| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 16, Number 2-3, March 2024, pages 106-117
Comparison of Clinical Features, Treatment and Outcomes of Lupus Nephritis Between Patients With Late- and Early-Onset Systemic Lupus Erythematosus: A Controlled Study
Jarukit Mongkolchaiarunyaa, b, Antika Wongthaneec, Nuntana Kasitanona, Worawit Louthrenooa, d
aDivision of Rheumatology, Department of Internal Medicine, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand
bCurrent address: Department of Internal Medicine, Sawanpracharak Hospital, Nakhonsawan 60000, Thailand
cDepartment of Internal Medicine, Faculty of Medicine, Chiang Mai University, Chiang Mai, Thailand
dCorresponding Author: Worawit Louthrenoo, Department of Internal Medicine, Faculty of Medicine, Chiang Mai University, Chiang Mai 50200, Thailand
Manuscript submitted December 20, 2023, accepted March 5, 2024, published online March 16, 2024
Short title: Lupus Nephritis in Late- and Early-Onset SLE
doi: https://doi.org/10.14740/jocmr5097
| Abstract | ▴Top |
Background: Studies have found that late-onset systemic lupus erythematosus (SLE) patients (age at diagnosis ≥ 50 years) had less severe disease and milder clinical course, but with higher organ damage and mortality rate than early-onset ones (age at diagnosis < 50 years). Unfortunately, direct comparison of renal manifestations and treatment outcomes between late- and early-onset SLE patients has been determined rarely. This study aimed to compare lupus nephritis (LN) manifestations, treatment, and outcomes between late- and early-onset in SLE patients.
Methods: Medical records of SLE patients in a lupus cohort at a tertiary care university hospital, seen between January 1994 and June 2020, were reviewed. Late- and early-onset patients were matched with year at SLE diagnosis at a ratio of 1:2 (62 and 124 patients, respectively). Those with LN were identified and analyzed.
Results: At SLE onset and end of the study, LN was identified in 29 and 33 late-onset patients, respectively, and 58 and 90 early-onset patients, respectively. At the end of the study, there were 39 and 214 LN flares in late- and early-onset patients, respectively: giving an incident rate (IR) (95% confidence interval (CI))/100 person-years of LN and active LN flares of 2.00 (0.75 - 5.33) vs. 6.11 (4.32 - 8.64), P = 0.020, and 5.78 (2.75 - 12.12) vs. 18.28 (13.93 - 24.00), P = 0.001, respectively. Late-onset patients received a higher proportion of moderate- to high-dose corticosteroids, but fewer immunosuppressive drugs. In all LN flares, no difference existed between the two groups in serum creatinine, degree of proteinuria, and proportion of patients with nephrotic range proteinuria or rapidly progressive glomerulonephritis, and outcomes in terms of complete, partial or no-remission were similar between them. Mortality rate was higher in late-onset patients (27.27% vs. 6.67%, P = 0.004).
Conclusion: This matched controlled study of year at SLE diagnosis showed that late-onset SLE patients had lower prevalence of LN and LN flares. Although they received fewer immunosuppressive drugs, their renal manifestations and treatment outcomes were no different from those in early-onset patients.
Keywords: Systemic lupus erythematosus; Lupus nephritis; Adult; Elderly; Treatment outcome
| Introduction | ▴Top |
Systemic lupus erythematosus (SLE) is a systemic autoimmune disease with a broad spectrum of clinical manifestations that predominantly affect young women. The disease has a highly variable clinical course, characterized by remission and relapse or exacerbation. Among the clinical manifestations, renal involvement or lupus nephritis (LN) is one of the most important causes of morbidity and mortality in patients with SLE [1-3].
Age at SLE diagnosis has a clear effect on clinical and laboratory manifestations, disease activity, organ damage, clinical outcomes, and mortality [4-18]. Studies have found that late-onset patients (age at SLE diagnosis ≥ 50 years) had insidious onset, less severe disease, and milder clinical course, but with higher organ damage and mortality rate when compared with early-onset ones (age at diagnosis < 50 years) [6, 10, 11, 18, 19]. Unfortunately, many of these studies compared late- and early-onset SLE patients directly, with few matching them with gender or disease duration [6, 10, 11, 18, 19], and a direct comparison of renal manifestations and treatment outcomes between late- and early-onset SLE patients has been determined rarely [20].
This study aimed to compare clinical features, serologic abnormalities, renal manifestations and flares, treatment, and treatment outcomes of LN between late- and early-onset SLE patients matched by the year of SLE diagnosis. This study was performed at Chiang Mai University Hospital (1,200 beds), the largest of four university hospitals in northern Thailand. The Division of Rheumatology takes care of patients with various rheumatic diseases and has approximately 400 admissions and 8,000 out-patient visits annually. SLE accounts for the majority of patients.
| Materials and Methods | ▴Top |
Clinical and laboratory data of the patients in this study were from the same cohort of SLE patients reported previously by the authors [18]. In brief, they were SLE patients diagnosed according to the 1997 updating of the American College of Rheumatology (ACR) for the classification of SLE [21], and followed up at the Rheumatology Clinic of Chiang Mai University Hospital between January 1994 and June 2020. The patients were followed up usually at 1- to 3-month intervals depending on disease activity. They were classified as late- and early-onset when their age at diagnosis was ≥ 50 and < 50 years, respectively. Inclusions comprised patients diagnosed SLE and followed up at the Rheumatology Clinic, aged ≥ 18 years at diagnosis, and followed up for ≥ 1 year (except for those who died within the first year of diagnosis). Exclusions included drug-induced SLE, SLE concomitant with other connective tissue diseases or malignancies, and patients with ≥ 25% of missing clinical or laboratory data. Late-onset patients were identified, and early-onset patients were matched with them by the year at diagnosis (± 1 year). From these matched patients, those with LN were identified and included in this analysis.
