| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 16, Number 2-3, March 2024, pages 46-55
Therapeutic Potential of Buprenorphine in Depression: A Meta-Analysis of Current Evidence
Siddhi Bhivandkara, n, Zouina Sarfrazb, Lakshit Jainc, Anil Bachud, Palash Kumar Maloe, Michael Hsuf, Shahana Ayubg, Laxmi Poudelh, Harendra Kumari, Hanyou Lohj, Faria Tazink, Saeed Ahmedl, Joji Suzukim
aDepartment of Psychiatry, Boston University Medical Center/Boston University School of Medicine, Boston, MA, USA
bDepartment of Psychiatry, Fatima Jinnah Medical University, Lahore, Pakistan
cDepartment of Psychiatry, Connecticut Valley Hospital, Middletown, CT, USA
dDepartment of Psychiatry, Baptist Health - UAMS Psychiatry Residency Program, North Little Rock, AR, USA
eDepartment of Psychiatry, Centre for Brain Research, Indian Institute of Science, Bengaluru, India
fDepartment of Psychiatry, Emory University School of Medicine, Atlanta, GA, USA
gInstitute of Living/Hartford Hospital Hartford, Hartford, CT, USA
hDepartment of Psychiatry, Nepalgunj Medical College, Nepalgunj, Banke, Nepal
iDow University of Health Sciences, Karachi, Pakistan
jNational University of Singapore, Singapore, Singapore
kDepartment of Psychiatry, East Liverpool City Hospital, East Liverpool, OH, USA
lSaint Francis Hospital and Medical Center, Hartford, CT, USA
mDepartment of Psychiatry, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, USA
nCorresponding Author: Siddhi Bhivandkar, Department of Psychiatry, Boston University Medical Center/Boston University School of Medicine, Boston, MA, USA
Manuscript submitted November 14, 2023, accepted March 6, 2024, published online March 16, 2024
Short title: Therapeutic Potential of BUP in Depression
doi: https://doi.org/10.14740/jocmr5050
| Abstract | ▴Top |
Background: Emerging research indicates buprenorphine, used in management of opioid use disorder, has attracted interest for its potential in treating a variety of psychiatric conditions. This meta-analysis aimed to determine the efficacy of buprenorphine in treating symptoms of depression.
Methods: Using Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines, a search was conducted of several databases until April 25, 2022, for English language articles related to buprenorphine and its use in treating various mental health conditions. Standardized mean differences (SMDs) and its 95% confidence intervals (CIs) were reported for the Hamilton Rating Scale for Depression (HAM-D) and the Montgomery-Asberg Depression Rating Scale (MADRS) scores. Statistical analyses were performed using Cochrane RevMan 5.
Results: Of the 1,347 identified studies, six clinical trials were included. MADRS-10 least square mean difference (LSMD) inter-group assessment favored buprenorphine over placebo, but it lacked statistical significance. Similarly, MADRS scores as well as HAM-D inter-group assessment were in favor of buprenorphine, however, were not statistically significant. These findings suggest a potential therapeutic role for buprenorphine in treating depression, albeit with caution due to the observed lack of statistical significance and the potential for confounding factors.
Conclusions: Preliminary evidence suggests potential efficacy of buprenorphine at lower doses in improving improving outcomes specifically related to depression. However, due to limitations in statistical significance and possible confounding factors, entail cautious interpretation. Further rigorous research is needed to investigate the long-term effects, optimal dosing, and determine the role of adjuvant drug therapy.
Keywords: Buprenorphine; Depression; Major depressive disorder; Treatment-resistant depression; Antidepressant; Buprenorphine/naloxone; Buprenorphine-based treatment
| Introduction | ▴Top |
The World Health Organization (WHO) estimates that over 320 million people suffer from major depression worldwide, with a prevalence that increased by 18.4% from 2005 to 2015 [1]. Despite having a large number of antidepressants available, it is reported that many patient with major depressive disorder (MDD) experience partial response (between 25% and 49% on a rating scale) or no response (less than 25% improvement on a depression rating scale), after receiving adequate doses for an adequate duration [2]. The STAR*D trial, the largest study on antidepressants, also reports that only 30% of patients with MDD achieve remission with initial treatment of citalopram (selective serotonin reuptake inhibitor (SSRI)), and remission rates drop even further in treatment-resistant depression (TRD) [3-5]. Without achieving remission, patients with MDD frequently demonstrate neuro-progressive clinical characteristics, such as recurring episodes of increasing severity, a reduced therapeutic response, and persistence of residual depressive symptoms, all of which lead to increased functional impairment, decreased quality of life, higher rates of chronicity in patients with MDD. These poor outcomes create a sense of urgency to look for alternative treatments for MDD [2, 6, 7].
The endogenous opioid system known for its role in mood regulation has recently gained popularity for its potential antidepressant properties. Buprenorphine, in particular, has been investigated for its therapeutic potential in the treatment of MDD [8]. While more research is needed to fully understand the mechanisms behind the antidepressant effects of opioids and to determine their safety and efficacy in the treatment of MDD, they may provide a useful option for patients who do not respond to other treatments.
Buprenorphine is a partial agonist of µ-opioid receptors and an antagonist of κ- and δ-opioid receptors. It binds to µ- and κ-opioid receptors with high affinity and to δ-opioid receptors with lower affinity [9]. Buprenorphine’s unique pharmacological profile, as a partial agonist of the µ-opioid receptor, makes it a standard of care for the management of opioid use disorder. Although the antidepressant and mood-elevating effects of buprenorphine have been recognized for decades [10], concerns over diversion, misuse potential, risk of initiating dependence, and the possibility of respiratory depression, even if low, have limited the broader application of buprenorphine as an antidepressant. Nevertheless, there is currently growing interest in investigating the involvement of the endogenous opioid system in affective disorders. Research on use of full µ-opioid receptors agonists, whose activation is known to mediate analgesic and euphoric effects, is limited due to their abuse and addiction potential.
