| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 16, Number 6, June 2024, pages 293-301
Comparison Between Twenty-Four-Hour Collection and Single Spot Urines for the Detection of Biogenic Amines by High-Performance Liquid Chromatography Tandem Mass Spectrometry
Chiara Rosatoa, d, Marilena Grecob, Giovanni Marciantec, Roberta Assunta Lazzaria, Floriano Indinoa, Giambattista Lobreglioa
aClinical Pathology and Microbiology Unit, “Vito Fazzi” Hospital, Lecce 73100, Italy
bClinical Pathology Unit, “Sacro Cuore di Gesu” Hospital, Gallipoli 73014, Italy
cOccupational Medicine Unit, University of Bari “Aldo Moro”, Bari 70121, Italy
dCorresponding Author: Chiara Rosato, Clinical Pathology and Microbiology Unit, “Vito Fazzi” Hospital, Lecce 73100, Italy
Manuscript submitted November 24, 2023, accepted January 13, 2024, published online June 30, 2024
Short title: Detection of Biogenic Amines by HPLC/MS-MS
doi: https://doi.org/10.14740/jocmr5070
| Abstract | ▴Top |
Background: Pheochromocytomas and paragangliomas (PPGL) are neuroendocrine tumors that originate from adrenal medulla or extra-adrenal chromaffin cells, respectively. They produce an excess of catecholamines and their metabolites. Abnormal levels of these biomolecules have been also found in pediatric patients with neuroblastoma (NB). Due to the diurnal fluctuation, the laboratory practice recommends the determination of biogenic amines in acidified 24-h urine samples. However, the collection and acidification of specimens cannot be performed easily, especially for children. Spot urines represent an attractive alternative for the detection of catecholamines and corresponding metabolites.
Methods: In our study, we enrolled 50 patients with symptoms related to PPGL and we determined the concentration values for both spot and 24-h urine samples using high-performance liquid chromatography tandem mass spectrometry (HPLC/MS-MS). Since day variations of the urinary concentration are due to fluctuations in renal excretion rather than in production, we normalized the concentration of biogenic amines in spot urine and in 24-h urine collection to urinary creatinine concentration. A correlation study between the normalized levels of biogenic amines was performed using a linear regression analysis model and Pearson’s correlation coefficients.
Results: We obtained a good correlation of values which suggests an interchangeability of the 24-h and random urine samples. Only for epinephrine a weak correlation was determined.
Conclusions: Our findings suggest that the sample collection as single spot urine may replace 24-h collection for the detection of urinary biogenic amines by HPLC/MS-MS.
Keywords: Catecholamines; Metanephrines; 24-hour collection urine; Single spot urine; Pheochromocytomas; Paragangliomas; Neuroblastoma
| Introduction | ▴Top |
Catecholamines are a class of biogenic amines characterized by a catechol group (formed by a benzene ring with adjacent hydroxyl groups) linked to an amine group. Dopamine (DA), norepinephrine (NE) and epinephrine (E) belong to this class. They act as neurotransmitters in both central and peripheral nervous system but they are also secreted as hormones by adrenal medulla or by extra-adrenal chromaffin cells [1].
After their release, catecholamines have a very short plasmatic half-life because they are rapidly converted by various enzymatic pathways into different inactive metabolites. In particular, DA is hydroxylated by the dopamine beta-hydroxylase (DBH) enzyme into NE. Then, the phenylethanolamine N-methyltransferase (PNMT) enzyme converts NE into E. The catecholamine catabolism can be divided into two steps: in the first step, DA, NE and E are converted by the enzyme catechol-O-methyltransferase (COMT) in their methylated metabolites 3-methoxytyramine (3-MT), normetanephrine (NMN) and metanephrine (MN), respectively. In the second step, these metabolites are catalyzed by multiple pathways involving a specific oxidative deamination catalyzed by the monoamine oxidase (MAO) enzyme into homovanillic acid (HVA) and vanillylmandelic acid (VMA) [2]. The 3-MT, NMN and MN are largely transformed in their sulphate esters in the gastrointestinal tract by specific sulfotransferases. The sulphate conjugation increases hydro-solubility of molecules, promoting their renal excretion [3].
Normal levels of catecholamines and corresponding catabolites in urine samples are very variable. They mainly depend on age and gender but a small increase of these biogenic amines is also observed in various physiological (excess body weight, smoking, eating habits and the way of life) or patho-physiological conditions (such as depression or anxiety) [4]. Consequently, published reference intervals for catecholamines and their metabolites are often contradictory so the establishment of cut-off points is crucial for a correct diagnosis and a prompt treatment monitoring [5-8].
In the current clinical practice, catecholamines and their related metabolites are considered important biomarkers for the biochemical diagnosis of neuroendocrine secreting tumors, such as pheochromocytomas and paragangliomas (PPGL) and NB [9]. In fact, elevated levels of these biogenic amines are produced in patients with these tumors, causing severe hypertension and cardiovascular consequences [10].