LN patients at the Rheumatology Clinic were treated usually with moderate- to high-dose corticosteroids (prednisolone ≥ 0.5 - 1.0 mg/kg/day), or intravenous methylprednisolone (IVMP) in the case of rapidly progressive glomerulonephritis (RPGN), together with immunosuppressive agents (usually cyclophosphamide or mycophenolate mofetil). Clinical manifestations and laboratory tests of LN were taken at every visit, as well as recordings of nephritic flares and LN treatment received for flares. In this study, LN treatment outcomes were assessed at the sixth and 12th month post treatment of each LN flare. Number of deaths and their cause also were determined.
This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration, and approved by the Ethics Committee of the Faculty of Medicine, Chiang Mai University, Thailand (No. 215/2020).
Definitions and measurement
LN was defined as persistent proteinuria > 0.5 g/day, or a 24-h or spot urine protein (mg/dL of urine protein) to urine creatinine (mg/dL of urine creatinine) (UPCR) of 0.5, together with active urine sediments [21, 22]. RPGN was defined as active nephritis (urine protein > 1 g/day) and active urine sediments together with ≥ 50% decline of the estimated glomerular filtration rate (eGFR) in less than 3 months. Complete remission was defined as UPCR < 500 mg/day and return of serum creatinine to the previous baseline level. Partial remission was defined as stabilization (±25%) or improvement of serum creatinine, but not to normal or ≥ 50% decrease of the UPCR. In the case of nephrotic-range proteinuria (UPCR ≥ 3.5), a reduction of ≥ 50% UPCR was required, and the UPCR must be < 3.0 [23]. No response referred to patients who did not achieve complete or partial remission. Those who had active nephritis flare before assessment at the sixth or 12th month were classified as having a new LN flare. LN duration lasted from first LN diagnosis to last visit, or study censor or death. The eGFR was calculated by Cockcroft-Gault equation [24]. In addition, other possible causes of proteinuria, and red or white blood cells in urine from renal calculi or urinary tract infection, were excluded before diagnosis of LN or assessment of renal response to treatment. SLE disease activity was determined by the modified systemic lupus erythematosus disease activity index-2000 (mSLEDAI-2K) [25], and organ damage accrual by the Systemic Lupus International Collaborating Clinics/ACR (SLICC/ACR) damage index (SDI) [26].
Statistical analysis
The STATA 16.0 for Windows computer software (Stata Corporation, College Station, TX, USA) was used to perform statistical analysis. Continuous variables were presented as mean (95% confidence interval (CI)), and Student’s t-test or Mann-Whitney U test was used for comparison. Categorical variables were defined as percentages, and the Chi-square or Fisher exact test was used for comparison. Non-normal distribution data were transformed to improve normality of data distributed for statistical analysis. To compare clinical manifestations, treatment, and LN outcomes between late- and early-onset patients with SLE, analysis of covariance (ANCOVA) and logistic regression were used for continuous and binary outcomes, respectively. Firth’s logistic regression was used for the binary outcomes of rare events, and Poisson regression for counting outcomes. To determine the survival difference between the two groups, after adjusting for confounding effects of other variables, Cox proportional hazards regression was used, and the proportional-hazards assumption was checked on the basis of Schoenfeld residuals. A P-value of < 0.05 was considered statistically significant.
| Results | ▴Top |
From 1,476 medical records of the included and excluded SLE patients reviewed, with matching year at SLE diagnosis, 62 and 124 patients with late- and early-onset, respectively, were identified (Fig. 1), of which 33 and 90, respectively, had LN, thus giving an LN prevalence of 53.23% and 72.58%, respectively (P = 0.009). However, 29 and 58 late- and early-onset patients, respectively, had LN at SLE diagnosis, thus giving an LN prevalence of 46.77% in both groups.