Several theories discuss dysregulation of the endogenous µ- and κ-opioid system in both depression and opioid use disorder [11]. The role of beta endorphins has been suggested in pathophysiology of MDD and opioid use disorder [12]. Postmortem studies have found an association between endorphin deficiencies and conditions such as depression and suicidality [11]. In the same context, as a potent κ-receptor antagonist, buprenorphine shows potential for treatment of suicidal ideation and depressive disorders [13]. Human studies have demonstrated antidepressant-like effects, showing promise beyond its primary use in opioid addiction treatment. Notably, it has been linked with a decrease in suicidal ideation, even in suicidal patients who do not have substance use disorders [14]. Buprenorphine has been investigated as a treatment option for chronically depressed patients who have not responded to antidepressants and electroconvulsive therapy (ECT). In one study, these patients showed rapid improvement under buprenorphine treatment over a 1-week period [15]. The response to treatment was even quicker in older patients with TRD who received low-dose buprenorphine [16]. Based on these findings, buprenorphine may provide a viable option for patients who have had limited success with other treatment alternatives, in the treatment of various psychological conditions such as MDD, suicidal ideation, and some psychotic and anxiety symptoms [13]. The purpose of this study was to evaluate the pharmacological potential of buprenorphine in the management of MDD and TRD.
| Materials and Methods | ▴Top |
Institutional Review Board approval and ethical compliance with human study are not applicable.
Identification and study selection
In accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 2020 statement guidelines, a search was conducted of databases PubMed, Scopus, EMBASE, Web of Science, and the Cochrane Library, and ClinicalTrials.Gov. Additional searches were conducted using Google Scholar. The search queries were from inception until April 25, 2022. Only English language articles were included. These keywords included “buprenorphine”, “depression”, “major depressive disorder”, “treatment-resistant depression”, “antidepressant”, “opioid substitution therapy”, “buprenorphine/naloxone”, “buprenorphine-based treatment”, “comorbidity”, “mood disorder”, “psychiatric symptoms”, “clinical trial”, “randomized controlled trial”. Randomized clinical trials with active interventional and placebo arms that examined the mental health outcomes with use of buprenorphine as primary or adjunct treatment of depression were included. Studies that did not assess buprenorphine were excluded. Participants were required to be 20 and older and diagnosed with MDD using the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), DSM-IV TR or DSM-V. Two investigators (SA, SB) determined the eligibility of the full-text studies obtained using the inclusion criteria. Any cohorts, case series, case reports, comments, opinion pieces, unpublished studies, conference posters, and abstracts were omitted. Any conflicts between the two investigators (SA, SB) regarding the eligibility of full-text studies obtained using the inclusion criteria were resolved by involving a third investigator (LJ). In case the data was incompatible with the outcomes of this study, the study was omitted. The PRISMA flowchart is attached in Figure 1.