Standard practices for the detection of urinary catecholamines and their metabolites requires the use of urine samples derived from 24-h collections, because circadian fluctuations in their excretion have been observed [11-14]. The collection of 24-h urine samples cannot be performed easily, especially for children: it is time-consuming, it is sometimes difficult to obtain a complete collection and for some patients it may require catheterization. Collecting 24-h urine specimens is not only problematic in infants but also for old patients (for numerous old patients, 24-h urine collections could be incomplete or they could represent collections for more than 24 h). Moreover, 24-h urine collections must be correctly acidified to preserve the basic degradation of molecules before their detection [15-17].
Spot urines represent an attractive alternative for the measurement of catecholamines and corresponding metabolites but, as previously described, their use is not recommended due to the existence of circadian cycle variations in the molecules excretion [13, 14]. Several studies demonstrate that the correction of spot urinary HVA and VMA outputs for creatinine excretion can be used for the diagnosis of NB in children [5, 18-20]. Little is known about the other biomarkers generally used for PPGL diagnosis such as NE, E, 3-MT, free and total NMN, and free and total MN and in most studies, they are especially quantified using older methods [20-24].
In our work, we enrolled 50 adult patients with clinical symptoms related to PPGL (episodic tachycardia, paroxysmal hypertension, anxiety, headache and sweating) and we determined the concentration values of catecholamines (DA, NE and E) and their methylated metabolites (3-MT, MN, and NMN) both in spot and 24-h urine samples, using high-performance liquid chromatography tandem mass spectrometry (HPLC/MS-MS). This analytical technique is considered the gold standard for the quantification of urinary catecholamines and metanephrines, due to its high specificity and sensitivity [25-31]. To eliminate the variability of the metabolite renal excretion, we expressed all the obtained results as ratio to urinary creatinine concentration, previously determined for each patient and for each type of sample. The aim of this study was to determine how closely correlated are spot and 24-h urinary DA, NE, E, 3-MT, free and total NMN, and free and total MN excretions, in order to estimate the loss of power due to the replacement of 24-h collection by spot collection.
| Materials and Methods | ▴Top |
Standards and specimens
A urine spot sample followed by a 24-h urine sample was collected from 50 patients with clinical symptoms related to PPGL (episodic tachycardia, paroxysmal hypertension, anxiety, headache and sweating) and delivered to the Clinical Pathology and Microbiology Unit of “Vito Fazzi” Hospital in Lecce (Puglia, Italy). Patients were included in the present study according to clinical suspicion of PPGL, in presence of signs and symptoms of catecholamine excess, which has been assayed to establish a diagnosis. The general clinical characteristics of the study population are reported in Table 1. To avoid possible exogenous interference, patients did not undergo any treatment nor take any medicines that may affect catecholamines metabolism within 48 h before sampling. Drinking, smoking or eating caffeinated foods were avoided. The 24-h urine samples were acidified by each patient adding 10 mL of hydrochloric acid (33% v/v), before the beginning of collection. On receipt in the laboratory, urine specimens were checked for adequate acidity (pH 2 - 5), stored at -20 °C and analyzed within 2 months. Samples not respecting all mentioned criteria of correct collection and storage were excluded from analysis. According to the manufacturer’s instructions, seven reconstituted urine standards (LC 77010, Eureka Chemicals Laboratories, Italy) were used for the calibration curve and three different concentrations (low, medium and high) of quality control (QC) samples (LC 77010, Eureka Chemicals Laboratories, Italy) were used for method validation. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
 Click to view | Table 1. General Clinical Characteristics of the Study Population |
HPLC/MS-MS conditions
Urinary DA, NE, E, 3-MT, free and total NMN, and free and total MN concentrations of standards, controls and patients were determined using a 4500 QTrap HPLC/MS-MS system (AB Sciex, USA). The acid hydrolysis of each urine sample was used to release the total NMN or MN from their sulphate-bound metabolites. Before HPLC, catecholamines and their metabolites were separated from the urine matrix by solid phase extraction (SPE). After sample pretreatment, 10 µL of each purified extract was injected on the HPLC column. The chromatography separation was performed on a ACQUITY UPLC® BEH Amide column (2.1 × 100 mm, 1.7 µm) with a temperature set of 30 °C and a total constant flow of 0.600 mL/min. Pre-injection equilibration lasted for 10 min. A binary gradient was used, as described in Table 2.
 Click to view | Table 2. Binary Gradient Used for HPLC |
Biomarkers eluted from the column at known retention times passed into the mass spectrometer where they were ionized by electrospray ionization (ESI). Analyst software from AB Sciex was used for instrument control and analysis. Each ion was separated according to the mass/charge ratio and appeared as a chromatographic peak. Its area was directly related to concentration. The MS/MS detection was conducted in positive ionization multiple reaction monitoring (MRM) mode. MRM transitions and retention time parameters of each ion are summarized in Table 3. For each biomarker, there was a stable internal standard, labelled with isotopes, which guaranteed reproducible and reliable quantitative results. The total run time including column re-equilibration was 8 min for each sample.