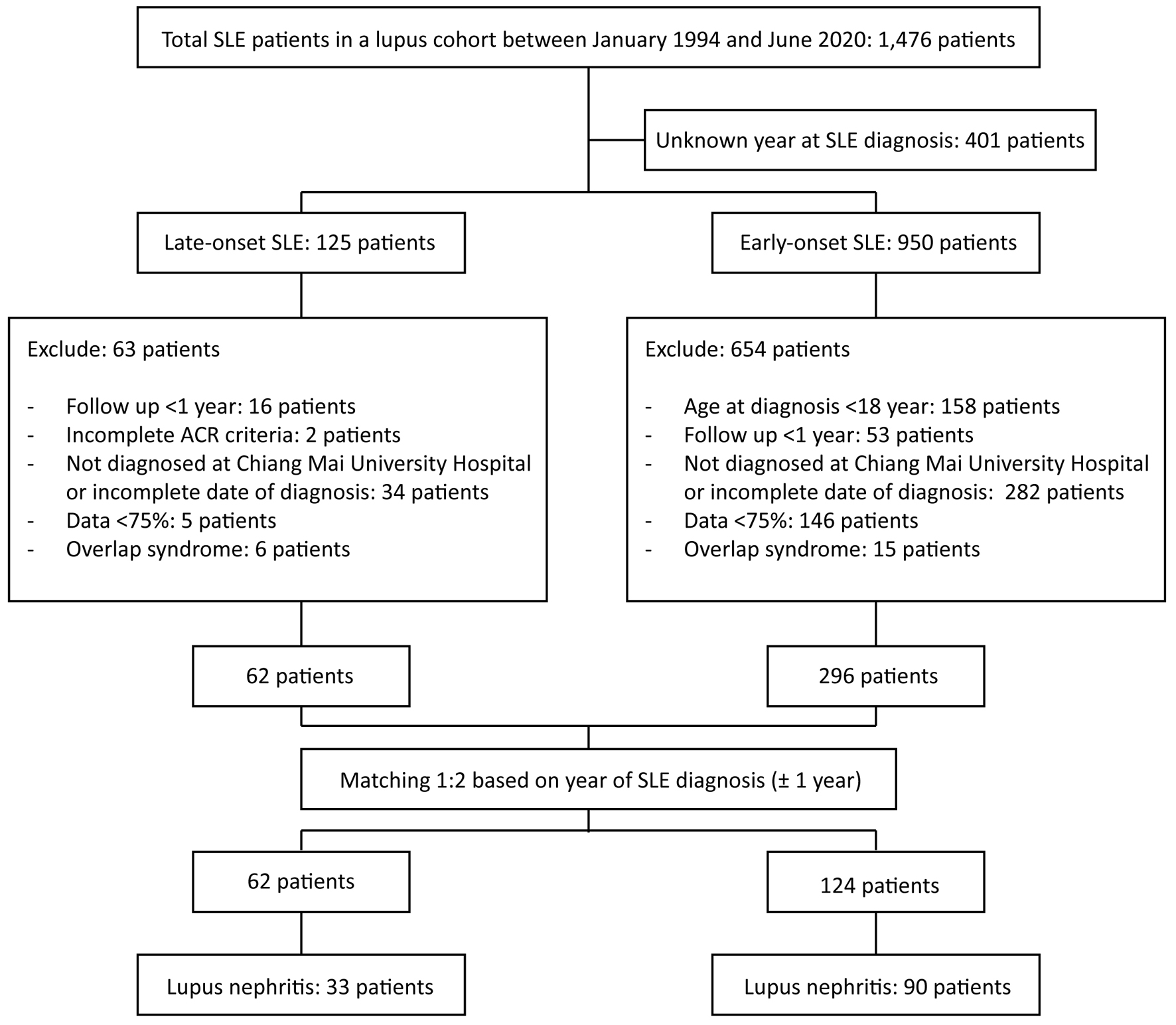 Click for large image | Figure 1. Flowchart showing the matching process. ACR: American College of Rheumatology; SLE: systemic lupus erythematosus. |
Demographic characteristics
Demographic characteristics of the patients studied are shown in Table 1. Twenty-eight (84.85%) of late-onset and 82 (91.11%) of early-onset patients were female, with their mean age at SLE and LN diagnosis being 56.27 years vs. 29.96 years, P < 0.001, and 56.78 years vs. 31.52 years, P < 0.001, respectively. Late-onset patients had shorter SLE and LN duration than early-onset patients (3.87 years vs. 6.46 years, P = 0.011, and 3.68 years vs. 5.39 years, P = 0.073). They also had higher prevalence of hypertension (27.27% vs. 2.22%, P < 0.001) and diabetes mellitus (9.09% vs. 1.11%, P = 0.059) at SLE diagnosis. Currently, none of them were smoking or drinking alcohol.
 Click to view | Table 1. Demographic Characteristics of Late- and Early-Onset SLE Patients With LN |
Clinical manifestations and laboratory abnormalities according to the 1997 ACR classification criteria, SLE disease activity, and damage index at SLE diagnosis between late- and early-onset LN patients are shown in Table 2. Compared with early-onset patients, late-onset ones had lower prevalence of malar rash (15.15% vs. 37.78%, P = 0.017), non-erosive arthritis (30.30% vs. 60.00%, P = 0.003), and positive anti-dsDNA antibody (68.97% vs. 87.14%, P = 0.033), but higher frequency of serositis (39.39% vs. 14.44%, P = 0.003), particularly pericarditis (24.24% vs. 8.89%, P = 0.025), nephritis (87.88% vs. 64.44%, P = 0.011) and hemolytic anemia (54.55% vs. 33.71%, P = 0.036). The mean number of the 1997 ACR classification criteria and SLE disease activity at SLE diagnosis were not different between the two groups. However, late-onset patients had higher damage scores (0.94 vs. 0.13, P < 0.001). Two (6.06%) and three (3.33%) of the late- and early-onset patients, respectively, had anti-phospholipid syndromes (P = 0.610).
 Click to view | Table 2. Comparison of 1997 ACR Classification Criteria for SLE, SLE Disease Activity and Damage Score at SLE Diagnosis Between Late- and Early-Onset SLE Patients With LN |
Renal manifestations of LN and treatment response
First LN episode
Details of the clinical parameters, treatment, and outcomes of treatment at the first LN episode, and at the sixth and 12th month are shown in Table 3. Late-onset patients had more hypertensive episodes during LN (24.24% vs. 10.00%, P = 0.235). Although serum creatinine was no different between the two groups, late-onset patients tended to have lower eGFR (81.43 mL/min/1.73 m2 vs. 93.97 mL/min/1.73 m2, P = 0.096). They also tended to have higher degree of proteinuria (UPCR) (2.57 vs. 1.94, P = 0.078), but lower serum albumin (2.3 g vs. 2.94 g, P < 0.001). Positive anti-dsDNA antibody was observed less frequently (65.52% vs. 86.21%, P = 0.025).
 Click to view | Table 3. Renal Manifestations and Response to Treatment of First LN Episode Between Late- and Early-Onset SLE Patients |
In terms of treatment, the mean dose of prednisolone was no different between the two groups, but late-onset patients received more moderate- to high-dose corticosteroids (prednisolone at ≥ 0.5 mg/kg/day) (96.97% vs. 78.89%, P = 0.016). They also received fewer immunosuppressive drugs, but without significance (54.54% vs. 58.89%, P = 0.666).
At the sixth month after treatment, five late-onset and three early-onset patients died before assessment (Table 3). Serum creatinine was higher, and eGFR was lower in the late-onset patients (mean: 0.99 mg/dL vs. 0.81 mg/dL, P = 0.019, and 74.02 mL/min/1.73 m2 vs. 103.11 mL/min/1.73 m2, P < 0.001, respectively). Although the UPCR was not different between the two groups, late-onset patients had lower serum albumin (mean: 3.49 g vs. 3.81 g, P = 0.020). There was no difference in proportion of patients with complete, partial or no response between the two groups. The same pattern of response was observed in assessment at the 12th month. One late-onset patient required hemodialysis during their LN episode.
