| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 16, Number 1, January 2024, pages 8-14
Cold Agglutinin Disease and COVID-19: A Scoping Review of Treatments and Outcomes
Jackson S. Musuuzaa, c , Silpa Kumara, Dheeraj Kumar Posaa, Aakash Hansa, Sankett Nayyara, Leslie Christensenb
, Gilbert-Roy Kamogaa
aDepartment of Internal Medicine, White River Health, Batesville, AR 72501, USA
bEbling Library for the Health Sciences, University of Wisconsin School of Medicine and Public Health, Madison, WI 53705, USA
cCorresponding Author: Jackson S. Musuuza, Department of Internal Medicine, White River Health, Batesville, AR 72501, USA
Manuscript submitted January 5, 2024, accepted January 19, 2024, published online January 31, 2024
Short title: Cold Agglutinin Disease and COVID-19
doi: https://doi.org/10.14740/jocmr5102
| Abstract | ▴Top |
Background: Reports suggest that patients with both acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and cold agglutinin disease (CAD) may experience poorer survival when treated with rituximab. We conducted a scoping review to evaluate severe outcomes, including intensive care unit (ICU) admission and mortality, in coronavirus disease 2019 (COVID-19) patients with CAD on various treatments, including rituximab.
Methods: This review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR). Four literature databases were searched on December 19, 2023, for studies reporting lab-confirmed SARS-CoV-2 and CAD, excluding rheumatological conditions.
Results: Of the 741 screened articles, 19 were included. Studies, predominantly case reports (17/19) or case series (2/19), were mainly from the USA (8/19) and India (3/19), with others across Europe and Asia. Among 23 patients (61% female, median age 61 years), 21/23 had a new CAD diagnosis; only two had pre-existing CAD. Overall, 74% recovered, 21% died, and outcomes for one were unreported. Nine (39%) were ICU-admitted. Of rituximab-treated patients (n = 4), 25% were ICU-admitted, none died. Non-rituximab treatments (n = 19) saw 42% ICU admissions and 26% mortality.
Conclusions: This review found no increased risk of severe outcomes in CAD and COVID-19 patients treated with rituximab.
Keywords: Cold agglutinin disease; COVID-19; Rituximab; Severe outcomes; SARS-CoV-2; Autoimmune cytopenia; Poor survival
| Introduction | ▴Top |
Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the virus that causes coronavirus disease 2019 (COVID-19), has been associated with several hematological disease processes, including cold agglutinin disease (CAD) [1]. CAD is a rare disorder accounting for 25-30% of autoimmune hemolytic anemias [2].
In CAD, autoantibodies (immunoglobulin (Ig)M and rarely IgA and IgM) bind on the I antigen on the surface of red blood cells and cause complement-mediated hemolysis [3]. Pathological cold agglutinin antibodies can cause this hemolysis even at temperatures close to room temperature [4, 5], which puts affected patients at risk of hemolysis during their day-to-day lives. CAD is commonly associated with autoimmune processes, lymphoid malignancy, or underlying infection such as Epstein-Barr virus, mycoplasma, and now SARS-CoV-2 infection [6, 7]. Current first-line treatment for CAD involves the use of rituximab as monotherapy or as duo therapy with bendamustine [8]. However, reports show poorer survival for SARS-CoV-2 infected patients who received rituximab [9]. Severe outcomes with risk ratios as high as 5.5 have been reported in COVID-19 patients treated with anti-CD20 therapy such as rituximab [10, 11]. These observations have been made among patients who were taking rituximab and had underlying autoimmune rheumatologic conditions. Whether the higher disease severity was due to the existing inflammatory disease versus rituximab treatment remains unclear. Our paper focuses on the effects of rituximab in patients who had CAD and COVID-19 without underlying rheumatological conditions. We decided to eliminate the confounding effect of immunosuppression that could have resulted from these primarily autoimmune rheumatological conditions. Due to the scarcity of data on this topic, we conducted a scoping review of the literature to assess severe outcomes, including intensive care unit (ICU) admission and mortality among COVID-19 patients with CAD on different treatments, including rituximab, and examined underlying comorbidities.
| Materials and Methods | ▴Top |
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) in reporting this review [12].
