| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Research
Volume 16, Number 2-3, March 2024, pages 94-105
Barriers to Exercise in Patients With Metabolic Dysfunction-Associated Steatotic Liver Disease: A Patient Survey
Kedar Deshpandea, f , John Olynykb, c
, Oyekoya Ayonrindeb, c, d, e
, Kazunori Nosakaa, e
aSchool of Medical and Health Sciences, Edith Cowan University, Joondalup, WA, Australia
bCurtin Medical School, Curtin University, Perth, Australia
cDepartment of Gastroenterology and Hepatology, Fiona Stanley Hospital, Perth, Australia
dMedical School, The University of Western Australia, Perth, Australia
eThese authors contributed equally to this article.
fCorresponding Author: Kedar Deshpande, School of Medical and Health Sciences, Edith Cowan University, Joondalup, WA 6027, Australia
Manuscript submitted January 17, 2024, accepted March 6, 2024, published online March 16, 2024
Short title: Barriers to Exercise in Patients With MASLD
doi: https://doi.org/10.14740/jocmr5113
| Abstract | ▴Top |
Background: Although adequate physical activity is an essential component of treatment for metabolic dysfunction-associated steatotic liver disease (MASLD), the majority of people with MASLD do not engage in regular exercise and lead sedentary lifestyles. We aimed to identify perceived barriers to exercise and to examine awareness about the role of exercise in the treatment of MASLD.
Methods: Individuals aged 18 years and above were recruited from a hepatology outpatient clinic. MASLD severity was assessed using controlled attenuation parameter (CAP) and transient elastography (TE) determined liver stiffness measurement (LSM) for the severity of hepatic steatosis and fibrosis, respectively. An online questionnaire was administered to record self-reported exercise patterns, barriers to exercise, and knowledge regarding effectiveness of different types of exercise for MASLD.
Results: Eighty-one participants (57% female) with a mean age of 55.3 ± 13.4 years and a mean body mass index (BMI) of 33.8 ± 6.4 answered the questionnaire. The mean CAP score was 335.7 ± 47.8 dB/m, and the median LSM was 12.45 kPa. While most patients (83%) considered MASLD to be a serious health concern, 73% did not achieve the recommended exercise levels of ≥ 150 min of moderate-intensity physical activity per week, and 54% were unsure about the role of exercise in the treatment of MASLD. Commonly reported barriers to exercise included physical and mental health issues (57%), lack of time (43%), lack of enjoyment in exercising (31%), fatigue caused by exercise (24%), and others (25%).
Conclusions: Most participants with MASLD were unaware of the role of exercise as a potential treatment option and were not achieving recommended exercise levels. Inadequate time, physical and mental health problems, lack of enjoyment in exercise, and fatigue were major barriers.
Keywords: Liver steatosis; Liver fibrosis; Challenges; Physical activity; Lifestyle
| Introduction | ▴Top |
Metabolic dysfunction-associated steatotic liver disease (MASLD) [1] is a new term for the entity previously known as non-alcoholic fatty liver disease (NAFLD), affecting over 30% of the global population [2, 3]. Approximately 25% of individuals with NAFLD progress to non-alcoholic steatohepatitis (NASH), characterized by liver inflammation and hepatocyte cell ballooning and are at higher risk of developing liver fibrosis and cirrhosis [4]. In Australia, the prevalent NAFLD and NASH cases are projected to increase by 25% and 40%, respectively, between 2019 and 2030. Furthermore, there is an expected rise of 85% in the incidence of advanced liver disease and NAFLD-related liver deaths by 2030 [5]. Given the evolution of the nomenclature of NAFLD and MASLD and the accepted interchangeability of the terms, findings pertaining to NAFLD and NASH may be extrapolated to MASLD and metabolic dysfunction-associated steatohepatitis (MASH), respectively [1, 6].
Currently, there is no approved pharmacological therapy for NAFLD, and lifestyle changes including diet and regular exercise form the core of its treatment [7]. The European Association for the Study of the Liver (EASL) and the American Association for the Study of Liver Diseases (AASLD) recommend patients with NAFLD to achieve 150 - 200 min of moderate-intensity exercise per week [8]. Extensive research has concluded that regular exercise, which is planned, structured, and purposeful, plays a pivotal role in the prevention and treatment of NAFLD, as well as in reducing the burden of extrahepatic comorbidities such as diabetes, obesity, cardiovascular disease, and cancer [7, 9, 10]. Studies indicate that both resistance and aerobic exercise are effective in reducing liver fat [11]. However, exercise advice and guidelines have failed to ameliorate the exponential growth in the incidence of hepatic steatosis for more than a century [12]. This may be attributed, in part, to a significant proportion of individuals with NAFLD struggling to initiate and maintain regular exercise habits, which poses a substantial challenge in implementing exercise programs as first-line therapy for NAFLD [13]. Furthermore, the optimal modality and intensity of exercise that should be performed by patients is still unclear [14]. The current consensus around the choice of exercise modality and intensity is that exercise should be personalized based on the patients’ fitness levels and preferences [14].
Despite guidelines and evidence supporting the importance of exercise, it has been reported that most patients with NAFLD fail to achieve these recommendations [15]. One possible reason for this could be that patients with NAFLD have poor cardiorespiratory fitness levels and face difficulties in performing regular physical activities [16, 17]. Another contributing factor is the disruption of energy metabolism caused by mitochondrial dysfunction, which is a common feature of advanced NAFLD. This leads to less energy being available for skeletal muscles to perform exercise [18]. To the best of our knowledge, only a few studies have focused on identifying the barriers to regular exercise other than fatigue that prevent patients with NAFLD from engaging in regular physical activity [4, 15]. However, these studies did not assess perceptions of patients regarding the significance of exercise as a first-line therapy in NAFLD.
Therefore, our aim was to characterize the barriers to exercise in patients with MASLD, which is crucial for successfully incorporating a program that improves exercise adherence and reduces sedentary behavior in these patients. We sought to identify perceived barriers to exercise in patients with MASLD. Additionally, we assessed physical activity levels, self-perceived awareness, and perception of exercise, and its significance in the treatment of MASLD.