All LN episodes
During the study period, including the first LN episode, there were overall 39 and 214 LN episodes in late- and early-onset patients, respectively (Table 4). Compared with early-onset patients, late-onset ones had lower prevalence of LN/person (1.18 vs. 2.38, P = 0.001), IR of patients with LN/100 person-years (2.00 vs. 6.11, P = 0.020), and occurrence rate of active LN/100 person-years (5.78 vs. 18.28, P = 0.001). Although serum creatinine was not different between the two groups, at the onset of each active LN flare, late-onset patients had significantly lower eGFR (79.33 mL/min/1.73 m2 vs. 92.57 mL/min/1.73 m2, P = 0.016). In addition, they also had lower serum albumin (2.43 g/dL vs. 3.13 g/dL, P < 0.001), and less positive anti-dsDNA (65.63% vs. 86.14%, P = 0.011). Both groups had no significant difference in hypertensive episodes during LN flares, degree of proteinuria, frequency of RPGN, and low complement levels. In terms of treatment, late-onset patients received slightly, but significantly, fewer mean daily doses of prednisolone (32.99 mg vs. 36.28 mg, P < 0.001), but more moderate- to high-dose corticosteroids (92.31% vs. 60.75%). However, they received fewer immunosuppressive drugs (58.97% vs. 78.50%, P = 0.009), particularly intravenous cyclophosphamide (12.82% vs. 28.50%, P = 0.040).
 Click to view | Table 4. Renal Manifestations and Response to Treatment of All Active Lupus Nephritis Episodes Between Late- and Early-Onset SLE Patients |
At the sixth month after treating all LN flares, including the first LN episode, five late- and four early-onset patients died before assessment. The outcomes were available for analysis in 33 and 200 late- and early-onset events, respectively. At the sixth and 12th month after each LN episode, late-onset patients only had significantly higher serum creatinine (1.01 mg/dL vs. 0.86 mg/dL, P = 0.011, and 0.95 mg/dL vs. 0.79 mg/dL, P = 0.001, respectively) and lower eGFR (72.93 mL/min/1.73 m2 vs. 97.47 mL/min/1.73 m2, P < 0.001, and 70.73 mL/min/1.73 m2 vs. 97.60 mL/min/1.73 m2, P < 0.001, respectively). However, there was no difference in the degree of proteinuria (UPCR) and serum albumin between the two groups. Interestingly, there was also no difference in the proportion of patients achieving complete, partial and no remission between the two groups. Three early-onset patients ended with chronic kidney disease that required long-term renal replacement therapy.
Renal pathology
Kidney biopsy was performed in only a small proportion of patients. Four late-onset patients (10.26%) had kidney biopsies, and the kidney pathological reports were class IV in three patients and class V in one patient. Nineteen early-onset patients (8.88%) had kidney biopsies, including eight with LN class IV (42.11%), seven with class V (36.84%), two with class IV/V (10.53%), and one each (5.26%) with class III/V and II.
Mortality and cause of death
Nine (27.27%) and six (6.67%) late- and early-onset patients, respectively, died during the study period (P = 0.002). After adjustment with LN duration or LN duration and underlying disease at SLE diagnosis, the mortality rate was still higher in late-onset patients (Table 5). Infection was the most common cause of death in both groups with no difference between them. The IR and 95% CI of death/100 patient-years were higher in late- than early-onset patients (5.61 (2.92 - 10.78) vs. 1.02 (0.46 - 2.26), P = 0.004) (Fig. 2).
 Click to view | Table 5. Death and Cause of Death |
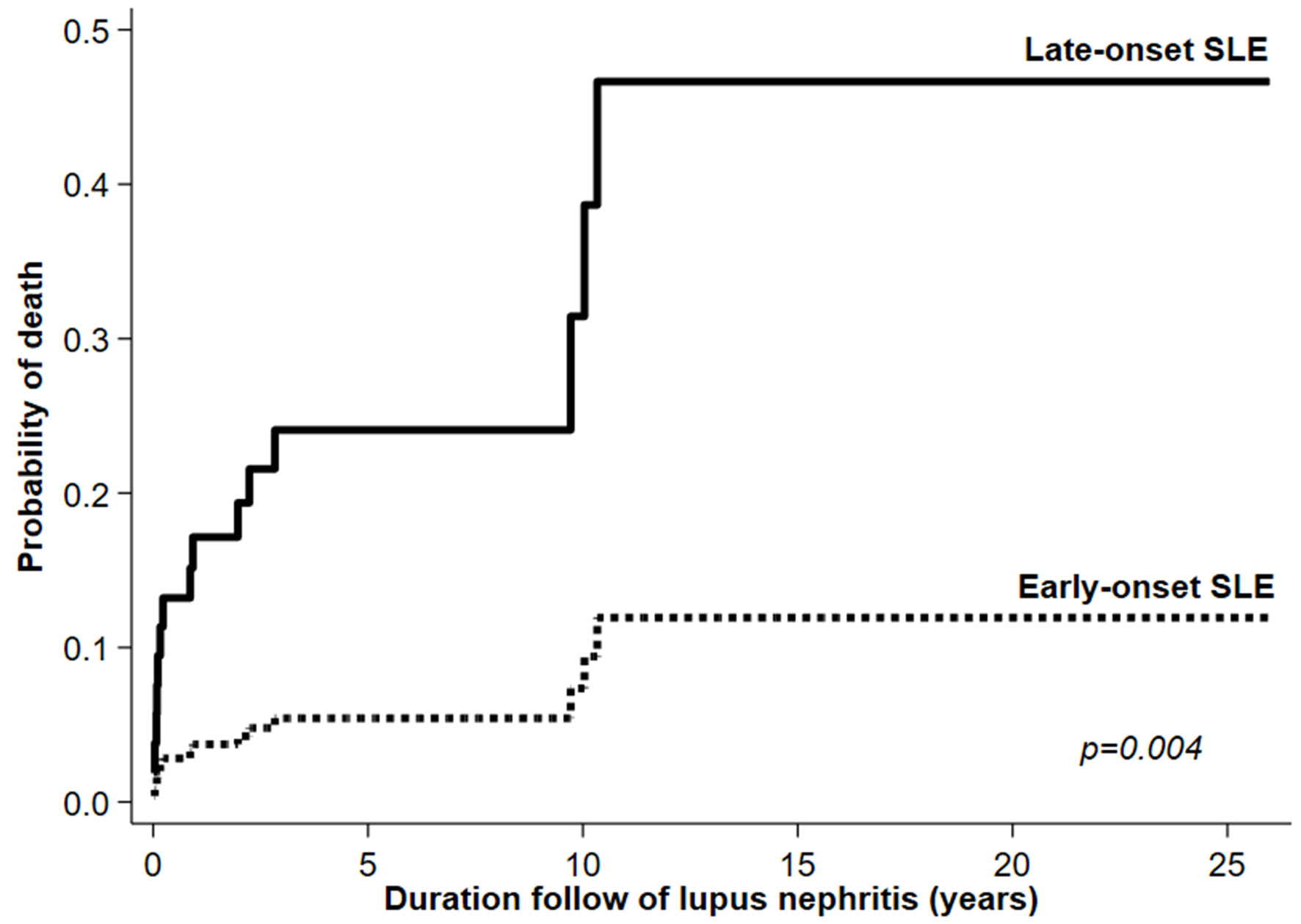 Click for large image | Figure 2. Kaplan-Meier curve showing mortality rate among late- and early-onset SLE patients. SLE: systemic lupus erythematosus. |
| Discussion | ▴Top |
This year at SLE diagnosis matched controlled study found that several clinical and laboratory manifestations and co-morbidities were different between late- and early-onset SLE patients. At SLE diagnosis, there was no difference in the prevalence of LN, mean number of ACR classification criteria and SLE disease activity between the two groups, but late-onset patients had higher organ damage. At the end of the study, late-onset patients had lower prevalence of LN and lower rate of LN flares. In terms of LN treatment, although there was no difference in the mean dose of corticosteroids and proportion of immunosuppressive drugs received between the two groups were not different at the first LN episode, a higher proportion of late-onset patients received moderate- to high-dose corticosteroids. However, at the end of the study, late-onset patients received less immunosuppressive therapy. Interestingly, LN treatment outcomes (complete, partial and no renal remission) were comparable between the two groups. Mortality was higher in late-onset patients.