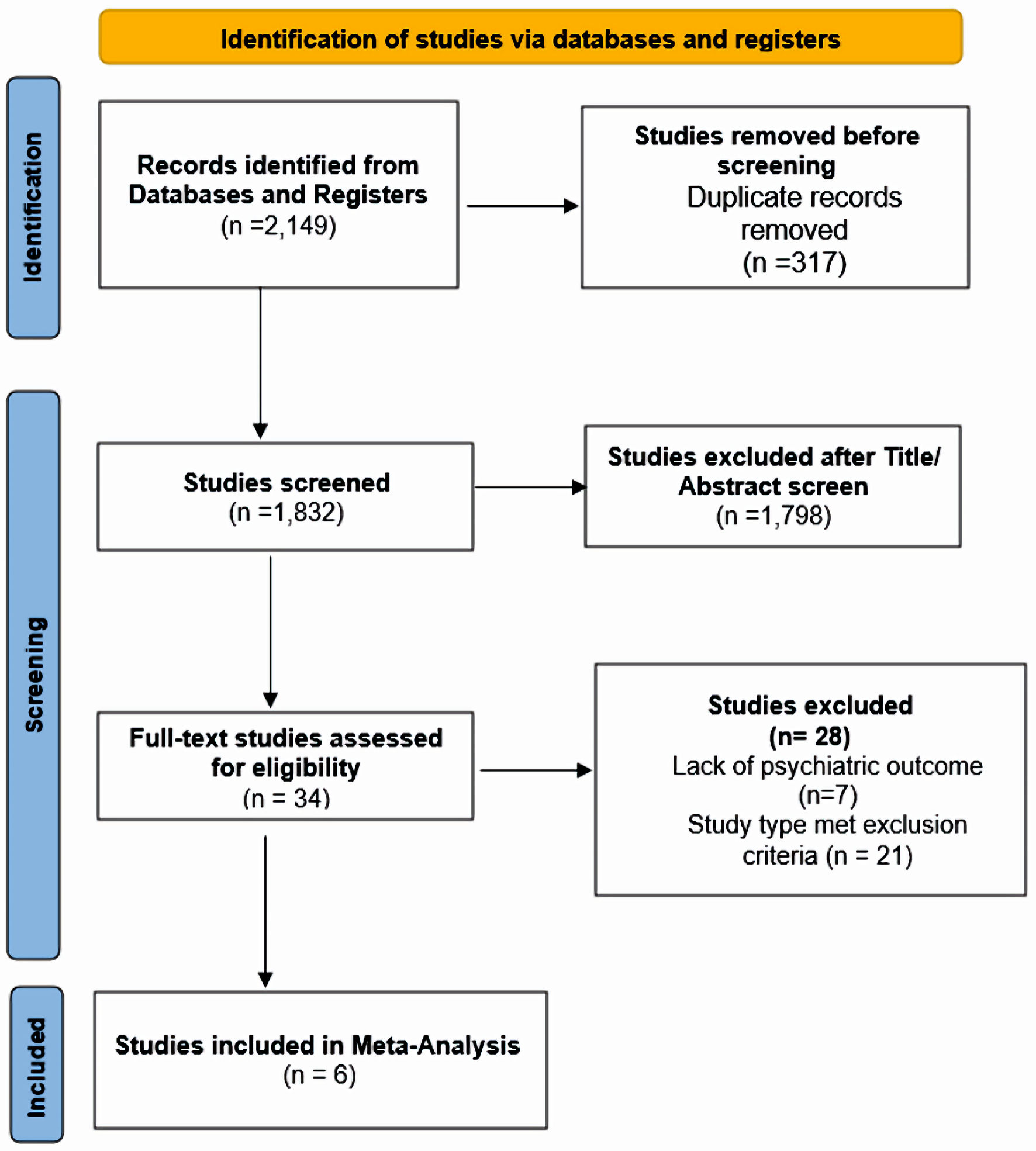 Click for large image | Figure 1. Search flowchart. |
Statistical analysis
Three investigators (SB, LJ and ZS) extracted the data onto a customized shared spreadsheet with the following headings: study and author, title, study design, sample characteristics, country, study outcomes, drug and dosage, and findings. Using quantitative analytical methodology, efficacy of buprenorphine on depression was determined. Hedge’s g standardized mean differences (SMDs) and its 95% confidence intervals (CIs) were calculated for the following: 1) Hamilton Rating Scale for Depression (HAM-D); 2) Montgomery-Asberg Depression Rating Scale (MADRS).
Meta-analysis
Six studies (Ehrich et al (2015) [11], Lee et al (2022) [8], Lin et al (2019) [17], Zajecka et al (2019) [18], Fava et al (2016) [19], and Fava et al (2018) [20]) were assessed using the Population, Intervention, Comparison and Outcomes (PICO) format recommended by the Cochrane Collaboration, and were found eligible to include in a meta-analysis [8, 11, 17-20]. Among these, two studies (Fava et al 2018 [20] and Zajecka et al (2019) [18]) reported least square mean difference (LSMD) of MADRS and its standard error. Thus, a generic inverse-variance method was adopted within the random-effects framework. In Fava et al (2018) [20], since FORWARD-4 could not achieve the primary endpoint, pooled MADRS-10 LSMD from FORWARD-4 and FORWARD-5 baseline to the end of treatment (EOT) was used. Hedge’s g SMDs and its 95% CIs were calculated for the studies reported MADRS (Ehrich et al (2015) [11], Fava et al (2016) [19], Lin et al (2019) [17], and Lee et al (2022) [8]) and HAM-D (Ehrich et al (2015) [11] and Fava et al (2016) [19]) using inverse-variance based DerSimonian and Laird’s estimation method. The intention-to-treat analysis was adopted, and analyses were performed using Cochrane RevMan 5.3. Finally, the statistical significance was checked at 5% level of significance, and the impact of heterogeneity was measured by I2-statistic.
Quality assessment
The Cochrane risk of bias tool was utilized to assess the risk of bias in studies included in this meta-analysis. The risk of bias tool assesses a specific set of domains, which includes trial design, conduct, and reporting. The findings were reported as “low risk”, “high risk”, or “unclear risk”.
| Results | ▴Top |
The characteristics of all included studies are listed in Table 1 [8, 11, 17-20]. Fava et al (2018) [20] and Zajecka et al (2019) [18] with a substantial large sample size, 122 and 295, respectively, reported MADRS-10 LSMD. Pooled LSMD from Figure 2 indicated that buprenorphine was more efficacious than placebo but was not statistically significant (pooled LSMD: -0.94, 95% CI: -2.31 to 0.43; z = 1.35; P value = 0.18; I2 = 0%). In addition, the overall summary measure across the four studies with MADRS (SMD (95% CI): -0.02 (-0.68, 0.63); z = 0.07, P value = 0.95; I2 = 75%) (Fig. 3) and two studies with HAM-D (SMD (95% CI): -0.98 (-2.61, 0.66); z = 1.17, P value = 0.24; I2 = 82%) (Fig. 4) were also found statistically insignificant. The HAM-D scores across studies were more heterogeneous compared to MADRS scores.