 Click to view | Table 3. MRM Transitions and Retention Time Parameters for Catecholamines and Their Metabolites |
Urinary creatinine quantification
Urinary creatinine was assayed in all urine samples by the automated enzymatic method (Roche Diagnostics, Mannheim, Germany), following manufacturer’s instructions.
Data analysis
Each analyte was quantified as µg per g of creatinine. The reference ranges for urinary catecholamines were provided by the company producing the HPLC kit. The correlation between the levels of each biogenic amine in spot and 24-h urine samples was performed using a linear regression analysis model. Pearson’s correlation coefficients (PCCs) were calculated to test relationships between levels of catecholamines in the two types of samples, which is considered strong for values > 0.7 or intermediate for values between 0.30 and 0.70.
| Results | ▴Top |
In our study, random urine sample levels of DA, NE, E, 3-MT, NMN (free and total), and MN (free and total) were compared with those of the 24-h collection urine and a good correlation has been obtained after normalization for the creatinine concentration of the sample. Only NE and E presented a greater diurnal variation of renal excretion. The correlation plots are shown in Figure 1. The PCCs are listed in Table 4.
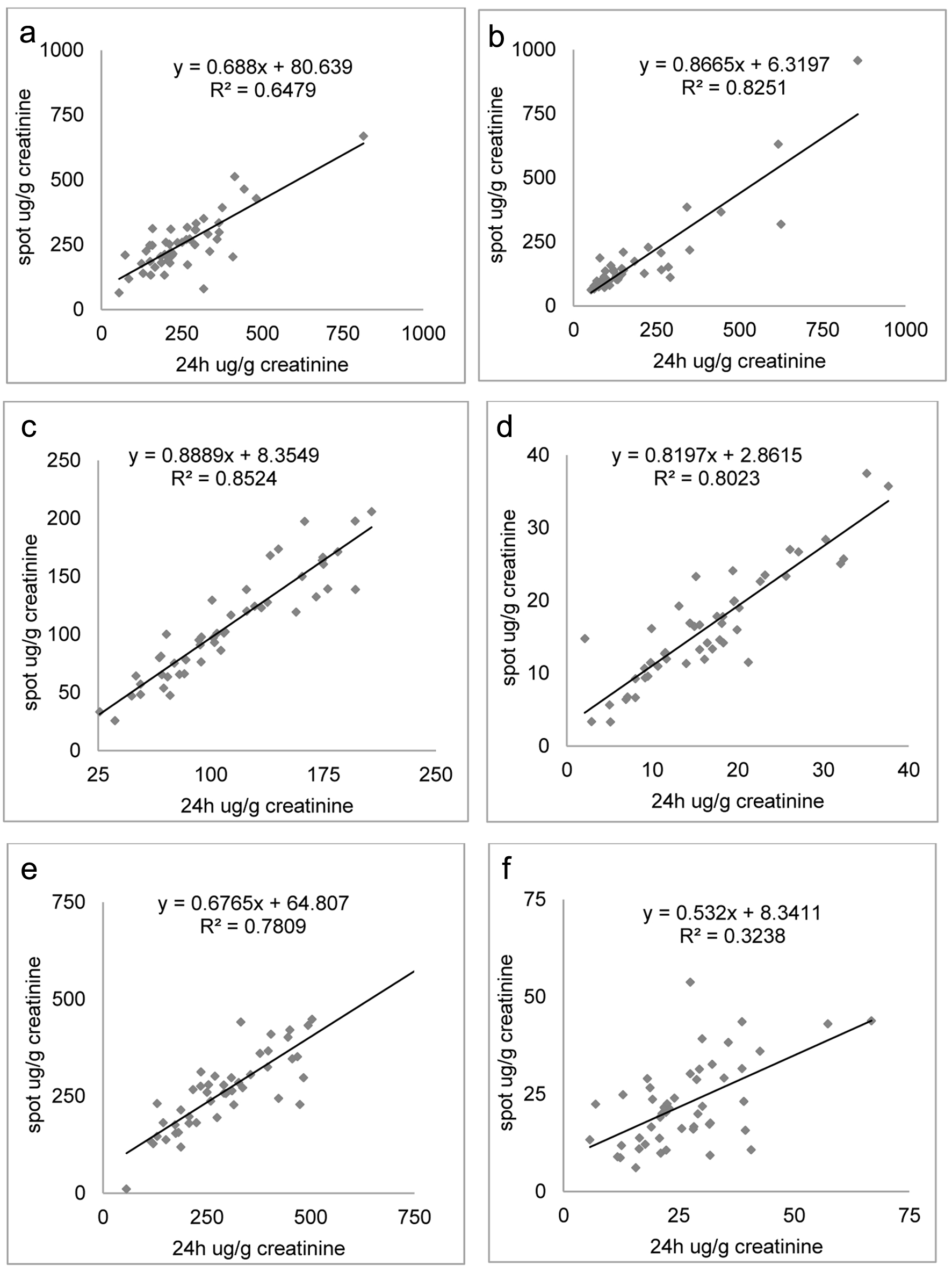 Click for large image | Figure 1. Correlation plots between dopamine (a), 3-methoxytyramine (b), total metanephrine (c), free metanephrine (d), total normetanephrine (e), and free normetanephrine (f) as a ratio to creatinine concentration measured in 24-h collection and spot urine samples. |
 Click to view | Table 4. PCC Obtained for DA, 3-MT, Total MN and Free MN, Total NMN and Free NMN and Type of Correlation Between Data of 24-h and Spot Urine Samples |
NE and E normalized quantification in random urine against 24-h urine (Fig. 2) showed weaker PCCs (0.573 for NE and 0.166 for E).