The prevalence of LN at SLE diagnosis between late- and early-onset SLE patients had been mentioned in only a few studies, which showed conflicting results. For example, studies from Spain [27] and Poland [28] found that late-onset patients had lower prevalence of LN, whereas studies from Hong Kong [29] and Korea [9] found LN prevalence comparable in both groups. Another study from Spain, by Alonso et al [30], found that late-onset patients had lower prevalence of LN, which almost reached statistical significance. Unfortunately, the aforementioned studies were uncontrolled. A controlled study in Canada, by Aljohani et al [6], found that late-onset patients had less LN at the time of diagnosis, while another controlled study from Brazil, by Appenzeller et al [10], found no difference in the prevalence of LN at SLE diagnosis between late- and early-onset patients. This difference between studies is unclear, which might be due to varied ethnic backgrounds, and possibility that the study center was much more interested in LN, or the period in which study patients were recruited. This study found that the prevalence of LN at SLE diagnosis was similar in both groups of patients, which was opposed to many previous studies, but similar to that reported by Appenzeller et al [10]. The reason for the similar prevalence of LN between late- and early-onset patients in this study is unclear, which might be due to this study being a controlled one, which matched the patients by year at diagnosis, or focused mainly on LN patients.
This study also found that although there were some differences in clinical manifestations at SLE diagnosis between late- and early-onset patients, SLE disease activity between these two groups was no different. However, many previous studies reported that late-onset patients usually had lower disease activity [6, 9, 10, 27]. This might be explained partially by the aforementioned studies, in that late-onset patients usually had lower prevalence of LN, or in other words, early-onset patients had higher prevalence of LN. The presence of active LN usually associates with positive anti-dsDNA antibody and low complement levels, the variables that have scores in determining SLE disease activity in the original SLEDAI-2K instrument [31]. This resulted in early-onset patients, with generally higher prevalence of LN, having higher SLEDAI-2K scores.
This study also found no difference in the mean number of ACR classification criteria at SLE diagnosis between the two groups, which contradicted many previous reports that found it lower generally in late-onset patients [6, 9, 16, 30, 32]. The reason for this discrepancy is unclear, but it might be due to this study focusing on SLE patients with LN, who tended to have more severe disease, whereas the other studies compared patients with SLE in general. However, a previous study by the authors, which compared late- and early-onset patients in general, also found no difference in the mean ACR classification criteria at diagnosis between the two groups [18]. The higher damage scores observed in late-onset patients at SLE diagnosis might be related partly to the higher co-morbidities in this group of patients.
Previous studies on the cumulative prevalence of LN between late- and early-onset patients also showed conflicting results. For example, studies from Canada [6, 16], Italy [15], South Africa [33], South America [13], Hong Kong [29], Spain [4], and Serbia [32] found that late-onset patients had lower LN prevalence, whereas other studies from Brazil [10], Hong Kong [34], Colombia [35], Korea [9], and the LUMINA group from the United States [11] found the cumulative prevalence of LN to be no different between the two groups. Unfortunately, these studies did not provide more details on LN. This study found that late-onset patients not only had a lower cumulative prevalence of LN than early-onset patients, which supported many previous studies, but also less frequency of LN flares.