Data sources and searches
The review team collaborated with a research librarian (LC) to develop and execute a comprehensive search of the literature. This search used controlled vocabulary and keywords to find studies related to rituximab therapy in patients with SARS-CoV-2 infection and CAD (Supplementary Material 1, www.jocmr.org). A search was developed in PubMed and translated into Scopus (Elsevier) and Web of Science Core Collection (Clarivate) as a multi-file search of Science Citation Index-Expanded and Emerging Sources Citation Index. All searches were performed on December 19, 2023. A date limit of November 1, 2019, to present was applied to eliminate studies prior to the emergence of COVID-19. No other limits or filters were used. A Google Scholar search was executed on December 19, 2023, and the first 200 results, sorted by relevance, were exported for screening.
Study selection
Results were downloaded to a citation management software (EndNote) and underwent manual de-duplication by the research librarian. Unique records were uploaded into Covidence (Veritas Health Information, Melbourne, Australia) for independent review by three team members using pre-determined inclusion/exclusion criteria.
Three authors (JSM, GRK, and AH) independently screened titles and abstracts and read the full texts to assess if they met the inclusion criteria. The authors met and discussed any articles where there was conflict and decided to either include or exclude such articles. Inclusion criteria were any study design (all were case reports/series) in which patients had both SARS-CoV-2 (laboratory-confirmed) and CAD. We excluded studies in pediatric populations, editorials, reviews, those published in a non-English language, articles where full texts were not available, and non-peer-reviewed preprints.
Data extraction
Using a standardized template, two reviewers (JSM and SK) independently abstracted data from individual studies. We abstracted data on the study author, year, country, study design, study population, CAD status, clinical presentation, comorbidities, rituximab treatment status, COVID-19 treatment, CAD treatment and mortality, and ICU admission status. Discrepancies were resolved by discussion between the two abstractors.
Data synthesis and analysis
In this scoping review, we assessed severe outcomes, including ICU admission and mortality among COVID-19 patients with CAD on different treatments, including rituximab, and examined underlying comorbidities. We calculated the proportions of patients treated with rituximab or other therapies who died or who were admitted to the ICU. In addition, we presented data on underlying comorbidities and demographics. Analyses were performed in SAS 9.4 (SAS Institute, Cary, NC). We did not register this study with PROSPERO nor conduct a quality assessment of the included studies, as these are unnecessary for a scoping review. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration. In addition, this scoping review was exempt from institutional review board approval.
| Results | ▴Top |
Our search yielded 1,065 records; we excluded 324 duplicates and screened 741 articles. At the abstract and title review stage, we excluded 716 articles, leaving 25 articles for full-text review. Of these, 19 met the inclusion criteria and were included in the scoping review. The most frequent reason for excluding studies at the full-text review stage was the study being in the wrong patient population, i.e., patients who had COVID-19 and were receiving rituximab for rheumatologic conditions but did not have CAD (Fig. 1). While the main reasons for exclusion at the abstract stage were studies about COVID-19 vaccinations, review articles such as systematic reviews or meta-analyses, studies on topics of other hemolytic anemias (but not CAD) and COVID-19, studies on the pathogenesis of CAD, but not treatment.