 Click to view | Table 1. Characteristics of Included Studies |
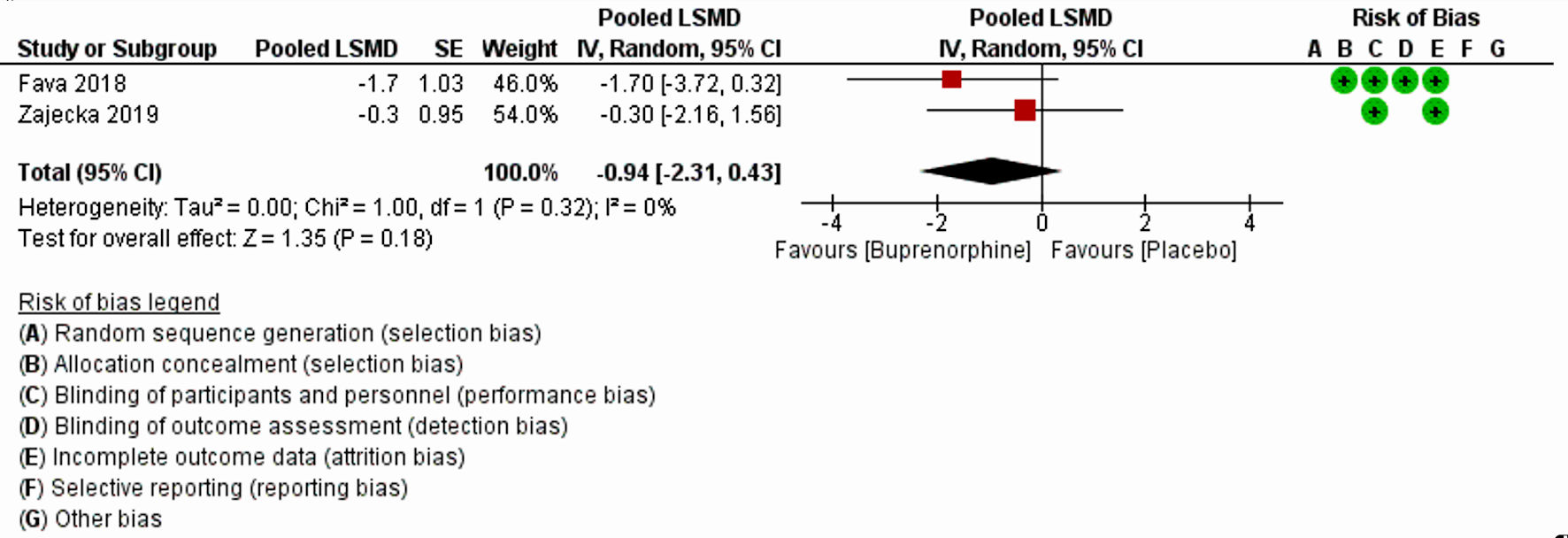 Click for large image | Figure 2. Forest plot for the studies reported MADRS least square mean difference. “+” and “-” signs indicate low and high risk of producing biased results, respectively. LSMD: least square mean difference; CI: confidence interval; SE: standard error; MADRS: Montgomery-Asberg Depression Rating Scale. |
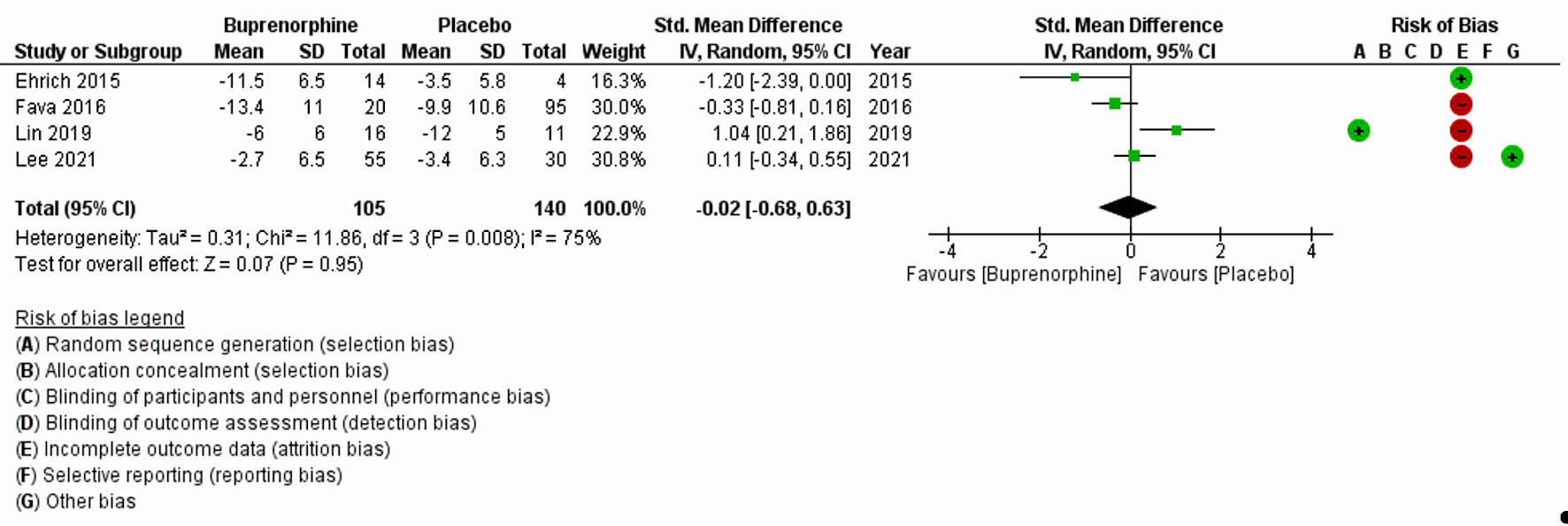 Click for large image | Figure 3. Forest plot for the studies reported MADRS. “+” and “-” signs indicate low and high risk of producing biased results, respectively. SD: standard deviation; CI: confidence interval; MADRS: Montgomery-Asberg Depression Rating Scale. |
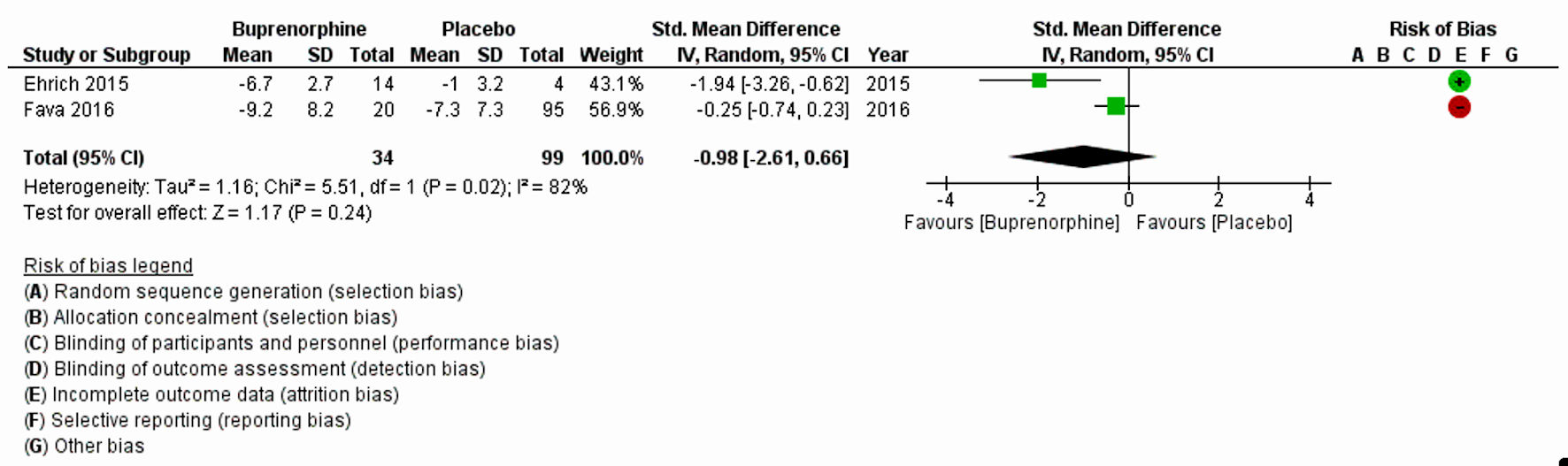 Click for large image | Figure 4. Forest plot for the studies reported HAM-D. “+” and “-” signs indicate low and high risk of producing biased results, respectively. SD: standard deviation; CI: confidence interval; HAM-D: Hamilton Rating Scale for Depression. |
Risk of bias assessments