Interestingly, this study found that late-onset patients had a degree of proteinuria, and a proportion of those with nephrotic range proteinuria or RPGN was no different from early-onset ones, both at the first LN episode or during the course of the disease. This finding indicated that despite late-onset patients having lower LN prevalence, the degree of nephritis was no different from that in early-onset patients. This finding supported reports from China [20] and Hong Kong [34], which found no difference between late- and early-onset patients in the proportions of those with LN and nephrotic range proteinuria.
The treatment of LN consists of induction to remission with moderate- to high-dose corticosteroids together with immunosuppressive and maintenance therapy [36, 37]. Although late-onset patients received a higher proportion of moderate- to high-dose corticosteroids in this study, there was no difference in the proportion of those receiving immunosuppressive drugs at the first LN episode. Cumulative treatment found that patients received the same pattern of corticosteroids, but lower proportion of immunosuppressive drugs. These findings were consistent with many previous reports in that late-onset patients received fewer corticosteroids or immunosuppressive drugs, which might be because they were found to have less severe disease than early-onset patients [4, 6, 10, 12, 13, 15, 16, 29, 38]. However, such results were based on treatment of generally clinical manifestation of SLE, and not specific to LN.
It should be noted that the use of immunosuppressive drugs, both at first and in recurrent LN episodes, was rather low in this study. In general, it is recommended that all patients with LN should receive immunosuppressive drugs. However, those with less active LN (mild degree of proteinuria and not very active urine sediments), of probably LN class I or II by renal biopsy, might not need immunosuppressive therapy, whereas those with active LN (high degree of proteinuria and very active urine sediments), suggesting LN class III and IV, usually require immunosuppressive drugs together with high-dose corticosteroids [37]. According to the authors’ experience, infection is the major cause of death in Thai SLE patients [39], therefore, the use of immunosuppressive drugs is avoided in SLE patients without very active LN if possible. If urine protein showed a significant reduction with treatment of corticosteroids alone, corticosteroids would be continued with gradual tapering according to renal response. However, if there is no improvement in the degree of proteinuria or an increase in it with more active urine sediments, immunosuppressive drugs would be added. In patients with very active LN (heavy proteinuria and active urine sediments), immunosuppressive drugs would be combined with corticosteroids at the beginning of treatment. Unfortunately, kidney biopsy was not carried out in the majority of patients in this study. Most of the patients in this study responded well to corticosteroids, therefore, this could explain the lower rate of immunosuppressive drug use in the LN patients. In addition, as late-onset patients had less frequency of flares, they tended to receive fewer immunosuppressive drugs. This could explain the use of less immunosuppressive drugs in the early-onset patients in this study.
A direct comparison of LN treatment outcomes between late- and early-onset patients has been reported rarely. A study from Hong Kong found no difference between late- and early-onset of LN in degree of proteinuria at presentation and at 12 months after treatment [40]. A study by Tang et al compared renal parameters and pathology between two groups, but unfortunately the renal outcomes of treatment were not provided [20]. This study found no difference in the proportion of LN patients, who achieved complete, partial or no remission between late- and early-onset patients, whether or not it was the first or recurrent LN episode. These findings indicated that no matter what treatment the patients received for LN (moderate- to high-dose corticosteroids and immunosuppressive drugs), the outcomes of treatment were no different; in other words, the response of LN therapy in late- and early-onset patients was similar. Although renal outcomes in this study support those from Hong Kong [40], more studies are required to confirm those findings. The significant difference in reduced renal function after treatment, observed in late- rather than early-onset patients, might be due to the presence of co-morbidities, which were observed more commonly in the former group.
In this study, the mortality rate was higher in late-onset patients, which was similar to that previously described [4, 9-13, 15, 30]. Interestingly, most of the deaths occurred within 6 months of the first LN episode. This might be related to use of intensive corticosteroids and immunosuppressive drugs during the induction period. The major causes of death were infections followed by associations with SLE conditions, which did not differ between the two groups.
This study had some limitations. It was a retrospective study; therefore, some data might be missing. However, cases with missing data of > 25% were excluded to ensure adequate data for analysis. The anti-dsDNA and complements level were not performed at every LN visit, due to high cost. Therefore, the mSLEDAI-2K instrument, which excluded the anti-dsDNA and complements level and assessed SLE disease activity in this study, might be unable to compare with studies that used the original SLEDAI-2K instrument [6, 9, 10, 13, 27]. Nevertheless, the mSLEDAI-2K instrument has shown very good correlation with the original SLEDAI-2K [25, 31, 41]. In addition, renal biopsy was performed only in 17% of the cases. Although not all the cases in this study had LN proven by tissue diagnosis, which could not be confirmed totally, the presence of other clinical manifestations, together with active urine sediments and abnormal serologies (where available), ensured that all the nephritis flare cases were due to LN. Furthermore, this study was performed only in a single center in Thailand, thus the results might not apply to other populations. More studies with larger sample size are needed to confirm these findings.
Despite these limitations, this study had strengths. It was probably the first study that compared LN manifestations, treatment, and LN outcomes between late- and early-onset SLE patients, starting from the first LN episode to the end of the study period. The cases (late-onset) and control (early-onset) patients being matched with the year of diagnosis was another important strength. In a case-controlled study, especially a retrospective one, matching the control with a particular year at disease onset or diagnosis is very important, as it does not only reflect ability of the investigation in obtaining a diagnosis, but also the treatment received as well as assessing the outcomes during the same period. The data related to LN were recorded in every LN episode, which was another strength that provided good comparison of renal manifestation between the two groups.