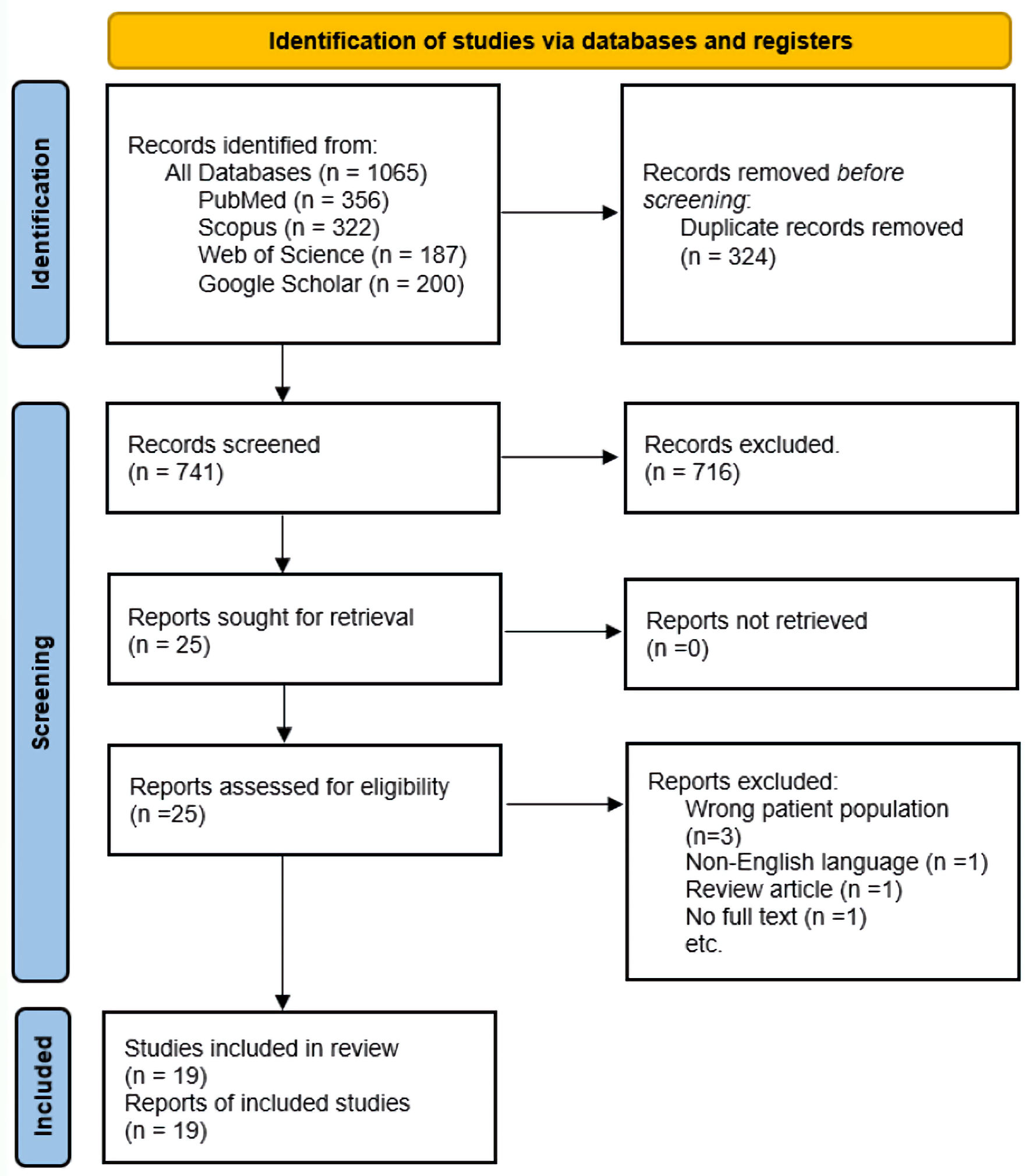 Click for large image | Figure 1. Study selection flow diagram: adapted from the PRISMA. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses. |
Characteristics of included studies are reported in Table 1 [13-31]. All the studies were case reports (17/19) or case series (2/19). Most of the studies were conducted in the USA (42% (8/19)) and India (15% (3/19)). The rest were conducted in various countries in Europe and Asia. Most of the patients were females (61% (14/23)), and the median age was 61 years (interquartile range (IQR): 24).
 Click to view | Table 1. Main Characteristics of Included Studies |
Most of the patients had a new diagnosis of CAD (21/23), and only two had pre-existing CAD before acquiring COVID-19. Anemia (7/23) and severe pneumonia (7/23) were the common diagnoses that the patients initially presented with. The other clinical presentations were thrombocytopenia, mild pneumonia, hypoxic respiratory failure, and nonspecific symptoms.
Examples of comorbidities included hypertension, type 2 diabetes mellitus, end-stage renal disease, cirrhosis, chronic obstructive airway disease, chronic lymphocytic leukemia, etc. Patients mostly presented with a combination of comorbidities co-existing. There was no difference in comorbidity prevalence among patients treated with rituximab compared to those treated with other therapies.
Treatments for CAD included the use of rituximab, packed red blood cell transfusions, plasma exchange, and steroids. These treatments were in combination; however, rituximab use occurred in only 4/23 patients.