The risk of bias assessment for the studies with LSMD (Fig. 2) showed no risk of performance and attrition biases but carried 50% risk of selection and detection biases. Moreover, studies with MADRS (Fig. 3) and HAM-D (Fig. 4) scores had a high risk of attrition bias, but other risks were unknown. Overall, Figure 5 indicates that less than 25% of the included studies adopted random sequence generation, allocation concealment and blinding of outcomes. Furthermore, 50% of the studies failed to use appropriate statistical models to accommodate missingness in the study outcomes. However, we adopted an intention-to-treat analysis, wherein the total sample size randomized for the buprenorphine and the placebo groups at the beginning of the study was considered instead of completers. Therefore, we made an attempt to minimize the effect of attrition bias in our findings. The reporting bias in all the studies were unclear; and other biases were unknown.
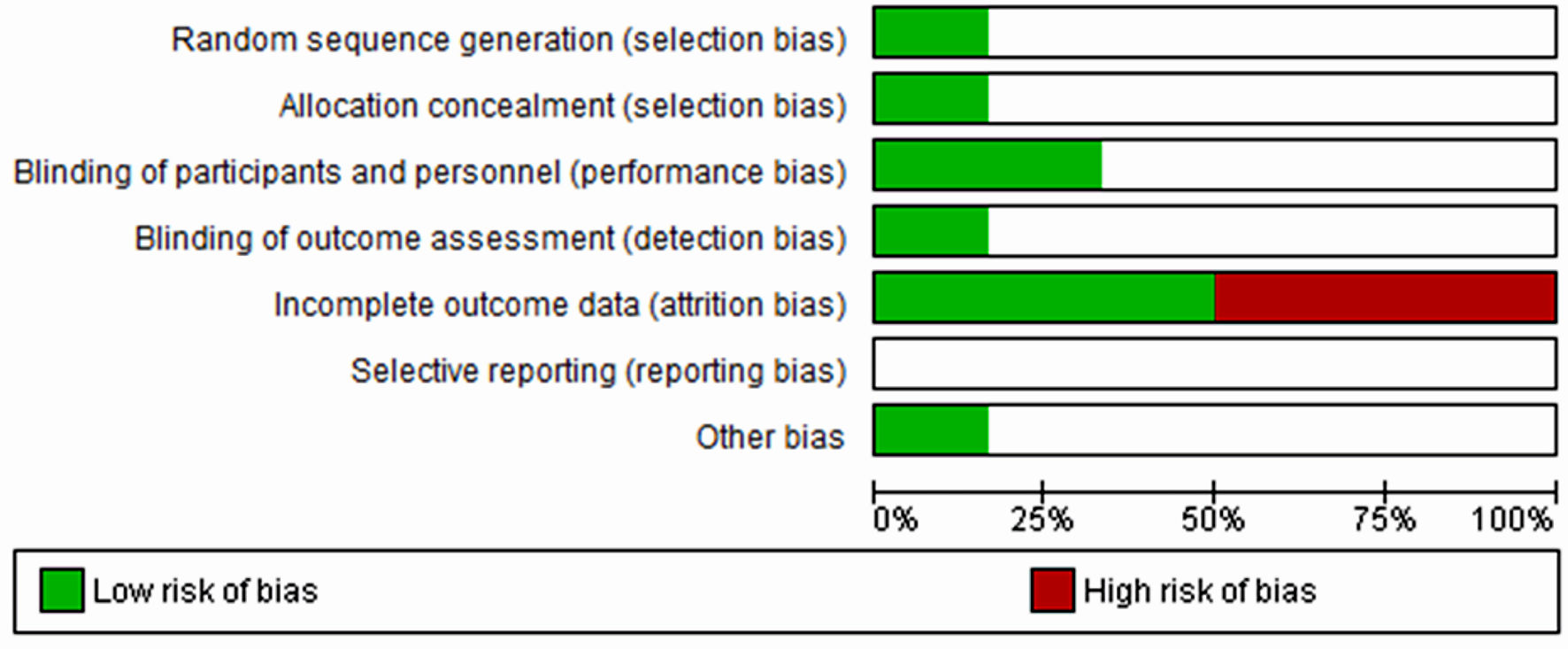 Click for large image | Figure 5. Overall risk of bias graph across all included studies. The white area indicated unclear risk of bias. |
| Discussion | ▴Top |
We conducted a meta-analysis reviewing randomized controlled trials (RCTs) investigating the therapeutic value of buprenorphine in the treatment of depression by evaluating the efficacy on various outcomes related to depression.