| Materials and Methods | ▴Top |
Study population
We enrolled adults aged above 18 years who were attending an outpatient hepatology clinic at a tertiary Australian hospital and had a clinical diagnosis of MASLD. Participants were assessed for the severity of hepatic steatosis using controlled attenuation parameter (CAP) and fibrosis by transient elastography (TE) with FibroScan® 502 Touch, as per routine clinical practice by an experienced consultant hepatologist, and MASLD diagnosed using recent consensus guidelines [1]. The TE and CAP assessments were considered valid if they fulfilled the manufacturer’s guidelines, i.e., at least 10 valid liver stiffness measurements (LSMs) with an interquartile range of < 30% and a success rate of ≥ 60% [19]. Participants with a CAP score of ≥ 275 dB/m, consistent with the approved threshold for hepatic steatosis diagnosis were invited to participate in the study. We defined MASLD as a steatotic liver with at least one metabolic risk factor without excessive alcohol consumption, according to the recent consensus nomenclature [1]. Participants with secondary causes of chronic liver disease such as autoimmune hepatitis, viral hepatitis, or significant alcohol consumption, and those with decompensated chronic liver disease with hepatic encephalopathy, ascites, esophageal varices, and hepatocellular carcinoma (HCC), were excluded. Participants were screened by the consultant hepatologist at the site where the present study was conducted using standard of care clinical (including specific quantification of alcohol consumption), biochemical, virological, immunological, ultrasound and liver stiffness assessments to exclude autoimmune hepatitis, viral hepatitis or significant alcohol consumption, hepatic encephalopathy, ascites, esophageal varices, and HCC. From these data, we were able to define a study population with the MASLD phenotype. Liver transaminase and platelet count results were extracted from the clinical records. From these, the fibrosis-4 (FIB-4) score was calculated. Ethics approval was obtained from the Human Research Ethics Committee at South Metropolitan Health Service (RGS0000004139) and Edith Cowan University (2020-01704-DESHPANDE). The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Study questionnaire
We created an online questionnaire using Qualtrics, which we electronically transmitted to the study participants following consultation with their hepatologist in the clinic (Supplementary Material 1, www.jocmr.org). Study participants completed the questionnaire during their clinic visit or virtually, which on average took approximately 5 - 7 min. The questionnaire consisted of various questions designed to assess physical activity levels, perception and knowledge about MASLD, and its seriousness as a health concern. Additionally, specific questions aimed to assess participant awareness regarding the role of exercise in the treatment of MASLD. The questionnaire included a list of 14 potential barriers to exercise, from which participants could choose multiple options. An open-text entry response was included to identify any additional barriers that were not listed. Responses to questions assessing perceptions and awareness were recorded as “yes/no/unsure”.
Statistical analyses
Statistical analyses were performed using IBM SPSS Statistics for Windows (Version 29.0, Armonk, NY, USA: IBM Corp.), and graphs were designed using GraphPad Prism 10. Descriptive statistics, using means, percentages, interquartile ranges, and standard deviations, were used to record the responses. Data are presented as mean ± standard deviation of the mean. Pearson’s and Spearman’s correlations were computed to assess the associations between baseline variables and questionnaire responses. One-way analysis of variance (ANOVA) and Student’s t-test with the Mann-Whitney U test, as appropriate, were used to assess differences between groups. Logistic regression analysis was used to assess the effects of age, gender, ethnicity, socioeconomic and education and occupation indices on the likelihood of the most common exercise barriers. The influence of liver-related variables on exercise behaviors was also assessed. Differences were considered statistically significant at a P value of less than 0.05.
| Results | ▴Top |
Study population
A total of 81 participants (57% female) with a mean age of 55.3 ± 13.4 (range: 20 - 81) years and a mean body mass index (BMI) of 33.8 ± 6.4 (22.8 - 60.6) answered the questionnaire. Table 1 summarizes the demographic and clinical characteristics of all the participants (n = 81) who answered the questionnaire. Based on their CAP and LSM scores, we categorized the participants into hepatic steatosis grade and hepatic fibrosis stages of MASLD. Quantification and grading of hepatic steatosis and hepatic fibrosis was performed as described in the study of Cao et al [20]. A high number of participants (63.1%) had grade 3 steatosis (S3), whereas 38% had suspected liver cirrhosis (F4). Although 81 participants responded to the questionnaire, the CAP and LSM scores for five participants were missing. We recorded their responses to all questions, but we were unable to classify them according to the severity of MASLD. Thirty participants (42.3%) were diabetic, while 41 were nondiabetic (57.7%).
 Click to view | Table 1. Demographic Characteristics of the Participants Who Participated in the Study |
Perceptions, awareness and exercise behaviors
The majority of participants (83%) agreed that a steatotic (or fatty) liver is a serious health concern. However, more than half (54%) were unsure about exercise as a therapeutic option for MASLD. A significant proportion of participants (73%) did not meet the recommended exercise guidelines of at least 150 min of moderate-intensity exercise or 75 min of vigorous exercise per week. When asked about their daily step count, 64% of them did not walk at least 10,000 steps per day. Additionally, 21% were unsure about their step count as they did not monitor this activity. We evaluated the influence of liver-related variables on exercise behaviors and found that severity of hepatic steatosis and fibrosis did not significantly influence exercise behaviors. Similarly, the Fib-4 index also had no significant impact on exercise behaviors (Table 2).
 Click to view | Table 2. Multinomial Logistic Regression Analysis of Liver-Related Variables Influencing the Exercise Behaviors |
In terms of exercise type, almost 36% of participants believed that aerobic exercise, such as walking and running, would be more effective in reversing MASLD, while only two participants considered resistance exercise to be helpful. However, 28% of the participants believed that both resistance and aerobic exercises could be beneficial. Nevertheless, almost 34% were unsure about the optimal type of exercise that they should engage in to manage their liver health (Fig. 1).