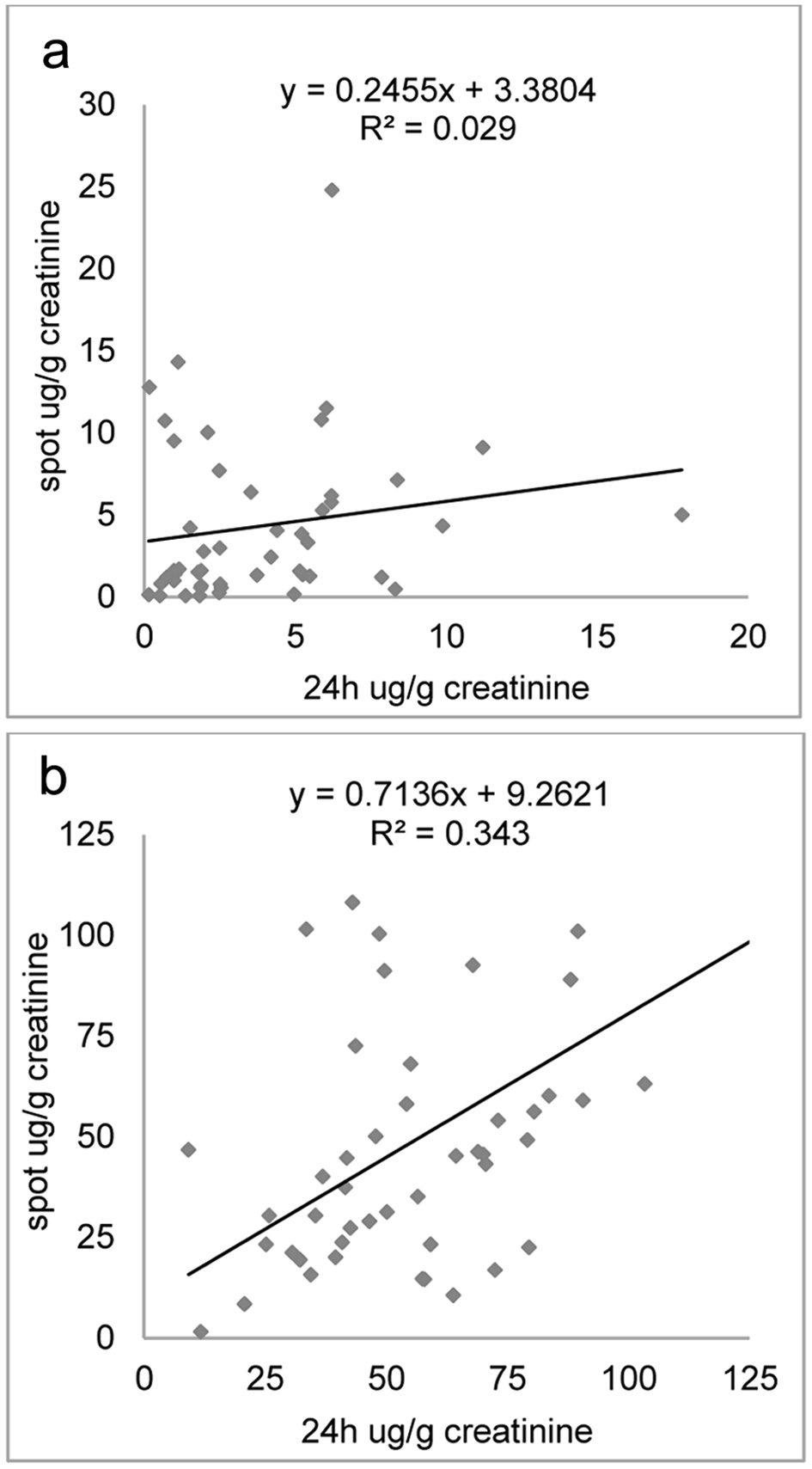 Click for large image | Figure 2. Correlation plots between epinephrine (a) and norepinephrine (b) as a ratio to creatinine concentration measured in 24-h collection and spot urine samples. |
Due to the rarity of neuroendocrine tumors (approximately estimated incidence: 0.8 per 100,000 persons/year [32]), only two of the analyzed patients showed elevated values for each biogenic amine, both for spot and 24-h urine samples related to creatinine. For these patients, the biochemical diagnosis of PPGL was confirmed by radiological images (Fig. 3) and histological analysis, with immunomorphological findings indicating the presence of adrenal pheochromocytomas. The results obtained for pathological patients are reported in Table 5.