The first analysis comprised of two RCTs (Zajecka et al (2019) [18] and Fava et al (2018) [20]), with 295 and 122 participants, respectively. Analysis based on MADRS-10 LSMD revealed that buprenorphine was more efficacious than placebo at treating depression, but this did not reach statistical significance (P = 0.18). The second analysis included four clinical trials (Ehrich et al (2015) [11], Fava et al (2016) [19], Lin et al (2019) [17] and Lee et al (2022) [8]), with a total of 245 participants and found that buprenorphine may be efficacious at improving symptoms of depression, as measured by the MADRS. The results were not statistically significant, but there was a high level of heterogeneity among the studies. The third analysis included two trials (Ehrich et al (2015) [11] and Fava et al (2016) [19]), with a total of 34 participants who received buprenorphine and 99 controls which reported that buprenorphine may be efficacious at improving depression scores, as measured by the HAM-D. However, the results were again not statistically significant (P = 0.24).
There are a few differences between the results measured using the MADRS, MADRS LSMD, and the HAM-D. First, the number of trials and participants varied across the three sets of results. The MADRS LSMD results were based on two trials with 417 participants, the MADRS results were based on four trials with 245 participants, and the HAM-D results were based on two trials with 133 participants. Second, the effect sizes of buprenorphine on the outcome measures differed. For the MADRS LSMD, the effect size favored the use of buprenorphine with a large effect (pooled LSMD = -0.94). For the MADRS, the effect size favored the use of buprenorphine with a small effect (Hedge’s g SMD = -0.02). For the HAM-D, the effect size favored the use of buprenorphine with a large effect (Hedge’s g SMD = -0.98). Third, the level of heterogeneity (differences among the studies) also differed. The MADRS-10 LSMD results had a low level of heterogeneity (I2 = 0.0%), MADRS results had a high level of heterogeneity (I2 = 75%) and the HAM-D results had a high level of heterogeneity (I2 = 82%).
The results of our study suggest that buprenorphine may have the potential to improve depressive symptoms. Buprenorphine’s therapeutic potential in opioid addiction and mood disorders can be attributed to its unique pharmacodynamic properties, primarily its interactions with µ- and κ-opioid receptors. As a partial agonist at µ-opioid receptors, buprenorphine effectively mitigates withdrawal symptoms and cravings associated with opioid addiction, offering a safer alternative due to its ceiling effect on respiratory depression. This contrasts with full agonists like morphine or fentanyl, which pose a higher risk of overdose and dependency. Concurrently, buprenorphine’s antagonist action at κ-opioid receptors is pivotal in its mood-modulating effects [8]. κ-receptor activity is generally linked to dysphoria and negative mood states; hence, buprenorphine’s antagonism at these sites may alleviate depressive and anxiety symptoms often co-occurring in opioid use disorders. This dual action places buprenorphine in a unique position, not only addressing the core issue of opioid dependency but also potentially ameliorating accompanying mood disturbances, an aspect not typically addressed by traditional opioid agonists. κ-opioid receptor antagonists are also being explored for their potential in treatment of depressive disorders [21, 22].
The results of our meta-analysis do not support the use of buprenorphine as monotherapy for depression. However, there is limited evidence to suggest that buprenorphine is a promising adjunctive therapy for the rapid relief of depressive symptoms among patients with MDD and TRD, especially for those with a comorbid opioid use disorder [23]. When administered in combination with samidorphan at a dose of 2 mg/2 mg, buprenorphine significantly improved depressive symptoms after 4 weeks among patients with inadequate response to one or two antidepressants [11, 19]. An open label trial with a dose of 0.4 mg showed a significant decrease in depression severity in the first 3 weeks [16]. However, one study suggests that micro doses of buprenorphine (0.2 - 1.2 mg) mono product may not confer the same degree of improvement [8, 17]. The antidepressant and suicide prevention effects of buprenorphine in humans may be mediated by its action at the µ-opioid receptor and its antagonism at the κ-opioid receptor [8, 11, 13, 17]. Buprenorphine is generally well tolerated, with the most common side effects being constipation, diarrhea, vomiting, and dizziness [24].
Selection of an adjunctive treatment for TRD requires careful weighing of risks and benefits. One head-to-head randomized control trial with buprenorphine at 16 mg and 32 mg doses and ketamine showed that both significantly reduced depression symptoms and suicidal ideation among adults with comorbid MDD and opioid use disorder (OUD) at the end of a 4-day study, with no significant difference across treatment groups. More head-to-head studies with other adjunctive antidepressant treatments such as aripiprazole, transcranial magnetic stimulation (TMS), psilocybin, and ketamine are needed to understand their comparative efficacy. Further studies are needed to understand the durability of buprenorphine’s antidepressant treatment effect and course of treatment needed to sustain remission. In terms of adverse effects, buprenorphine is not associated with sexual or metabolic side effects, or onset of psychosis, which are potential advantages compared to other adjunctive treatments for depression such as SSRIs, second-generation antipsychotics, and ketamine, respectively [25-27]. Clinicians should be aware of possible respiratory depression especially when combined with other sedatives like benzodiazepines, though the relative risk is lower when using lower doses of buprenorphine.