Conclusion
This year at SLE diagnosis matched controlled study found no difference in the prevalence of LN and degree of LN at SLE diagnosis between late- and early-onset patients. Late-onset patients had lower prevalence of LN and rate of LN flares during the follow-up period. Although the first LN treatment pattern was similar between the two groups, late-onset patients received less immunosuppressive drugs for cumulative treatment during LN flares. Interestingly, renal outcomes in terms of renal remission were comparable in both groups, either at the first LN or at the end of the study. These findings indicated that LN in late-onset SLE patients might not be benign, as believed initially. However, renal impairment and mortality rate were higher in late-onset patients.
Acknowledgments
The authors thank Mrs. Waraporn Sukitawut for her secretarial assistance.
Financial Disclosure
This study had no funding support.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Informed consent was waived due to the retrospective nature of the study.
Author Contributions
Study design: JM, AW, NK, and WL. Data collection: JM and WL. Data analysis: JM and AW. Interpretation of statistical results: JM, AW, NK, and WL. Writing the first and final draft of the manuscript: JW and WL. All of the authors provided a critical review on intellectual content and approved the final version to be submitted for publication. Dr. Louthrenoo had full access to all of the data in the study and took responsibility for the integrity and accuracy of the data and analysis.
Data Availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
| References | ▴Top |
- Mok CC. Prognostic factors in lupus nephritis. Lupus. 2005;14(1):39-44.
doi pubmed - Hocaoglu M, Valenzuela-Almada MO, Dabit JY, Osei-Onomah SA, Chevet B, Giblon RE, Zand L, et al. Incidence, prevalence, and mortality of lupus nephritis: a population-based study over four decades using the lupus midwest network. Arthritis Rheumatol. 2023;75(4):567-573.
doi pubmed pmc - Ocampo-Piraquive V, Nieto-Aristizabal I, Canas CA, Tobon GJ. Mortality in systemic lupus erythematosus: causes, predictors and interventions. Expert Rev Clin Immunol. 2018;14(12):1043-1053.
doi pubmed - Riveros Frutos A, Holgado S, Sanvisens Berge A, Casas I, Olive A, Lopez-Longo FJ, Calvo-Alen J, et al. Late-onset versus early-onset systemic lupus: characteristics and outcome in a national multicentre register (RELESSER). Rheumatology (Oxford). 2021;60(4):1793-1803.
doi pubmed - Medhat BM, Behiry ME, Sobhy N, Farag Y, Marzouk H, Mostafa N, Khalifa I, et al. Late-onset systemic lupus erythematosus: characteristics and outcome in comparison to juvenile- and adult-onset patients-a multicenter retrospective cohort. Clin Rheumatol. 2020;39(2):435-442.
doi pubmed - Aljohani R, Gladman DD, Su J, Urowitz MB. Disease evolution in late-onset and early-onset systemic lupus erythematosus. Lupus. 2017;26(11):1190-1196.
doi pubmed - Sassi RH, Hendler JV, Piccoli GF, Gasparin AA, da Silva Chakr RM, Brenol JC, Monticielo OA. Age of onset influences on clinical and laboratory profile of patients with systemic lupus erythematosus. Clin Rheumatol. 2017;36(1):89-95.
doi pubmed - das Chagas Medeiros MM, Bezerra MC, Braga FN, da Justa Feijao MR, Gois AC, Reboucas VC, de Carvalho TM, et al. Clinical and immunological aspects and outcome of a Brazilian cohort of 414 patients with systemic lupus erythematosus (SLE): comparison between childhood-onset, adult-onset, and late-onset SLE. Lupus. 2016;25(4):355-363.
doi pubmed - Sohn IW, Joo YB, Won S, Bae SC. Late-onset systemic lupus erythematosus: Is it "mild lupus"? Lupus. 2018;27(2):235-242.
doi pubmed - Appenzeller S, Pereira DA, Costallat LT. Greater accrual damage in late-onset systemic lupus erythematosus: a long-term follow-up study. Lupus. 2008;17(11):1023-1028.
doi pubmed - Bertoli AM, Alarcon GS, Calvo-Alen J, Fernandez M, Vila LM, Reveille JD, LUMINA Study Group. Systemic lupus erythematosus in a multiethnic US cohort. XXXIII. Clinical [corrected] features, course, and outcome in patients with late-onset disease. Arthritis Rheum. 2006;54(5):1580-1587.
doi pubmed - Boddaert J, Huong DLT, Amoura Z, Wechsler B, Godeau P, Piette JC. Late-onset systemic lupus erythematosus: a personal series of 47 patients and pooled analysis of 714 cases in the literature. Medicine (Baltimore). 2004;83(6):348-359.
doi pubmed - Catoggio LJ, Soriano ER, Imamura PM, Wojdyla D, Jacobelli S, Massardo L, Chacon Diaz R, et al. Late-onset systemic lupus erythematosus in Latin Americans: a distinct subgroup? Lupus. 2015;24(8):788-795.
doi pubmed - Choi JH, Park DJ, Kang JH, Yim YR, Lee KE, Lee JW, Wen L, et al. Comparison of clinical and serological differences among juvenile-, adult-, and late-onset systemic lupus erythematosus in Korean patients. Lupus. 2015;24(12):1342-1349.
doi pubmed - Cartella S, Cavazzana I, Ceribelli A, Inverardi F, Tincani A, Franceschini F. Evaluation of mortality, disease activity, treatment, clinical and immunological features of adult and late onset systemic Lupus erythematosus. Autoimmunity. 2013;46(6):363-368.
doi pubmed - Lalani S, Pope J, de Leon F, Peschken C, Members of Ca NFoL. Clinical features and prognosis of late-onset systemic lupus erythematosus: results from the 1000 faces of lupus study. J Rheumatol. 2010;37(1):38-44.
doi pubmed - Kang JH, Park DJ, Lee KE, Lee JS, Choi YD, Lee SS. Comparison of clinical, serological, and prognostic differences among juvenile-, adult-, and late-onset lupus nephritis in Korean patients. Clin Rheumatol. 2017;36(6):1289-1295.