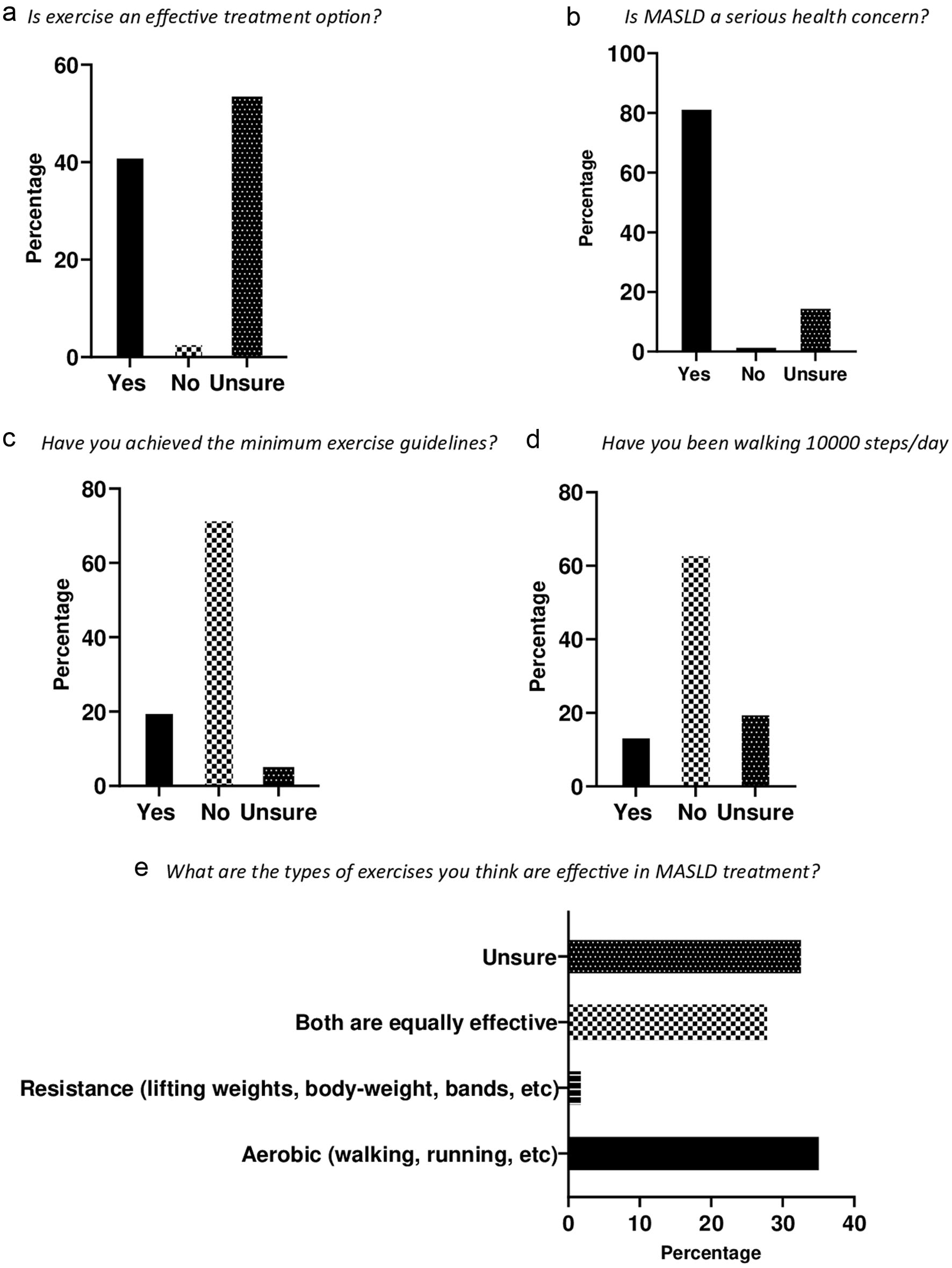 Click for large image | Figure 1. Perceptions, awareness and exercise behaviors. (a) More than half (54%) of the participants were unsure about exercise as a therapeutic option for MASLD. (b) Majority of the participants (83%) agreed that fatty liver is a serious health concern. (c) Majority of the participants (73%) did not achieve the recommended exercise guidelines. (d) Sixty-four percent of the participants did not walk at least 10,000 steps per day. (e) Aerobic exercise was picked as the most effective exercise (36%), and 28% thought that both aerobic and resistance exercise were effective in MASLD therapy. However, a noteworthy proportion (36%) were unsure about the optimal exercise type for MASLD therapy. MASLD: metabolic dysfunction-associated steatotic liver disease. |
We examined whether there were any significant correlations between the questionnaire responses and the baseline CAP and LSM scores. Our findings revealed that BMI was positively correlated with CAP (Pearson’s r = 0.44, P < 0.01) and LSM (r = 0.24, P < 0.05) (Fig. 2a, b). Additionally, we observed a positive correlation between age and liver stiffness (r = 0.30, P < 0.01) (Fig. 2c). However, we did not find any significant correlation between age and liver fat content as measured by the CAP score. We found no significant difference in the mean CAP scores between diabetic and nondiabetic participants (331 vs. 336, P = 0.07). Likewise, the mean LSM scores showed no significant difference between diabetics and nondiabetics (17.8 vs. 13.63, P = 0.2).
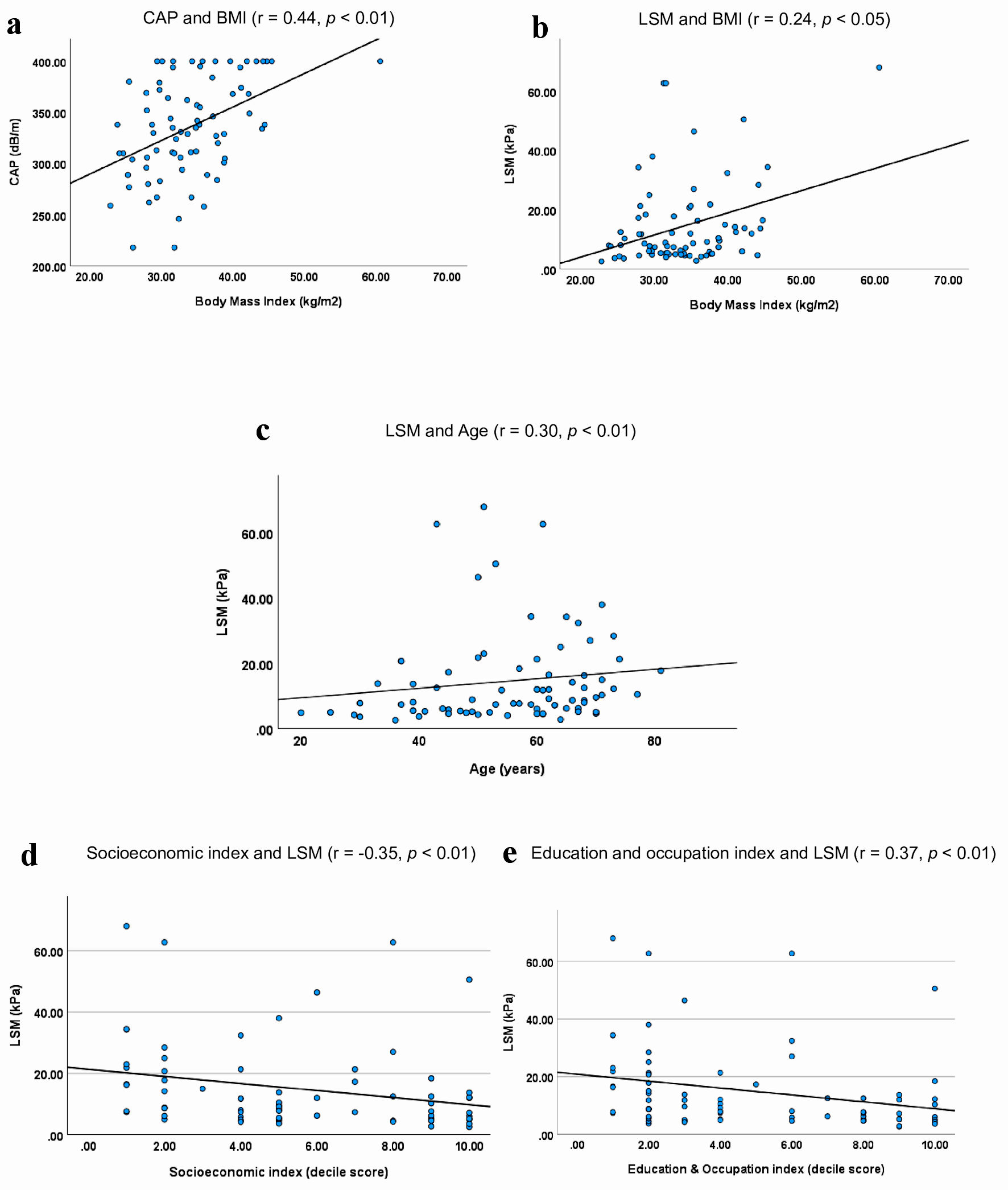 Click for large image | Figure 2. Correlations of CAP and LSM with age, BMI, socioeconomic and education and occupation indices. (a). Participants with high BMI had high CAP scores (r = 0.44, P < 0.01). (b) Participants with high BMI also appeared to have elevated LSM scores (r = 0.24, P < 0.05). (c) Older participants appeared to have higher LSMs (r = 0.30, P < 0.01). (d) LSM was inversely correlated with the socioeconomic status of the participants (r = -0.35, P < 0.01). (e) LSM was inversely correlated with the education and occupation indices of participants (r = -0.35, P < 0.01). Decile scores: 1 - most disadvantaged, 5 - average, 10 - most advantaged. CAP: controlled attenuation parameter; LSM: liver stiffness measurement; BMI: body mass index. |
Associations of exercise behavior and liver fat severity with relative socioeconomic and education and occupation status
We used the Socio-Economic Indexes for Areas (SEIFA) data in 2021 from the Australian Bureau of Statistics (ABS) and examined the exercise behaviors and severity of CAP and LSM scores of patients based on their relative socioeconomic and education and occupation indices using their residential post codes in Perth. SEIFA combines census data, such as income, education, employment, occupation, housing, and family structure, to summarize the socioeconomic characteristics of an area. We used their decile scoring system, in which each area (suburb) receives a SEIFA decile score out of 10, indicating how relatively advantaged or disadvantaged that area is compared to other areas. Decile 1 is the most disadvantaged, and decile 10 is the most advantaged area relative to other deciles. Decile 5 shows the average ranking. This analysis was only performed for 74 patients, as the residential information for seven patients was missing.