The use of buprenorphine in clinical settings is accompanied by several challenges. Firstly, prescribing sublingual (SL) buprenorphine off-label for conditions like MDD or other than OUD may have legal implications in the United States. The legality and permissibility of off-label prescribing can vary depending on specific regulations, individual state laws, and insurance coverage policies. Secondly, the utilization of buprenorphine carries the risk of misuse, diversion [28], and the potential to induce physiological opioid dependence in individuals without OUD. These concerns raise significant safety issues. However, the addition of a µ-opioid receptor antagonist, such as samidorphan, can help mitigate these risks [11, 19]. This combination has been shown to be efficacious in reducing depressive symptoms in individuals with MDD and TRD [11, 19]. However, caution must be taken to not administer high doses of buprenorphine in combination with samidorphan, as adverse effects like nausea, vomiting, and dizziness have been observed [11, 19]. Finally, clinicians may not feel comfortable prescribing an opioid-based medication like buprenorphine in the treatment of depression. Further educational efforts may help address stigma and improve adoption of medications like buprenorphine.
Given these considerations, it is important for healthcare providers and policymakers to carefully evaluate the potential benefits and risks of using buprenorphine as an antidepressant. Healthcare providers should assess the individual’s risk of misuse before starting buprenorphine treatment and monitor their use closely.
Our meta-analysis has several limitations. First, the analysis included a small number of trials [8, 16, 18, 19, 24, 25], which may limit the generalizability of the findings. Second, the level of heterogeneity (differences among the studies) was moderate to high, which suggests that there may be factors contributing to the differences in the efficacy of buprenorphine among the studies included in the pool. Third, the sample sizes of the individual trials were relatively small, which may limit the statistical power of the meta-analysis to detect significant differences between the treatment and placebo groups. Despite the lack of statistical significance, the findings of our study may carry clinical implications in practical settings. While our study did not yield statistically significant results, it is worth considering the potential clinical implications in practical settings. Although LSMD and SMD were not statistically significant, results trended towards favoring buprenorphine over placebo. These trends may indicate some potential for therapeutic benefits in clinical context, particularly for patients with TRD, who often face a significant burden of disease and require immediate symptom relief. However, further research at a larger level with a larger sample size and rigorous methodology is needed to validate these findings, subsequently establishing convincing evidence in support of buprenorphine’s efficacy in these specific populations. An important methodological consideration in our analysis was the application of the intention-to-treat (ITT) principle across included studies. While the majority of studies adhered to ITT principles, enhancing our findings’ robustness, a few did not explicitly state their use of ITT. This variation in analytical approaches, reflecting a mix of strict and loose adherence to ITT principles, was thoughtfully considered in our conclusions. The diversity underscores the complexity of analyzing and interpreting pooled data from multiple studies and emphasizes the need for cautious interpretation of our meta-analysis results.
Conclusions
Current evidence suggests that low-dose buprenorphine may improve mood symptoms. The role of the µ-opioid receptor and κ-receptor antagonism in providing favorable outcomes is postulated. However, due to the lack of statistical significance and the high heterogeneity observed in our analysis, clinicians should exercise caution and carefully weigh the risks and benefits when selecting an adjunctive treatment for severe or TRD. The possibility of the observed results being due to chance or other factors has influenced our results and should be considered as well. Further research, larger, rigorous studies including RCTs, is needed to examine the long-term effects, optimize the use of subtherapeutic versus therapeutic doses, and determine the role of adjuvant drug therapy in the treatment of depression.
Acknowledgments
Thanks to Azza Sarfraz, Bibek Dhungana, Rizwan Ahmed, and Ali Mahmood Khan.
Financial Disclosure
This research received no external funding.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
No human subjects were involved in our study.
Author Contributions
Siddhi Bhivandkar: conception, data collection and/or processing, literature review, and writing. Zouina Sarfraz: data collection and/or processing, analysis and/or interpretation, and literature review. Lakshit Jain: literature review, and writing. Anil Bachu: literature review, and writing. Palash Kumar Malo: analysis and/or interpretation, writing, and critical review. Michael Hsu, Shahana Ayub, Laxmi Poudel, Harendra Kumar, Hanyou Loh, and Faria Tazin contributed to writing. Saeed Ahmed: design, supervision, and writing. Joji Suzuki: supervision.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- World Health Organization (WHO). Depression and other common mental disorders: Global Health Estimates; WHO: Geneva, Switzerland. 2017.
- Papakostas GI, Jackson WC, Rafeyan R, Trivedi MH. Inadequate response to antidepressant treatment in major depressive disorder. J Clin Psychiatry. 2020;81(3):OT19037COM5.
doi pubmed - Valenstein M. Keeping our eyes on STAR*D. Am J Psychiatry. 2006;163(9):1484-1486.
doi pubmed - Dunner DL, Rush AJ, Russell JM, Burke M, Woodard S, Wingard P, Allen J. Prospective, long-term, multicenter study of the naturalistic outcomes of patients with treatment-resistant depression. J Clin Psychiatry. 2006;67(5):688-695.
doi pubmed - Sinyor M, Schaffer A, Levitt A. The sequenced treatment alternatives to relieve depression (STAR*D) trial: a review. Can J Psychiatry. 2010;55(3):126-135.
doi pubmed - Sibille E, French B. Biological substrates underpinning diagnosis of major depression. Int J Neuropsychopharmacol. 2013;16(8):1893-1909.