Overall, 74% (17/23) of the patients recovered, while 21% (5/23) died, and no outcome was reported for one patient. Nine patients (39%) were admitted to the ICU. Among the four patients who were treated with rituximab, only one (25%) was admitted to the ICU, and none died. Among those treated with other treatments without rituximab, 42% (8/19) patients were admitted to the ICU, and 26% (5/19) died.
| Discussion | ▴Top |
We did not find an increased risk of severe outcomes among patients with CAD infected with COVID-19 who were treated with rituximab compared to those treated with other therapies. No difference in comorbidity prevalence among these two groups of patients (treated with rituximab vs. those treated with other therapies) was identified.
Previous studies showed that there was an increased risk of severe outcomes among patients with underlying rheumatological conditions presenting with COVID-19 and CAD and being treated with rituximab [9, 11, 32, 33]. Hence, a confounding effect of immunosuppression that could have resulted from these primary autoimmune rheumatological conditions could have erroneously attributed the increased risk of severe outcomes to rituximab therapy.
As seen in our systematic literature search, there is a paucity of studies specifically describing the effect of rituximab use among COVID-19 patients with CAD. We speculate that this is likely because after the publication of the rheumatological studies as mentioned above, the use of rituximab for CAD among COVID-19 patients might have decreased.
This precautionary use of rituximab for the treatment of CAD in patients with COVID-19 is likely to continue. Hence, there is a need for further studies examining the effect of rituximab use among COVID-19 patients with CAD and rheumatological conditions while adjusting for underlying immune suppression of these autoimmune conditions.
Our study faced limitations due to the scarcity of existing research on this topic, prompting us to conduct a scoping review. The included studies in this review primarily consisted of case reports or case series, as no retrospective or prospective studies (stronger studies) were published in the literature on this subject. Another limitation of our study is that we did not have data on confounding factors such as length of hospital stay and all the therapies the patients received while hospitalized. However, these risk factors are likely to have been more common among patients that required treatment with rituximab, and it would bias the results of our analysis towards the null.
In accordance with our scoping review, a multicenter observational study by Sorin et al did not show any discernible increase in the risk of severe COVID-19 among patients with autoimmune cytopenia undergoing rituximab treatment. This study evaluated the incidence and risk factors associated with severe COVID-19 in a cohort of patients and demonstrated a low occurrence. Out of the 308 patients studied, only 11 had COVID-19 necessitating oxygen therapy, and only two died [34].
Conclusions
This scoping review did not reveal an elevated risk of severe outcomes in CAD patients with COVID-19 treated with rituximab. Nonetheless, caution is advised when using rituximab in CAD patients with COVID-19 and underlying rheumatological conditions due to the heightened risk of severe outcomes in this subgroup, as reported in the literature.
| Supplementary Material | ▴Top |
Suppl 1. Search strategies.
Acknowledgments
Thanks to the Graduate Medical Education Department, White River Medical Center, Batesville, AR, USA.
Financial Disclosure
This work was not supported by any funding agency.
Conflict of Interest
Investigators will receive only normal scholarly gains from taking part in this study. The authors declare no competing interests.
Informed Consent
Not applicable.
Author Contributions
Conception and design: J.S. Musuuza, G.R. Kamoga. Literature search: L. Christensen, J.S Musuuza. Literature review and data abstraction from publications: J.S. Musuuza, S. Kumar, D.K. Posa, A. Hans, S. Nayyar, G.R. Kamoga. Analysis and interpretation of the data: J.S. Musuuza, G.R. Kamoga. Drafting of first draft: J.S. Musuuza, G.R. Kamoga. Critical revision for important intellectual content: all authors. Reading and final approval of the manuscript: all authors.
Data Availability
All data used in analysis of this manuscript are freely available by contacting the corresponding author.