We observed that the number of people with MASLD was evenly distributed based on their relative socioeconomic indices. However, based on the education and occupation index, the number of participants in the low-decile group slightly exceeded those in the high or above-average decile group. Approximately 55% of the participants resided in areas near the most disadvantaged decile (2) and in below-average regions (Fig. 3). LSM severity scores showed a negative correlation with both socioeconomic (r = -0.35, P < 0.01) and education and occupation (r = -0.37, P < 0.01) decile scores (Fig. 2d, e). However, socioeconomic and education and occupation indices did not exhibit a significant correlation with CAP scores. Inactive participants who did not meet the recommended exercise guidelines were also evenly distributed between the high and low decile score areas based on their socioeconomic and education and occupation indices.
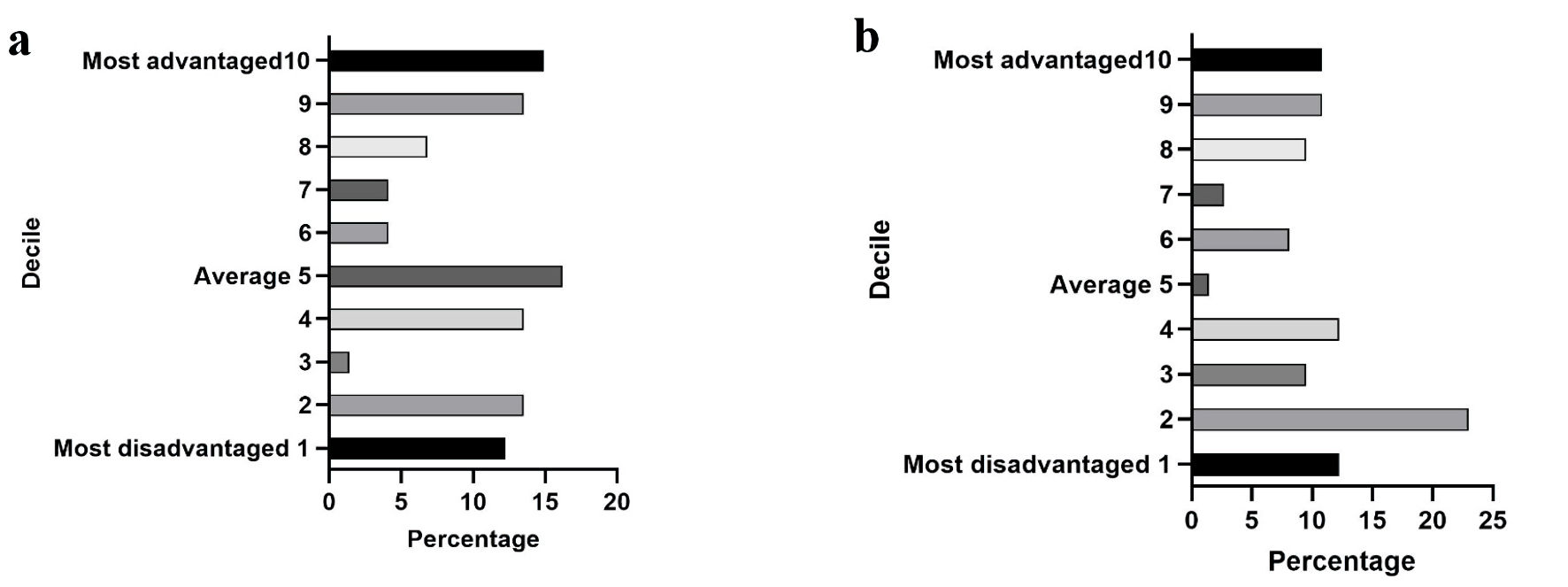 Click for large image | Figure 3. Socioeconomic, education and occupation indices for all participants. (a) Socioeconomic index. The socioeconomic status of participants did not differ significantly and was evenly distributed across most-advantaged and most-disadvantaged regions. (b) Education and occupation index. The number of participants residing in the low decile score regions (55% in decile 2) exceeded those in the high or above-average decile score regions based on their education and occupation index. |
We categorized the decile scores for both the indices into three groups. The first group, labeled “Top 3”, included participants with decile scores of 1, 2, and 3. The second group was termed “Bottom 3” and consisted of decile scores of 8, 9, and 10. The third group, termed the “Average score group”, included scores of 4, 5, 6, and 7. One-way ANOVA was used to assess whether there were any significant differences in the CAP and LSM scores between the three groups. For the CAP scores, no significant differences were observed among the three groups for socioeconomic, education and occupation indices. Similarly, LSM scores did not differ significantly among the groups based on the education and occupation index. However, a notable difference emerged in LSM scores concerning the socioeconomic index. The sample size (n) and means (M) for the three groups were significantly different (F2,70 = 3.5, P < 0.05) between the Top 3 (n = 20, M = 22.10), Bottom 3 (n = 25, M = 12.27), and Rest (n = 28, M = 12.40). Despite this significance, the post-hoc analysis did not specify the exact differences between the groups.