doi pubmed - Mongkolchaiarunya J, Wongthanee A, Kasitanon N, Louthrenoo W. Comparison of clinical features, disease activity, treatment and outcomes between late-onset and early-onset patients with systemic lupus erythematosus. A sex- and year at diagnosis-matched controlled study. Adv Rheumatol. 2023;63(1):20.
doi pubmed - Maddison P, Farewell V, Isenberg D, Aranow C, Bae SC, Barr S, Buyon J, et al. The rate and pattern of organ damage in late onset systemic lupus erythematosus. J Rheumatol. 2002;29(5):913-917.
pubmed - Tang Z, Chen D, Yang S, Zhang H, Hu W, Liu Z, Li L. Late onset lupus nephritis: analysis of clinical manifestations and renal pathological features in Chinese patients. Rheumatol Int. 2011;31(12):1625-1629.
doi pubmed - Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40(9):1725.
doi pubmed - Dooley MA, Aranow C, Ginzler EM. Review of ACR renal criteria in systemic lupus erythematosus. Lupus. 2004;13(11):857-860.
doi pubmed - Kidney Disease: Improving Global Outcomes Glomerular Diseases Work Group. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100(4S):S1-S276.
doi pubmed - Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31-41.
doi pubmed - Uribe AG, Vila LM, McGwin G, Jr., Sanchez ML, Reveille JD, Alarcon GS. The Systemic Lupus Activity Measure-revised, the Mexican Systemic Lupus Erythematosus Disease Activity Index (SLEDAI), and a modified SLEDAI-2K are adequate instruments to measure disease activity in systemic lupus erythematosus. J Rheumatol. 2004;31(10):1934-1940.
pubmed - Gladman D, Ginzler E, Goldsmith C, Fortin P, Liang M, Urowitz M, Bacon P, et al. The development and initial validation of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for systemic lupus erythematosus. Arthritis Rheum. 1996;39(3):363-369.
doi pubmed - Formiga F, Moga I, Pac M, Mitjavila F, Rivera A, Pujol R. Mild presentation of systemic lupus erythematosus in elderly patients assessed by SLEDAI. SLE Disease Activity Index. Lupus. 1999;8(6):462-465.
doi pubmed - Jeleniewicz R, Suszek D, Majdan M. Clinical picture of late-onset systemic lupus erythematosus in a group of Polish patients. Pol Arch Med Wewn. 2015;125(7-8):538-544.
doi pubmed - Ho CT, Mok CC, Lau CS, Wong RW. Late onset systemic lupus erythematosus in southern Chinese. Ann Rheum Dis. 1998;57(7):437-440.
doi pubmed pmc - Alonso MD, Martinez-Vazquez F, de Teran TD, Miranda-Filloy JA, Dierssen T, Blanco R, Gonzalez-Juanatey C, et al. Late-onset systemic lupus erythematosus in Northwestern Spain: differences with early-onset systemic lupus erythematosus and literature review. Lupus. 2012;21(10):1135-1148.
doi pubmed - Gladman DD, Ibanez D, Urowitz MB. Systemic lupus erythematosus disease activity index 2000. J Rheumatol. 2002;29(2):288-291.
pubmed - Tomic-Lucic A, Petrovic R, Radak-Perovic M, Milovanovic D, Milovanovic J, Zivanovic S, Pantovic S, et al. Late-onset systemic lupus erythematosus: clinical features, course, and prognosis. Clin Rheumatol. 2013;32(7):1053-1058.
doi pubmed - Budhoo A, Mody GM, Dubula T, Patel N, Mody PG. Comparison of ethnicity, gender, age of onset and outcome in South Africans with systemic lupus erythematosus. Lupus. 2017;26(4):438-446.
doi pubmed - Mak SK, Lam EK, Wong AK. Clinical profile of patients with late-onset SLE: not a benign subgroup. Lupus. 1998;7(1):23-28.
doi pubmed - Penaranda-Parada E, Quintana G, Yunis JJ, Mantilla R, Rojas W, Panqueva U, Caminos JE, et al. Clinical, serologic, and immunogenetic characterization (HLA-DRB1) of late-onset lupus erythematosus in a Colombian population. Lupus. 2015;24(12):1293-1299.
doi pubmed - Hahn BH, McMahon MA, Wilkinson A, Wallace WD, Daikh DI, Fitzgerald JD, Karpouzas GA, et al. American College of Rheumatology guidelines for screening, treatment, and management of lupus nephritis. Arthritis Care Res (Hoboken). 2012;64(6):797-808.
doi pubmed pmc - Fanouriakis A, Kostopoulou M, Cheema K, Anders HJ, Aringer M, Bajema I, Boletis J, et al. 2019 Update of the Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann Rheum Dis. 2020;79(6):713-723.
doi pubmed - Merola JF, Bermas B, Lu B, Karlson EW, Massarotti E, Schur PH, Costenbader KH. Clinical manifestations and survival among adults with (SLE) according to age at diagnosis. Lupus. 2014;23(8):778-784.
doi pubmed pmc - Kasitanon N, Louthrenoo W, Sukitawut W, Vichainun R. Causes of death and prognostic factors in Thai patients with systemic lupus erythematosus. Asian Pac J Allergy Immunol. 2002;20(2):85-91.
pubmed - Mak A, Mok CC, Chu WP, To CH, Wong SN, Au TC. Renal damage in systemic lupus erythematosus: a comparative analysis of different age groups. Lupus. 2007;16(1):28-34.
doi pubmed - Bombardier C, Gladman DD, Urowitz MB, Caron D, Chang CH. Derivation of the SLEDAI. A disease activity index for lupus patients. The Committee on Prognosis Studies in SLE. Arthritis Rheum. 1992;35(6):630-640.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


