, et al. Cardiovascular diseases burden in COVID-19: Systematic review and meta-analysis. Am J Emerg Med. 2021;46:82-391.
doi pubmed pmc - Podzolkov VI, Tarzimanova AI, Bragina AE, Loriya IZh, Pokrovskaya AE, Bykova EE, Ivannikov AA, et al. Predictors of atrial fibrillation in patients with COVID-19. Russian Journal of Cardiology. 2022;27(7):5095.
- Podzolkov VI, Bragina AE, Tarzimanova AI, Vasil’eva LV, Ogibenina ES, Bykova EE, Shvedov II, et al. Arterial hypertension and severe COVID-19 in hospitalized patients: data from a cohort study. Rational Pharmacotherapy in Cardiology. 2023;19(1):4-10
- Violi F, Novella A, Pignatelli P, Castellani V, Tettamanti M, Mannucci PM, Nobili A, et al. Low serum albumin is associated with mortality and arterial and venous ischemic events in acutely ill medical patients. Results of a retrospective observational study. Thromb Res. 2023;225:-10.
doi pubmed - Seidu S, Kunutsor SK, Khunti K. Serum albumin, cardiometabolic and other adverse outcomes: systematic review and meta-analyses of 48 published observational cohort studies involving 1,492,237 participants. Scand Cardiovasc J. 2020;54(5):280-293.
doi pubmed - Viana-Llamas MC, Arroyo-Espliguero R, Silva-Obregon JA, Uribe-Heredia G, Nunez-Gil I, Garcia-Magallon B, Toran-Martinez CG, et al. Hypoalbuminemia on admission in COVID-19 infection: An early predictor of mortality and adverse events. A retrospective observational study. Med Clin (Barc). 2021;156(9):428-436.
doi pubmed pmc - Chi G, Gibson CM, Liu Y, Hernandez AF, Hull RD, Cohen AT, Harrington RA, et al. Inverse relationship of serum albumin to the risk of venous thromboembolism among acutely ill hospitalized patients: Analysis from the APEX trial. Am J Hematol. 2019;94(1):21-28.
doi pubmed - Li J, Li M, Zheng S, Li M, Zhang M, Sun M, Li X, et al. Plasma albumin levels predict risk for nonsurvivors in critically ill patients with COVID-19. Biomark Med. 2020;14(10):827-837.
doi pubmed pmc - Paliogiannis P, Mangoni AA, Cangemi M, Fois AG, Carru C, Zinellu A. Serum albumin concentrations are associated with disease severity and outcomes in coronavirus 19 disease (COVID-19): a systematic review and meta-analysis. Clin Exp Med. 2021;21(3):343-354.
doi pubmed pmc - Ronit A, Kirkegaard-Klitbo DM, Dohlmann TL, Lundgren J, Sabin CA, Phillips AN, Nordestgaard BG, et al. Plasma albumin and incident cardiovascular disease: results from the CGPS and an updated meta-analysis. Arterioscler Thromb Vasc Biol. 2020;40(2):473-482.
doi pubmed - Wu MA, Fossali T, Pandolfi L, Carsana L, Ottolina D, Frangipane V, Rech R, et al. Hypoalbuminemia in COVID-19: assessing the hypothesis for underlying pulmonary capillary leakage. J Intern Med. 2021;289(6):861-872.
doi pubmed - Violi F, Ceccarelli G, Cangemi R, Alessandri F, D'Ettorre G, Oliva A, Pastori D, et al. Hypoalbuminemia, coagulopathy, and vascular disease in COVID-19. Circ Res. 2020;127(3):400-401.
doi pubmed - Paar M, Rossmann C, Nusshold C, Wagner T, Schlagenhauf A, Leschnik B, Oettl K, et al. Anticoagulant action of low, physiologic, and high albumin levels in whole blood. PLoS One. 2017;12(8):e0182997.
doi pubmed pmc - Arques S. Serum albumin and cardiovascular disease: State-of-the-art review. Ann Cardiol Angeiol (Paris). 2020;69(4):192-200.
doi pubmed - Ramadori G. Albumin infusion in critically ill COVID-19 patients: hemodilution and anticoagulation. Int J Mol Sci. 2021;22(13):7126.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