Barriers to exercise
Table 3 provides a description of all the exercise barriers listed in the questionnaire. Among the predefined options, the most commonly reported barriers were health issues (57%), lack of time (43%), lack of enjoyment in exercising (31%), other barriers (25%) and energy requirement and fatigue caused by exercise (24%). The other barriers described by participants in the free text option were as follows: depression, laziness, lack of motivation, lack of energy to exercise after work, joint pain from osteoarthritis, lack of companionship, and other commitments taking precedence. Factors such as peer pressure (0%), safety, accessibility and traffic issues (4%), discouragement and body shaming by others (9%), and lack of self-confidence to exercise (14%) were less commonly reported barriers.
 Click to view | Table 3. Responses to Barriers to Exercise |
When gender differences were accounted for, lack of time, health issues, and other barriers were common barriers for both men and women. However, women also reported lack of money and energy requirement and fatigue caused by exercise (28%) as their significant barriers to exercise. On the other hand, lack of enjoyment in exercise (40%) and boredom or nothing innovative in exercising (23%) were also reported as two of their leading barriers to exercise. We performed binomial logistic regression analyses to assess the influence of the independent variables such as age, gender, ethnicity, socioeconomic status, and education and occupation indices on the most significant barriers to exercise reported by participants (Table 4). We found that age was negatively associated with lack of time as a barrier. The odds for people choosing lack of time as their barrier to exercise went down with increasing age (P = 0.002, OR = 0.93). Similarly, as age increased, the likelihood of energy requirement and fatigue caused by exercise as a barrier was reduced (P = 0.02, OR = 0.94). Nonetheless, the likelihood of people choosing health issues as their barrier went up with age (P = 0.03, OR = 1.04).
 Click to view | Table 4. Binomial Logistic Regression Analysis of Factors Influencing the Leading Barriers to Exercise |
| Discussion | ▴Top |
While nearly all the participants in our study acknowledge the serious health consequences of MASLD, a considerable number lacked awareness regarding the significance of exercise and the optimal exercise interventions for MASLD treatment. A previous study examined the perception of people with NAFLD regarding exercise as a foundational treatment for NAFLD and found that the majority of participants agreed that exercise was important, and they also preferred exercise over medication to treat their NAFLD [15]. This differs from our observations, where we found that most participants were unsure about the significance of exercise as a treatment option for MASLD. Furthermore, many participants in our study were uncertain about the optimal modality of exercise that would be beneficial for them in improving their MASLD. This indicates that raising awareness among individuals regarding the role of exercise and optimal types of exercise in treating MASLD is crucial.
Almost three-quarters of our participants did not meet the recommended physical activity guidelines [8]. Our observations confirm and extend previous observations, which indicate that 80% of patients with NAFLD do not exercise for at least 150 min of moderate-intensity exercise per week [13, 21]. The exercise behaviors were not influenced by the severity of hepatic steatosis, liver fibrosis, and the Fib-4 scores. Fib-4 is a simple noninvasive tool which can reliably diagnose patients who are at higher risk of having advanced fibrosis [22]. Nevertheless, the association of Fib4 scores needs to be interpreted with caution as we only had scores for 57 participants out of 81. We also found that the majority of participants in our study were not walking at least 10,000 steps per day. Recent studies have shown a strong association between increased step count and a reduction in the risk of diabetes and all-cause mortality [23, 24]. Although the effects of daily step count have not been investigated in MASLD, the underlying metabolic risk factors in diabetes are the same in MASLD. Thus, it is highly likely that increasing the daily step count could help mitigate the burden of MASLD.
We found a significant positive correlation between BMI and both the CAP and LSM scores. This suggests that a higher BMI is associated with increased liver fat content and worsened liver stiffness, indicating a potential link between weight and MASLD severity. Several studies have reported similar observations, demonstrating that weight gain and high BMI are independent risk factors for the development and progression of NAFLD, applicable to both obese and non-obese individuals [25, 26]. Indeed, many studies have shown that even a modest degree of weight loss is significantly effective in improving NAFLD [27]. Furthermore, studies indicate that the onset and progression of NAFLD increases with age due to chronic inflammation, oxidative stress, and mitochondrial dysfunction, which alters the normal metabolic profile and drives the progression of liver fibrosis [28, 29]. This reinforces the importance of MASLD assessment across the age spectrum.
The presence of diabetes in 30 participants highlighted the bidirectional relationship between NAFLD and type 2 diabetes mellitus (T2DM), which has been attributed to underlying inflammation and insulin resistance [30]. Studies have shown that diabetes promotes the advancement of fatty liver to NASH, further elevating the risk of developing cirrhosis and liver cancer [31, 32]. However, a recent study showed that measuring the amount of liver fat can help predict the risk of overall mortality and development of T2DM [33]. It has been observed that reducing liver fat can significantly reduce the risk of developing T2DM [33, 34]. The severity of hepatic steatosis has also been associated with an increased risk of cardiovascular disease in middle-aged adults with T2DM [35]. As the majority of participants in our study did not have diabetes, this could indicate that the development of fatty liver may precede the onset of T2DM. Moreover, regular screening for T2DM in individuals with NAFLD is important for predicting and managing the risk of future T2DM development. The exercise barriers reported by people with T2DM are also similar to people with MASLD. A study found that the majority of T2DM patients were physically inactive, and factors related to low motivation such as lack of time, lack of willpower, and lack of energy were the main barriers to exercise. These barriers are almost identical to what we found in our study and may reflect existing knowledge that approximately 55-68% of those with T2DM have coexisting hepatic steatosis [36].
We assessed the influence of relative socioeconomic and education and occupation indices on the behaviors and disease severity of people with NAFLD. While we found a balanced distribution of patients based on their socioeconomic status, there was a slightly higher incidence of MASLD among individuals residing in areas characterized as less advantaged based on the education and occupation index. This suggests that individuals with lower education levels or those engaged in low-skilled occupations are more susceptible to developing fatty liver, potentially due to limited awareness of MASLD, lack of access to healthcare facilities, and unhealthy dietary choices such as affordable junk food options.