Abbreviations
CAD: cold agglutinin disease; ICU: intensive care unit; PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses
| References | ▴Top |
- Rahi MS, Jindal V, Reyes SP, Gunasekaran K, Gupta R, Jaiyesimi I. Hematologic disorders associated with COVID-19: a review. Ann Hematol. 2021;100(2):309-320.
doi pubmed pmc - Jager U, Barcellini W, Broome CM, Gertz MA, Hill A, Hill QA, Jilma B, et al. Diagnosis and treatment of autoimmune hemolytic anemia in adults: Recommendations from the First International Consensus Meeting. Blood Rev. 2020;41:100648.
doi pubmed - Berentsen S. Cold agglutinin disease. Hematology Am Soc Hematol Educ Program. 2016;2016(1):226-231.
doi pubmed pmc - Berentsen S, Tjonnfjord GE. Diagnosis and treatment of cold agglutinin mediated autoimmune hemolytic anemia. Blood Rev. 2012;26(3):107-115.
doi pubmed - Ulvestad E, Berentsen S, Bo K, Shammas FV. Clinical immunology of chronic cold agglutinin disease. Eur J Haematol. 1999;63(4):259-266.
doi pubmed - Berentsen S, Randen U, Tjonnfjord GE. Cold agglutinin-mediated autoimmune hemolytic anemia. Hematol Oncol Clin North Am. 2015;29(3):455-471.
doi pubmed - Dawudi Y, Federici L, Debus J, Zucman N. Cold agglutinin disease secondary to severe SARS-CoV-2 treated with eculizumab. BMJ Case Rep. 2022;15(4):e242937.
doi pubmed pmc - Berentsen S. How I manage patients with cold agglutinin disease. Br J Haematol. 2018;181(3):320-330.
doi pubmed - Loarce-Martos J, Garcia-Fernandez A, Lopez-Gutierrez F, Garcia-Garcia V, Calvo-Sanz L, Del Bosque-Granero I, Teran-Tinedo MA, et al. High rates of severe disease and death due to SARS-CoV-2 infection in rheumatic disease patients treated with rituximab: a descriptive study. Rheumatol Int. 2020;40(12):2015-2021.
doi pubmed pmc - Boekel L, Wolbink GJ. Rituximab during the COVID-19 pandemic: time to discuss treatment options with patients. Lancet Rheumatol. 2022;4(3):e154-e155.
doi pubmed pmc - Andersen KM, Bates BA, Rashidi ES, Olex AL, Mannon RB, Patel RC, Singh J, et al. Long-term use of immunosuppressive medicines and in-hospital COVID-19 outcomes: a retrospective cohort study using data from the National COVID Cohort Collaborative. Lancet Rheumatol. 2022;4(1):e33-e41.
doi pubmed pmc - Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, Moher D, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med. 2018;169(7):467-473.
doi pubmed - Zagorski E, Pawar T, Rahimian S, Forman D. Cold agglutinin autoimmune haemolytic anaemia associated with novel coronavirus (COVID-19). Br J Haematol. 2020;190(4):e183-e184.
doi pubmed pmc - Tsukamoto Y, Umeda M, Muto Y, Sugimoto T, Yamauchi M, Ando K, Ariyoshi K. Severe Anemia Due to Cold Agglutinin Syndrome in a COVID-19 Patient with IgM Monoclonal Gammopathy of Undetermined Significance Successfully Treated with Corticosteroids. Intern Med. 2022;61(11):1789-1793.
doi pubmed pmc - Ramos-Ruperto L, Garcia-Perez E, Hernandez-Maraver D, Kerguelen-Fuentes A, Viejo-Llorente A, Robles-Marhuenda A, Busca-Arenzana C. A 3-Case Series of Autoimmune Haemolytic Anaemia and COVID-19: Is Plasma Exchange an Alternative? SN Compr Clin Med. 2021;3(6):1420-1423.
doi pubmed pmc - Raghuwanshi B. Serological Blood Group Discrepancy and Cold Agglutinin Autoimmune Hemolytic Anemia Associated With Novel Coronavirus. Cureus. 2020;12(11):e11495.
doi pubmed pmc - Priyadarshini SG, Pasupulati S. Cold agglutinin disease in COVID-19 causing severe intravascular hemolysis. Journal of Applied Hematology. 2022;13:154-156.