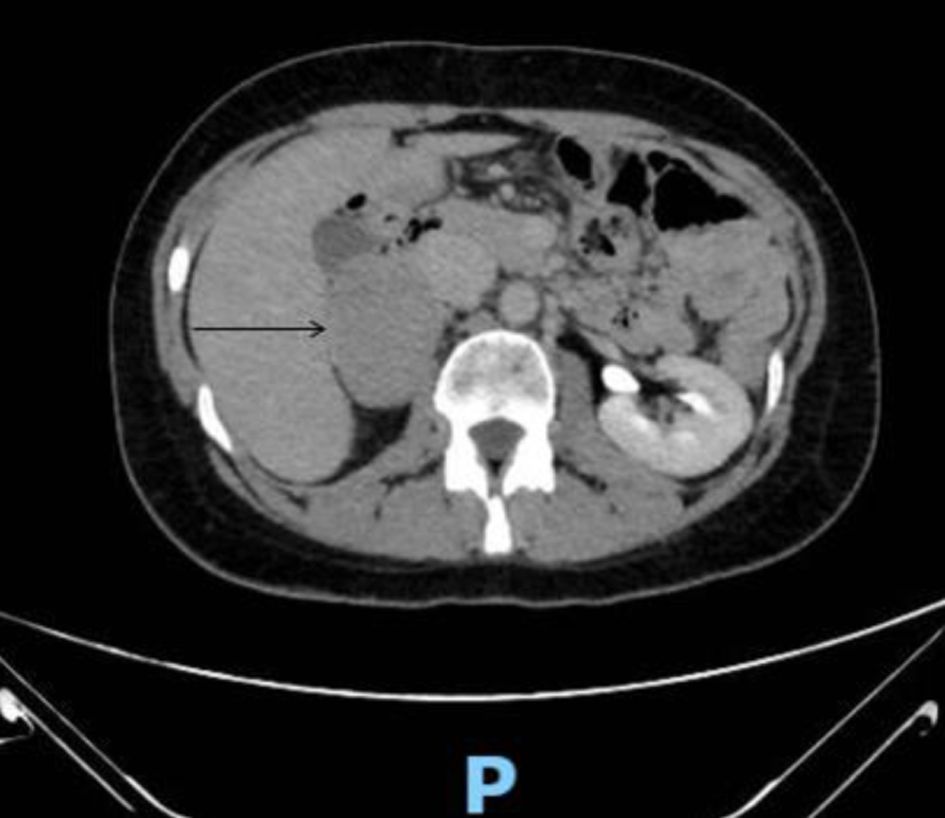 Click for large image | Figure 3. Computed tomography (CT) performed with the administration of an intravenous contrast media showed a well-defined, large and roundish mass (50 × 60 mm) in the right adrenal gland. The neoformation had a weak and uneven enhancement in the dynamic phases (arrow). |
 Click to view | Table 5. Biogenic Amines Concentrations (µg/g Creatinine) of the Two Pathological Patients, Obtained Analyzing Both Spot and 24-h Urine Samples |
Due to the small pathological sample size, these results need to be confirmed in larger studies. However, they suggest the reliability of the HPLC/MS-MS catecholamine quantification in spot urine samples by creatinine normalization also for pathological patients, when 24-h collection is not easy to be performed.
| Discussion | ▴Top |
In the present study, the correlation between urinary catecholamine concentrations and creatinine excretion was investigated both for 24-h and spot urine samples. Compared to most of the previous studies which mainly evaluated VMA and HVA [15, 16, 18, 19], in this work we also measured DA, E, NE, 3-MT, free and total MN, and free and total NMN. As demonstrated by Matser et al (2023), the accuracy of the eight catecholamine metabolites assessment was significantly higher compared to the only HVA and VMA one, for a correct diagnosis of NB, reducing false positive rate [33].