Interestingly, our analysis revealed a uniform prevalence of participants not meeting the recommended exercise guidelines, irrespective of their socioeconomic and education and occupation indices. This highlights the widespread issue of insufficient physical activity among individuals with NAFLD [13]. We identified a negative correlation between LSM scores and both socioeconomic and education and occupation indices. This indicates that individuals residing in socioeconomically disadvantaged areas or those employed in low-skilled occupations, and with lower education levels, exhibit higher liver stiffness, potentially making them more susceptible to progressing to advanced stages of MASLD. A study of middle-aged Korean adults found that low socioeconomic scores were independently associated with an increased risk of NAFLD [37]. Koutny et al [38] found a significantly higher prevalence of NAFLD based on the fatty liver index (FLI) and liver FIB-4 scores in the low education group than in the high education group. However, a recent study found that people who were categorized in the highest wealth group were significantly more likely to have high FLI scores compared to those in the low wealth category [39]. The authors speculated that this could be a result of low physical activity and high calorie intake among these individuals. These findings underscore the urgent need for increased awareness of MASLD and its management, particularly among vulnerable populations. The uniform distribution of CAP scores across all the relative socioeconomic, education and occupation indices indicates that MASLD is prevalent in all the regions encompassed by our study population. While the influence of education attainment and occupation on LSMs was not statistically significant, socioeconomic factors significantly affected the extent of liver stiffness. However, the data do not reveal where the exact differences lie within the three groups or whether the socioeconomic index has a more pronounced influence on a specific demographic.
Physical and mental health issues were the leading barriers to exercise. This may be due to the extrahepatic comorbidities associated with NAFLD pathogenesis. The pathophysiology of NAFLD involves multiple organs, leading to comorbidities such as T2DM, obstructive sleep apnea, cardiovascular disease, chronic kidney disease, polycystic ovarian syndrome, and osteoporosis [40]. Recent studies have shown a strong correlation between NAFLD pathogenesis and the presence of rheumatoid arthritis, hypothyroidism, psoriasis, and male sexual dysfunction [40]. Joint pain, fatigue, and muscle loss are commonly observed symptoms of these disorders, which are responsible for the poor physical function capacity of individuals with NAFLD and may result in sarcopenia [41]. Our results also showed that the likelihood of health issues as a significant exercise barrier increased with increasing age. Furthermore, there is a strong mechanistic link between NAFLD, mental health disorders, and metabolic syndrome mediated by oxidative stress, inflammation, and mitochondrial dysfunction [42]. Thus, mental health issues are leading barriers to exercise, as these conditions can lead to severe fatigue, pain, and loss of physical function.
Insufficient time to exercise was the second most commonly reported barrier, consistent with other studies conducted in healthy and obese adults [43-45]. Busy lifestyles, work commitments, and no time to go to the gym are common reasons for poor exercise habits. Lack of time was a more common barrier for people who were less old. This probably stands true as most of the older people are retired and may not be as busy as those who are working. Simple resistance exercises, such as push-ups and squats using only bodyweight, are helpful in improving the biomarkers of metabolic syndrome in NAFLD [46]. Furthermore, a few studies have shown that home-based exercise programs could help improve the metabolic profile of patients who have undergone liver and lung transplants or those awaiting liver transplantation [47, 48]. Nonetheless, the efficacy of a home-based exercise program delivered via videoconferencing in individuals with MASLD and MASH has not yet been evaluated. If such an exercise program, with minimal to no equipment, could be delivered online to individuals with MASLD by exercise professionals, it could encourage patients to participate in regular exercise and improve their exercise habits. This type of exercise program might also help in making the exercises more palatable and enjoyable for patients, given that a lack of enjoyment in exercising was also reported as a major barrier to exercise.
We found that fatigue experienced during and after exercise was also a common barrier that prevented participants from engaging in regular exercise. This is likely related to the physical discomfort experienced by these participants during and after the exercise. Studies suggest that participants with NAFLD have poor cardiorespiratory fitness levels and a greater rate of perceived exertion during exercise, significantly impacting their ability and willingness to participate in exercise [16, 49]. The negative association between age and energy requirement and fatigue as a barrier was interesting. This may indicate that fatigue caused by MASLD is not restricted to old and frail people. Administering a personalized exercise program that is physically less demanding and easier to perform could be an effective way to address this barrier. One such option is eccentric muscle contractions with eccentric exercise, in which the muscles generate force while lengthening. Studies have found that eccentric contractions are metabolically less demanding, thus producing less fatigue and cardiorespiratory stress, making them a suitable option for chronically ill individuals [50, 51].
It has been observed that sex differences contribute significantly to the prevalence and development of MASLD [52]. We also found differences between men and women regarding their significant barriers to exercise (Table 4). Women documented fatigue and energy requirement in exercise as one of their common barriers. Menopause could be a reason behind this as the average age of the women cohort in our study was 58 years. The metabolic and hormonal changes associated with menopause can lead to fatigue and lowered tolerance to exercise [53].
There are several limitations to our study. Our sample size was not sufficiently large to detect more associations and to gain a deeper understanding of the perceptions of exercise in participants with MASLD. Furthermore, self-reported physical activity levels and disease awareness may be subject to overestimation or underestimation by participants. Some of the participants answered the questionnaire virtually as opposed to the others who did it in person. While this can be a source of bias, we ensured that no additional or different information was provided to participants who answered the questionnaire in person to minimize any potential biases. Both in-person and virtual participants answered the questionnaire via an online link provided to them via text or email. Although Fibroscan is a reliable tool for detecting liver fibrosis, accurately assessing liver steatosis (the fat content in the liver), particularly in obese participants, could pose challenges and potentially impact the results. It is possible that participation in, and enjoyment of exercise could be improved by paying attention to features of the built environment and opportunities for physical activity in sedentary occupations.
In conclusion, we found that participants were aware of MASLD and its serious health consequences but lacked awareness of the significance of exercise in its treatment. The presence of various physiological and behavioral barriers to exercise highlights the necessity for a nuanced approach to therapy by understanding these barriers to enhance the overall health and quality of life in individuals with MASLD. Developing personalized training programs tailored to individual fitness levels and patient preferences can promote long-term adherence and sustainability. Prioritizing future research to identify such effective exercise interventions is crucial for improving morbidity and mortality rates in MASLD and reducing the overall burden on the healthcare system.
| Supplementary Material | ▴Top |
Suppl 1. Study questionnaire.
Acknowledgments
The authors thank all the study participants, as well as hepatology nurses; Wendy Lam, Crystal Connelly, Marcelle Scagliotta, and Huirong Ma for their assistance with the project.
Financial Disclosure
This work was supported by a PhD scholarship from Edith Cowan University. The university did not have any role in the study design, data collection, analysis or interpretation of the data; in writing this manuscript, or in the decision to submit the article for publication.
Conflict of Interest
The authors declare no conflict of interest that pertains to this work.
Informed Consent
Patient consent was obtained.