doi pubmed pmc - Fekadu A, Wooderson SC, Rane LJ, Markopoulou K, Poon L, Cleare AJ. Long-term impact of residual symptoms in treatment-resistant depression. Can J Psychiatry. 2011;56(9):549-557.
doi pubmed - Lee HH, Blumberger DM, Lenze EJ, Anderson SJ, Barch DM, Black KJ, Cristancho P, et al. Low-dose augmentation with buprenorphine for treatment-resistant depression: a multisite randomized controlled trial with multimodal assessment of target engagement. Biol Psychiatry Glob Open Sci. 2022;2(2):127-135.
doi pubmed pmc - Kumar R, Viswanath O, Saadabadi A. Buprenorphine. In: StatPearls. Treasure Island (FL): StatPearls Publishing. 2024.
pubmed - Emrich HM, Vogt P, Herz A. Possible antidepressive effects of opioids: action of buprenorphine. Ann N Y Acad Sci. 1982;398:108-112.
doi pubmed - Ehrich E, Turncliff R, Du Y, Leigh-Pemberton R, Fernandez E, Jones R, Fava M. Evaluation of opioid modulation in major depressive disorder. Neuropsychopharmacology. 2015;40(6):1448-1455.
doi pubmed pmc - Hegadoren KM, O'Donnell T, Lanius R, Coupland NJ, Lacaze-Masmonteil N. The role of beta-endorphin in the pathophysiology of major depression. Neuropeptides. 2009;43(5):341-353.
doi pubmed - Ahmadi J, Jahromi MS, Ehsaei Z. The effectiveness of different singly administered high doses of buprenorphine in reducing suicidal ideation in acutely depressed people with co-morbid opiate dependence: a randomized, double-blind, clinical trial. Trials. 2018;19(1):462.
doi pubmed pmc - Yovell Y, Bar G, Mashiah M, Baruch Y, Briskman I, Asherov J, Lotan A, et al. Ultra-low-dose buprenorphine as a time-limited treatment for severe suicidal ideation: a randomized controlled trial. Am J Psychiatry. 2016;173(5):491-498.
doi pubmed - Nyhuis PW, Gastpar M, Scherbaum N. Opiate treatment in depression refractory to antidepressants and electroconvulsive therapy. J Clin Psychopharmacol. 2008;28(5):593-595.
doi pubmed - Karp JF, Butters MA, Begley AE, Miller MD, Lenze EJ, Blumberger DM, Mulsant BH, et al. Safety, tolerability, and clinical effect of low-dose buprenorphine for treatment-resistant depression in midlife and older adults. J Clin Psychiatry. 2014;75(8):e785-793.
doi pubmed pmc - Lin C, Karim HT, Pecina M, Aizenstein HJ, Lenze EJ, Blumberger DM, Mulsant BH, et al. Low-dose augmentation with buprenorphine increases emotional reactivity but not reward activity in treatment resistant mid- and late-life depression. Neuroimage Clin. 2019;21:101679.
doi pubmed pmc - Zajecka JM, Stanford AD, Memisoglu A, Martin WF, Pathak S. Buprenorphine/samidorphan combination for the adjunctive treatment of major depressive disorder: results of a phase III clinical trial (FORWARD-3). Neuropsychiatr Dis Treat. 2019;15:795-808.
doi pubmed pmc - Fava M, Memisoglu A, Thase ME, Bodkin JA, Trivedi MH, de Somer M, Du Y, et al. Opioid modulation with buprenorphine/samidorphan as adjunctive treatment for inadequate response to antidepressants: a randomized double-blind placebo-controlled trial. Am J Psychiatry. 2016;173(5):499-508.
doi pubmed - Fava M, Thase ME, Trivedi MH, Ehrich E, Martin WF, Memisoglu A, Nangia N, et al. Opioid system modulation with buprenorphine/samidorphan combination for major depressive disorder: two randomized controlled studies. Mol Psychiatry. 2020;25(7):1580-1591.
doi pubmed pmc - Carlezon WA, Jr., Krystal AD. Kappa-opioid antagonists for psychiatric disorders: from bench to clinical trials. Depress Anxiety. 2016;33(10):895-906.
doi pubmed pmc - Li W, Sun H, Chen H, Yang X, Xiao L, Liu R, Shao L, et al. Major depressive disorder and kappa opioid receptor antagonists. Transl Perioper Pain Med. 2016;1(2):4-16.
pubmed pmc - Kosten TR, O'Connor PG, Schottenfeld RS. Buprenorphine maintenance for opioid dependence: a review. Am J Addict. 2002;11(1):2-20
- Zoorob R, Kowalchuk A, Mejia de Grubb M. Buprenorphine therapy for opioid use disorder. Am Fam Physician. 2018;97(5):313-320.
pubmed - Thase ME, Trivedi MH, Stack JA, Dube S, Sorter M. Antidepressant pharmacotherapy for patients with comorbid depression and substance use disorders. Journal of Clinical Psychiatry. 2004;65(5):627-635.
- McElroy SL, Kotwal R, Keck PE, Hudson JI. Sexual dysfunction associated with antipsychotic and antidepressant medications. Journal of Clinical Psychiatry. 2010;71(12):1665-1672.
- Beck K, Hindley G, Borgan F, Ginestet C, McCutcheon R, Brugger S, Driesen N, et al. Association of ketamine with psychiatric symptoms and implications for its therapeutic use and for understanding schizophrenia: a systematic review and meta-analysis. JAMA Netw Open. 2020;3(5):e204693.
doi pubmed pmc - Foti K, Heyward J, Tajanlangit M, Meek K, Jones C, Kolodny A, Alexander GC. Primary care physicians' preparedness to treat opioid use disorder in the United States: A cross-sectional survey. Drug Alcohol Depend. 2021;225:108811.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