In our results, urinary creatinine levels showed a positive correlation with the excretion of DA, 3-MT, free and total MN, and free and total NMN both for 24-h and spot urines samples. When biogenic amines concentration measurements were normalized to urinary creatinine values, no difference between 24-h and spot urine samples has been found. This result suggests that the renal excretion of DA, 3-MT, free and total MN, and free and total NMN could be considered continuous. Figures 1 and 2 reveal significant correlations among these metabolites between 24-h urine collection and spot urine samples. This suggests a potential interchangeability or similarity between the two collection methods. However, for E and also NE, a weaker correlation was observed, indicating less consistency between the 24-h and random urine samples for this specific metabolite. Probably, the lack of storage granules of NE and E and their rapid conversion into VMA explained their frequent increased excretion [34].
Patients diagnosed with PPGL via radiological and histological confirmation exhibited elevated urinary catecholamine levels in both spot and 24-h urine samples (Table 5). Subsequent surgical removal of the tumors resulted in the restoration of healthy conditions for both patients.
Using spot urine samples for diagnostic purposes could offer advantages in situations in which collecting urine over a complete 24-h period proves challenging or unfeasible, particularly in cases involving pediatric patients or instances of inaccurate sample collection and storage. This approach has the potential to simplify the diagnostic pathway and enable prompt intervention for patients diagnosed with PPGL.
Our findings are in accordance with those obtained by Peitzsch et al (2020). They measured urinary catecholamine excretions at different time of the day in patients with PPGL and healthy controls and they demonstrated that HVA, VMA, DA, 3-MT, NMN and MN showed similar diagnostic sensitivities in spot urine and 24-h urine. For these biogenic amines, there were no significant differences in their day-to-night excretion. They also found that only E and NE showed an important day-to-night fluctuation in their urinary excretion for both patients and controls [35].
Sbardella et al (2020) showed no differences in the diagnostic sensitivity of spot urine sampling compared to 24-h urine collections and plasma samples when 3-MT, MN and NMN urinary concentrations were measured by HPLC-MS/MS and normalized to creatinine, although they did not distinguish total and free MN and NMN [20]. Regarding 3-MT, it was poorly investigated and our results appear very promising. In fact, spot urinary 3-MT outputs corrected for creatinine excretions could be particularly useful in NB diagnosis for children. The 3-MT is considered an important biomarker for NB because it is associated with MYC activity: elevated levels of urinary 3-MT at NB diagnosis are associated with a poor prognosis [36, 37].
Our results are supported by a recent work published by Matser et al (2023) in a retrospective study, although they performed catecholamine metabolites measurements in 24-h urine and spot urine which were not sampled within the same patients [33].
Our study had several limitations. First, as previously described, due to the rarity of neuroendocrine tumors, only two of the 50 analyzed patients were pathological and showed abnormal catecholamines levels, in both spot and 24-h urine samples normalized to creatinine. For these patients, the biochemical diagnosis of PPGL was confirmed by radiological images and histological analysis. Nonetheless, due to the small sample size, our results must be confirmed in larger studies. Second, the feasibility of catecholamine metabolite analysis in spot urine was not investigated during therapy and follow-up of the patients. Lastly, it cannot be excluded that differences in sample handling and storage may have led to differences in catecholamine concentrations.
Conclusions
The present work suggests that spot urine specimens could be used for the diagnosis of PPGL and NB, when 24-h urine sample collection is complicated. Since variations in the urinary concentration of catecholamines and corresponding metabolites during different periods of the day are due to variations in renal excretion rather than variation in production, the catecholamine/metabolite assay has to be normalized to urinary creatinine concentration for all biomolecules. Only E and NE show a weak correlation between results.
Acknowledgments
This research was supported by Eureka Chemicals Laboratories (Italy).
Financial Disclosure
None to declare.
Conflict of Interest
All other authors have no conflict of interest to disclose.
Informed Consent
A signed informed consent form was obtained from all subjects for research data collection.
Author Contributions
Conceptualization: CR, MG, GM and GL; methodology: CR, MG and GM; formal analysis: CR, GM and MG; investigation: CR, MG, GM, RAL and FI; data curation: CR, MG, GM, RAL and FI; writing - original draft preparation: CR, MG and GM; writing - review and editing: CR, MG and GM; supervision: CR, MG, GM and GL. All authors have read and agreed to the published version of the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
3-MT: 3-methoxytyramine; COMT: catechol-O-methyltransferase; DA: dopamine; DBH: dopamine beta-hydroxylase; E: epinephrine; ESI: electrospray ionization; HPLC/MS-MS: high-performance liquid chromatography tandem mass spectrometry; HVA: homovanillic acid; MAO: monoamine oxidase; MN: metanephrine; MRM: multiple reaction monitoring; NB: neuroblastoma; NE: norepinephrine; NMN: normetanephrine; PCC: Pearson’s correlation coefficient; PNMT: phenylethanolamine N-methyltransferase; PPGL: pheochromocytomas and paragangliomas; SPE: solid phase extraction; VMA: vanillylmandelic acid
| References | ▴Top |
- Lenders JW, Eisenhofer G, Mannelli M, Pacak K. Phaeochromocytoma. Lancet. 2005;366(9486):665-675.
doi pubmed - Eisenhofer G, Peitzsch M, Bechmann N, Huebner A. Biochemical diagnosis of catecholamine-producing tumors of childhood: neuroblastoma, pheochromocytoma and paraganglioma. Front Endocrinol (Lausanne). 2022;13:901760.
doi pubmed pmc - Pamporaki C, Darr R, Bursztyn M, Stephan G, Bornstein SR, Lenders JWM, Pacak K, et al. Plasma free versus deconjugated metanephrines for diagnosis of phaeochromocytoma. Clin Endocrinol (Oxf). 2013;79(4):476-483.