Author Contributions
Substantial contributions to study concept and design: KD, OA, KN, and JO. Acquisition of data: KD and OA. Analysis and interpretation of data: KD and OA. Drafting of the manuscript: KD. Statistical analysis: KD and OA. Figures and tables: KD, OA, KN. Critical revision of the manuscript for important intellectual content: KD, OA, KN and JO.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
AASLD: American Association for the Study of Liver Diseases; ABS: Australian Bureau of Statistics; BMI: body mass index; CAP: controlled attenuation parameter; EASL: European Association for the Study of the Liver; FLI: fatty liver index; LSM: liver stiffness measurement; MASH: metabolic dysfunction-associated steatohepatitis; MASLD: metabolic dysfunction-associated steatotic liver disease; NASH: non-alcoholic steatohepatitis; NAFLD: non-alcoholic fatty liver disease; SEIFA: Socio-Economic Indexes for Areas; SMHS: South Metropolitan Health Services; T2DM: type 2 diabetes mellitus; TE: transient elastography
| References | ▴Top |
- Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, Romero D, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;79(6):1542-1556.
doi pubmed - Younossi ZM, Golabi P, Paik JM, Henry A, Van Dongen C, Henry L. The global epidemiology of nonalcoholic fatty liver disease (NAFLD) and nonalcoholic steatohepatitis (NASH): a systematic review. Hepatology. 2023;77(4):1335-1347.
doi pubmed pmc - Devarbhavi H, Asrani SK, Arab JP, Nartey YA, Pose E, Kamath PS. Global burden of liver disease: 2023 update. J Hepatol. 2023;79(2):516-537.
doi pubmed - Glass O, Liu D, Bechard E, Guy CD, Pendergast J, Mae Diehl A, Abdelmalek MF. Perceptions of exercise and its challenges in patients with nonalcoholic fatty liver disease: a survey-based study. Hepatol Commun. 2022;6(2):334-344.
doi pubmed pmc - Adams LA, Roberts SK, Strasser SI, Mahady SE, Powell E, Estes C, Razavi H, et al. Nonalcoholic fatty liver disease burden: Australia, 2019-2030. J Gastroenterol Hepatol. 2020;35(9):1628-1635.
doi pubmed pmc - Thornton J. Associations rename fatty liver disease to reduce stigma. BMJ. 2023;382:1587.
doi pubmed - Heinle JW, DiJoseph K, Sabag A, Oh S, Kimball SR, Keating S, Stine JG. Exercise is medicine for nonalcoholic fatty liver disease: exploration of putative mechanisms. Nutrients. 2023;15(11):2452.
doi pubmed pmc - European Association for the Study of the Liver, European Association for the Study of Diabetes, European Association for the Study of Obesity. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64(6):1388-1402.
doi pubmed - Stine JG, Long MT, Corey KE, Sallis RE, Allen AM, Armstrong MJ, Conroy DE, et al. American College of Sports Medicine (ACSM) International Multidisciplinary Roundtable report on physical activity and nonalcoholic fatty liver disease. Hepatol Commun. 2023;7(4):e0108.
doi pubmed pmc - Zhang HJ, Pan LL, Ma ZM, Chen Z, Huang ZF, Sun Q, Lu Y, et al. Long-term effect of exercise on improving fatty liver and cardiovascular risk factors in obese adults: A 1-year follow-up study. Diabetes Obes Metab. 2017;19(2):284-289.
doi pubmed - Hashida R, Kawaguchi T, Bekki M, Omoto M, Matsuse H, Nago T, Takano Y, et al. Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: A systematic review. J Hepatol. 2017;66(1):142-152.
doi pubmed - Ayonrinde OT. Historical narrative from fatty liver in the nineteenth century to contemporary NAFLD - Reconciling the present with the past. JHEP Rep. 2021;3(3):100261.
doi pubmed pmc - Gerber L, Otgonsuren M, Mishra A, Escheik C, Birerdinc A, Stepanova M, Younossi ZM. Non-alcoholic fatty liver disease (NAFLD) is associated with low level of physical activity: a population-based study. Aliment Pharmacol Ther. 2012;36(8):772-781.
doi pubmed - Hallsworth K, Adams LA. Lifestyle modification in NAFLD/NASH: facts and figures. JHEP Rep. 2019;1(6):468-479.
doi pubmed pmc - Stine JG, Soriano C, Schreibman I, Rivas G, Hummer B, Yoo E, Schmitz K, et al. Breaking down barriers to physical activity in patients with nonalcoholic fatty liver disease. Dig Dis Sci. 2021;66(10):3604-3611.
doi pubmed pmc - Mitchell T, McKinnon E, Ayonrinde O, Adams LA, Trinder D, Chua ACG, Newton RU, et al. Decreased physical working capacity in adolescents with nonalcoholic fatty liver disease associates with reduced iron availability. Clin Gastroenterol Hepatol. 2020;18(7):1584-1591.
doi pubmed - Golabi P, Otgonsuren M, Cable R, Felix S, Koenig A, Sayiner M, Younossi ZM. Non-alcoholic Fatty Liver Disease (NAFLD) is associated with impairment of Health Related Quality of Life (HRQOL). Health Qual Life Outcomes. 2016;14:18.
doi pubmed pmc - Prasun P, Ginevic I, Oishi K. Mitochondrial dysfunction in nonalcoholic fatty liver disease and alcohol related liver disease. Transl Gastroenterol Hepatol. 2021;6:4.
doi pubmed pmc - Armstrong MJ, Corbett C, Hodson J, Marwah N, Parker R, Houlihan DD, Rowe IA, et al. Operator training requirements and diagnostic accuracy of Fibroscan in routine clinical practice. Postgrad Med J. 2013;89(1058):685-692.
doi pubmed pmc - Cao YT, Xiang LL, Qi F, Zhang YJ, Chen Y, Zhou XQ. Accuracy of controlled attenuation parameter (CAP) and liver stiffness measurement (LSM) for assessing steatosis and fibrosis in non-alcoholic fatty liver disease: A systematic review and meta-analysis. EClinicalMedicine. 2022;51:101547.
doi pubmed pmc - Krasnoff JB, Painter PL, Wallace JP, Bass NM, Merriman RB. Health-related fitness and physical activity in patients with nonalcoholic fatty liver disease. Hepatology. 2008;47(4):1158-1166.
doi pubmed pmc - McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59(9):1265-1269.
doi pubmed - Paluch AE, Bajpai S, Bassett DR, Carnethon MR, Ekelund U, Evenson KR, Galuska DA, et al. Daily steps and all-cause mortality: a meta-analysis of 15 international cohorts. Lancet Public Health. 2022;7(3):e219-e228.
doi pubmed pmc - Garduno AC, LaCroix AZ, LaMonte MJ, Dunstan DW, Evenson KR, Wang G, Di C, et al. Associations of daily steps and step intensity with incident diabetes in a prospective cohort study of older women: the OPACH study. Diabetes Care. 2022;45(2):339-347.
doi pubmed pmc - Yamada G, Hagiwara Y, Kimura T, Takeuchi Y, Oba K, Masuda K, Matsuyama Y. Impact of body weight gain on the incidence of nonalcoholic fatty liver disease in nonobese Japanese individuals. Am J Gastroenterol. 2021;116(4):733-740.
doi pubmed - Kim Y, Chang Y, Cho YK, Ahn J, Shin H, Ryu S. Obesity and weight gain are associated with progression of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2019;17(3):543-550.e542.