- Patil NR, Herc ES, Girgis M. Cold Agglutinin Disease and Autoimmune Hemolytic Anemia with Pulmonary Embolism as a Presentation of COVID-19 Infection. Hematol Oncol Stem Cell Ther. 2022;15(4):213-216.
doi pubmed pmc - Moonla C, Watanaboonyongcharoen P, Suwanpimolkul G, Paitoonpong L, Jantarabenjakul W, Chanswangphuwana C, Polprasert C, et al. Cold agglutinin disease following SARS-CoV-2 and Mycoplasma pneumoniae co-infections. Clin Case Rep. 2020;8(12):2402-2405.
doi pubmed pmc - Maslov DV, Simenson V, Jain S, Badari A. COVID-19 and Cold Agglutinin Hemolytic Anemia. TH Open. 2020;4(3):e175-e177.
doi pubmed pmc - Lazarian G, Quinquenel A, Bellal M, Siavellis J, Jacquy C, Re D, Merabet F, et al. Autoimmune haemolytic anaemia associated with COVID-19 infection. Br J Haematol. 2020;190(1):29-31.
doi pubmed pmc - Kaur J, Mogulla S, Khan R, Krishnamoorthy G, Garg S. Transient Cold Agglutinins in a Patient With COVID-19. Cureus. 2021;13(1):e12751.
doi pubmed pmc - Jacobs J, Eichbaum Q. COVID-19 associated with severe autoimmune hemolytic anemia. Transfusion. 2021;61(2):635-640.
doi pubmed pmc - Gupta R, Singh S, Anusim N, Gupta S, Gupta S, Huben M, Howard G, et al. Coronavirus Disease 2019 and Cold Agglutinin Syndrome: An Interesting Case. Eur J Case Rep Intern Med. 2021;8(3):002387.
doi pubmed pmc - Chang CY, Chin HH, Chin PW, Zaid M. Cold agglutinin-mediated autoimmune haemolytic anaemia associated with COVID-19 infection: a case report. Hong Kong Med J. 2022;28(3):257-259.
doi pubmed - Capes A, Bailly S, Hantson P, Gerard L, Laterre PF. COVID-19 infection associated with autoimmune hemolytic anemia. Ann Hematol. 2020;99(7):1679-1680.
doi pubmed pmc - Cabana VG, Teodorescu M, Dray S. Identification of basophils as the cells bearing both allelic immunoglobulin allotypes among white blood cells from the peripheral blood of heterozygous rabbits. J Immunol. 1980;124(5):2268-2280.
pubmed - Bhagat YV, Hussien S, Queenan H, Michael MB. Exacerbation of Secondary Cold Agglutinin Syndrome in the Setting of SARS-CoV-2. Cureus. 2021;13(11):e19387.
doi pubmed pmc - Aldaghlawi F, Shammah A, Kio E. SARS-CoV-2 infection complicated with cold agglutinin disease and myositis. Clin Case Rep. 2021;9(4):2196-2199.
doi pubmed pmc - Ahmed Y, Khandelwal A, Walker L. Cold agglutinin disease and COVID-19 requiring therapeutic plasma exchange. BMJ Case Rep. 2021;14(7):e244227.
doi pubmed pmc - Ahmadnezhad M, Mosleh M, Ferdowsi S, Mohammadi S, Eshghi P, Oodi A. Cold agglutinin associated with COVID-19 infection in a thalassemia patient with multiple alloantibodies: A case of cold hemagglutinin disease (CAD) with complex antibody detection. Hematol Transfus Cell Ther. 2021;43(3):361-363.
doi pubmed pmc - Brooks J, Montgomery A, Dalbeth N, Sapsford M, Ngan Kee R, Cooper A, Quincey V, et al. Omicron variant infection in inflammatory rheumatological conditions - outcomes from a COVID-19 naive population in Aotearoa New Zealand. Lancet Reg Health West Pac. 2023;38:100843.
doi pubmed pmc - Al-Adhoubi NK, Ali M, Wahshi HA, Salmi IA, Al-Balushi F, Lawati TA, Mohammed A, et al. COVID-19 Mortality in Patients with Rheumatic Diseases: A Real Concern. Curr Rheumatol Rev. 2022;18(3):234-242.
doi pubmed - Sorin B, Gaigne L, Garzaro M, Moulis G, Mageau A, Lopez C, Roy-Peaud F, et al. Severe SARS-CoV-2 infection in rituximab-treated patients with autoimmune cytopenia: A multicenter observational study. Am J Hematol. 2023;98(9):E259-E262.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