- Paine NJ, Watkins LL, Blumenthal JA, Kuhn CM, Sherwood A. Association of depressive and anxiety symptoms with 24-hour urinary catecholamines in individuals with untreated high blood pressure. Psychosom Med. 2015;77(2):136-144.
doi pubmed pmc - Pussard E, Neveux M, Guigueno N. Reference intervals for urinary catecholamines and metabolites from birth to adulthood. Clin Biochem. 2009;42(6):536-539.
doi pubmed - Eisenhofer G, Peitzsch M, Kaden D, Langton K, Mangelis A, Pamporaki C, Masjkur J, et al. Reference intervals for LC-MS/MS measurements of plasma free, urinary free and urinary acid-hydrolyzed deconjugated normetanephrine, metanephrine and methoxytyramine. Clin Chim Acta. 2019;490:46-54.
doi pubmed - Zeng HL, Wang X, Li HJ, Yang Q. Quantitative analysis of catecholamines and their metabolites in 491 patients with adrenal tumors: a retrospective single-center cohort study. J Cancer Res Clin Oncol. 2023;149(8):4979-4989.
doi pubmed - Boyle JG, Davidson DF, Perry CG, Connell JM. Comparison of diagnostic accuracy of urinary free metanephrines, vanillyl mandelic Acid, and catecholamines and plasma catecholamines for diagnosis of pheochromocytoma. J Clin Endocrinol Metab. 2007;92(12):4602-4608.
doi pubmed - van Berkel A, Lenders JW, Timmers HJ. Diagnosis of endocrine disease: Biochemical diagnosis of phaeochromocytoma and paraganglioma. Eur J Endocrinol. 2014;170(3):R109-119.
doi pubmed - Galetta F, Franzoni F, Bernini G, Poupak F, Carpi A, Cini G, Tocchini L, et al. Cardiovascular complications in patients with pheochromocytoma: a mini-review. Biomed Pharmacother. 2010;64(7):505-509.
doi pubmed - Peaston RT, Weinkove C. Measurement of catecholamines and their metabolites. Ann Clin Biochem. 2004;41(Pt 1):17-38.
doi pubmed - Brodeur GM, Pritchard J, Berthold F, Carlsen NL, Castel V, Castelberry RP, De Bernardi B, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11(8):1466-1477.
doi pubmed - Fibiger W, Singer G, Miller AJ, Armstrong S, Datar M. Cortisol and catecholamines changes as functions of time-of-day and self-reported mood. Neurosci Biobehav Rev. 1984;8(4):523-530.
doi pubmed - Faucheux B, Kuchel O, Cuche JL, Messerli FH, Buu NT, Barbeau A, Genest J. Circadian variations of the urinary excretion of catecholamines and electrolytes. Endocr Res Commun. 1976;3(5):257-272.
doi pubmed - Gregianin LJ, McGill AC, Pinheiro CM, Brunetto AL. Vanilmandelic acid and homovanillic acid levels in patients with neural crest tumor: 24-hour urine collection versus random sample. Pediatr Hematol Oncol. 1997;14(3):259-265.
doi pubmed - Cangemi G, Barco S, Reggiardo G, Viscardi E, Di Cataldo A, Garaventa A, Melioli G, et al. Interchangeability between 24-hour collection and single spot urines for vanillylmandelic and homovanillic acid levels in the diagnosis of neuroblastoma. Pediatr Blood Cancer. 2013;60(12):E170-172.
doi pubmed - Monsaingeon M, Perel Y, Simonnet G, Corcuff JB. Comparative values of catecholamines and metabolites for the diagnosis of neuroblastoma. Eur J Pediatr. 2003;162(6):397-402.
doi pubmed - Barco S, Gennai I, Reggiardo G, Galleni B, Barbagallo L, Maffia A, Viscardi E, et al. Urinary homovanillic and vanillylmandelic acid in the diagnosis of neuroblastoma: report from the Italian Cooperative Group for Neuroblastoma. Clin Biochem. 2014;47(9):848-852.
doi pubmed - Tuchman M, Ramnaraine ML, Woods WG, Krivit W. Three years of experience with random urinary homovanillic and vanillylmandelic acid levels in the diagnosis of neuroblastoma. Pediatrics. 1987;79(2):203-205.