doi pubmed - Koutoukidis DA, Koshiaris C, Henry JA, Noreik M, Morris E, Manoharan I, Tudor K, et al. The effect of the magnitude of weight loss on non-alcoholic fatty liver disease: A systematic review and meta-analysis. Metabolism. 2021;115:154455.
doi pubmed - Li Y, Adeniji NT, Fan W, Kunimoto K, Torok NJ. Non-alcoholic fatty liver disease and liver fibrosis during aging. Aging Dis. 2022;13(4):1239-1251.
doi pubmed pmc - Alqahtani SA, Schattenberg JM. NAFLD in the elderly. Clin Interv Aging. 2021;16:1633-1649.
doi pubmed pmc - Fujii H, Kawada N, Japan Study Group of NAFLD (JSG-NAFLD). The role of insulin resistance and diabetes in nonalcoholic fatty liver disease. Int J Mol Sci. 2020;21(11):3863.
doi pubmed pmc - Tomah S, Alkhouri N, Hamdy O. Nonalcoholic fatty liver disease and type 2 diabetes: where do Diabetologists stand? Clin Diabetes Endocrinol. 2020;6:9.
doi pubmed pmc - Targher G, Corey KE, Byrne CD, Roden M. The complex link between NAFLD and type 2 diabetes mellitus - mechanisms and treatments. Nat Rev Gastroenterol Hepatol. 2021;18(9):599-612.
doi pubmed - Nasr P, Fredrikson M, Ekstedt M, Kechagias S. The amount of liver fat predicts mortality and development of type 2 diabetes in non-alcoholic fatty liver disease. Liver Int. 2020;40(5):1069-1078.
doi pubmed - Cho HJ, Hwang S, Park JI, Yang MJ, Hwang JC, Yoo BM, Lee KM, et al. Improvement of nonalcoholic fatty liver disease reduces the risk of type 2 diabetes mellitus. Gut Liver. 2019;13(4):440-449.
doi pubmed pmc - Kuo SZ, Cepin S, Bergstrom J, Siddiqi H, Jung J, Lopez S, Huang DQ, et al. Clinical utility of liver fat quantification for determining cardiovascular disease risk among patients with type 2 diabetes. Aliment Pharmacol Ther. 2023;58(6):585-592.
doi pubmed pmc - Ciardullo S, Muraca E, Perra S, Bianconi E, Zerbini F, Oltolini A, Cannistraci R, et al. Screening for non-alcoholic fatty liver disease in type 2 diabetes using non-invasive scores and association with diabetic complications. BMJ Open Diabetes Res Care. 2020;8(1):e000904.
doi pubmed pmc - Cho J, Lee I, Park DH, Kwak HB, Min K. Relationships between socioeconomic status, handgrip strength, and non-alcoholic fatty liver disease in middle-aged adults. Int J Environ Res Public Health. 2021;18(4):1892.
doi pubmed pmc - Koutny F, Aigner E, Datz C, Gensluckner S, Maieron A, Mega A, Iglseder B, et al. Relationships between education and non-alcoholic fatty liver disease. Eur J Intern Med. 2023;118:98-107.
doi pubmed - Sadeghianpour Z, Cheraghian B, Farshchi HR, Asadi-Lari M. Non-alcoholic fatty liver disease and socioeconomic determinants in an Iranian cohort study. BMC Gastroenterol. 2023;23(1):350.
doi pubmed pmc - Rosato V, Masarone M, Dallio M, Federico A, Aglitti A, Persico M. NAFLD and extra-hepatic comorbidities: current evidence on a multi-organ metabolic syndrome. Int J Environ Res Public Health. 2019;16(18):3415.
doi pubmed pmc - Linge J, Ekstedt M, Dahlqvist Leinhard O. Adverse muscle composition is linked to poor functional performance and metabolic comorbidities in NAFLD. JHEP Rep. 2021;3(1):100197.
doi pubmed pmc - Soto-Angona O, Anmella G, Valdes-Florido MJ, De Uribe-Viloria N, Carvalho AF, Penninx B, Berk M. Non-alcoholic fatty liver disease (NAFLD) as a neglected metabolic companion of psychiatric disorders: common pathways and future approaches. BMC Med. 2020;18(1):261.
doi pubmed pmc - Ashton LM, Hutchesson MJ, Rollo ME, Morgan PJ, Collins CE. Motivators and barriers to engaging in healthy eating and physical activity. Am J Mens Health. 2017;11(2):330-343.
doi pubmed pmc - Burgess E, Hassmen P, Pumpa KL. Determinants of adherence to lifestyle intervention in adults with obesity: a systematic review. Clin Obes. 2017;7(3):123-135.
doi pubmed - Hoare E, Stavreski B, Jennings GL, Kingwell BA. Exploring motivation and barriers to physical activity among active and inactive Australian adults. Sports (Basel). 2017;5(3):47.
doi pubmed pmc - Takahashi A, Abe K, Usami K, Imaizumi H, Hayashi M, Okai K, Kanno Y, et al. Simple resistance exercise helps patients with non-alcoholic fatty liver disease. Int J Sports Med. 2015;36(10):848-852.
doi pubmed - Rozenberg D, Santa Mina D, Nourouzpour S, Camacho Perez E, Stewart BL, Wickerson L, Tsien C, et al. Feasibility of a home-based exercise program for managing posttransplant metabolic syndrome in lung and liver transplant recipients: protocol for a pilot randomized controlled trial. JMIR Res Protoc. 2022;11(3):e35700.
doi pubmed pmc - Williams FR, Vallance A, Faulkner T, Towey J, Kyte D, Durman S, Johnson J, et al. Home-based exercise therapy in patients awaiting liver transplantation: protocol for an observational feasibility trial. BMJ Open. 2018;8(1):e019298.
doi pubmed pmc - Weinstein AA, Escheik C, Oe B, Price JK, Gerber LH, Younossi ZM. Perception of effort during activity in patients with chronic hepatitis C and nonalcoholic fatty liver disease. PM R. 2016;8(1):28-34.
doi pubmed - Julian V, Thivel D, Costes F, Touron J, Boirie Y, Pereira B, Perrault H, et al. Eccentric training improves body composition by inducing mechanical and metabolic adaptations: a promising approach for overweight and obese individuals. Front Physiol. 2018;9:1013.
doi pubmed pmc - Roig M, Shadgan B, Reid WD. Eccentric exercise in patients with chronic health conditions: a systematic review. Physiother Can. 2008;60(2):146-160.
doi pubmed pmc - Nagral A, Bangar M, Menezes S, Bhatia S, Butt N, Ghosh J, Manchanayake JH, et al. Gender differences in nonalcoholic fatty liver disease. Euroasian J Hepatogastroenterol. 2022;21(Suppl 1):S19-S25.
doi pubmed pmc - Lurati AR. Menopause and exercise intolerance. Nurs Womens Health. 2017;21(2):130-136.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