pubmed - Sbardella E, Maunsell Z, May CJH, Tadman M, James T, Jafar-Mohammadi B, Isidori AM, et al. Random 'spot' urinary metanephrines compared with 24-h-urinary and plasma results in phaeochromocytomas and paragangliomas. Eur J Endocrinol. 2020;183(2):129-139.
doi pubmed - White IR, Brunner EJ, Barron JL. A comparison of overnight and 24 hour collection to measure urinary catecholamines. J Clin Epidemiol. 1995;48(2):263-267.
doi pubmed - Sullivan JM, Solomon HS. The diagnosis of pheochromocytoma. Overnight excretion of catecholamine metabolites. JAMA. 1975;231(6):618-619.
pubmed - Ganguly A, Henry DP, Yune HY, Pratt JH, Grim CE, Donohue JP, Weinberger MH. Diagnosis and localization of pheochromocytoma. Detection by measurement of urinary norepinephrine excretion during sleep, plasma norepinephrine concentration and computerized axial tomography (CT-scan). Am J Med. 1979;67(1):21-26.
doi pubmed - Kaplan NM, Kramer NJ, Holland OB, Sheps SG, Gomez-Sanchez C. Single-voided urine metanephrine assays in screening for pheochromocytoma. Arch Intern Med. 1977;137(2):190-193.
pubmed - Xie Z, Lorkiewicz P, Riggs DW, Bhatnagar A, Srivastava S. Comprehensive, robust, and sensitive UPLC-MS/MS analysis of free biogenic monoamines and their metabolites in urine. J Chromatogr B Analyt Technol Biomed Life Sci. 2018;1099:83-91.
doi pubmed pmc - Taylor RL, Singh RJ. Validation of liquid chromatography-tandem mass spectrometry method for analysis of urinary conjugated metanephrine and normetanephrine for screening of pheochromocytoma. Clin Chem. 2002;48(3):533-539.
pubmed - Whiting MJ. Simultaneous measurement of urinary metanephrines and catecholamines by liquid chromatography with tandem mass spectrometric detection. Ann Clin Biochem. 2009;46(Pt 2):129-136.
doi pubmed - Gabler J, Miller A, Wang S. A simple liquid chromatography-tandem mass spectrometry method for measuring metanephrine and normetanephrine in urine. Clin Chem Lab Med. 2011;49(7):1213-1216.
doi pubmed - Clark ZD, Frank EL. Urinary metanephrines by liquid chromatography tandem mass spectrometry: using multiple quantification methods to minimize interferences in a high throughput method. J Chromatogr B Analyt Technol Biomed Life Sci. 2011;879(31):3673-3680.
doi pubmed - Lu H, Yu J, Wang J, Wu L, Xiao H, Gao R. Simultaneous quantification of neuroactive dopamine serotonin and kynurenine pathway metabolites in gender-specific youth urine by ultra performance liquid chromatography tandem high resolution mass spectrometry. J Pharm Biomed Anal. 2016;122:42-51.
doi pubmed - Kushnir MM, Urry FM, Frank EL, Roberts WL, Shushan B. Analysis of catecholamines in urine by positive-ion electrospray tandem mass spectrometry. Clin Chem. 2002;48(2):323-331.
pubmed - Haap M, Blaschka F, Lehmann R, Hoyer A, Mussig K. Association Between Urinary Catecholamine Excretion and Urine Volume. Horm Metab Res. 2019;51(8):531-538.
doi pubmed - Matser YAH, Verly IRN, van der Ham M, de Sain-van der Velden MGM, Verhoeven-Duif NM, Ash S, Cangemi G, et al. Optimising urinary catecholamine metabolite diagnostics for neuroblastoma. Pediatr Blood Cancer. 2023;70(6):e30289.
doi pubmed - Itoh T, Omori K. Biosynthesis and storage of catecholamines in pheochromocytoma and neuroblastoma cells. J Lab Clin Med. 1973;81(6):889-896.
pubmed - Peitzsch M, Kaden D, Pamporaki C, Langton K, Constantinescu G, Conrad C, Fliedner S, et al. Overnight/first-morning urine free metanephrines and methoxytyramine for diagnosis of pheochromocytoma and paraganglioma: is this an option? Eur J Endocrinol. 2020;182(5):499-509.
doi pubmed - Verly IRN, Matser YAH, Leen R, Meinsma R, Fiocco M, Koster J, Volckmann R, et al. Urinary 3-methoxytyramine is a biomarker for MYC activity in patients with neuroblastoma. JCO Precis Oncol. 2022;6:e2000447.
doi pubmed pmc - Verly IRN, van Kuilenburg ABP, Abeling N, Goorden SMI, Fiocco M, Vaz FM, van Noesel MM, et al. 3-Methoxytyramine: An independent prognostic biomarker that associates with high-risk disease and poor clinical outcome in neuroblastoma patients. Eur J Cancer. 2018;90:102-110.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


