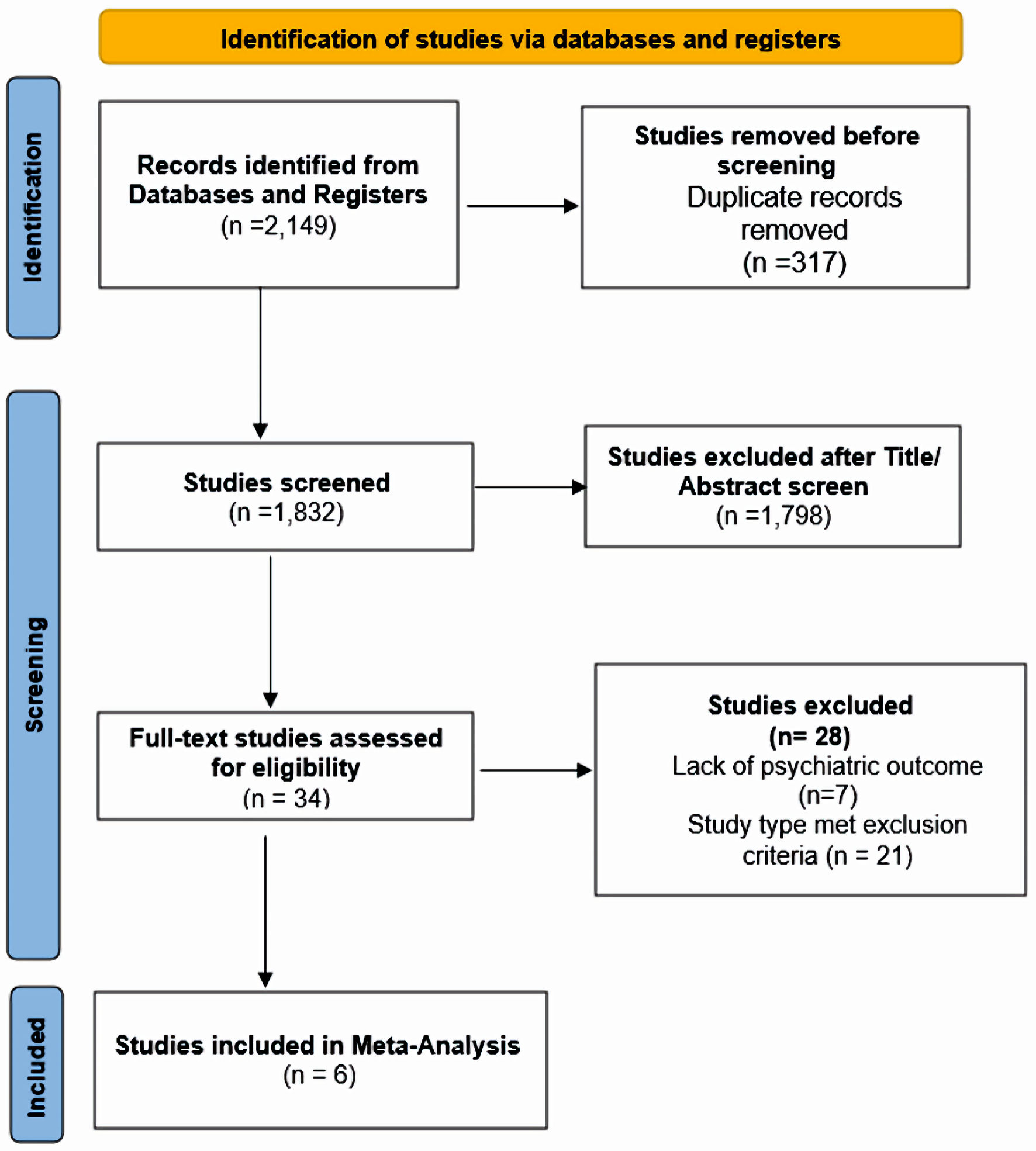
Figure 1. Search flowchart.
| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 16, Number 2-3, March 2024, pages 46-55
Therapeutic Potential of Buprenorphine in Depression: A Meta-Analysis of Current Evidence
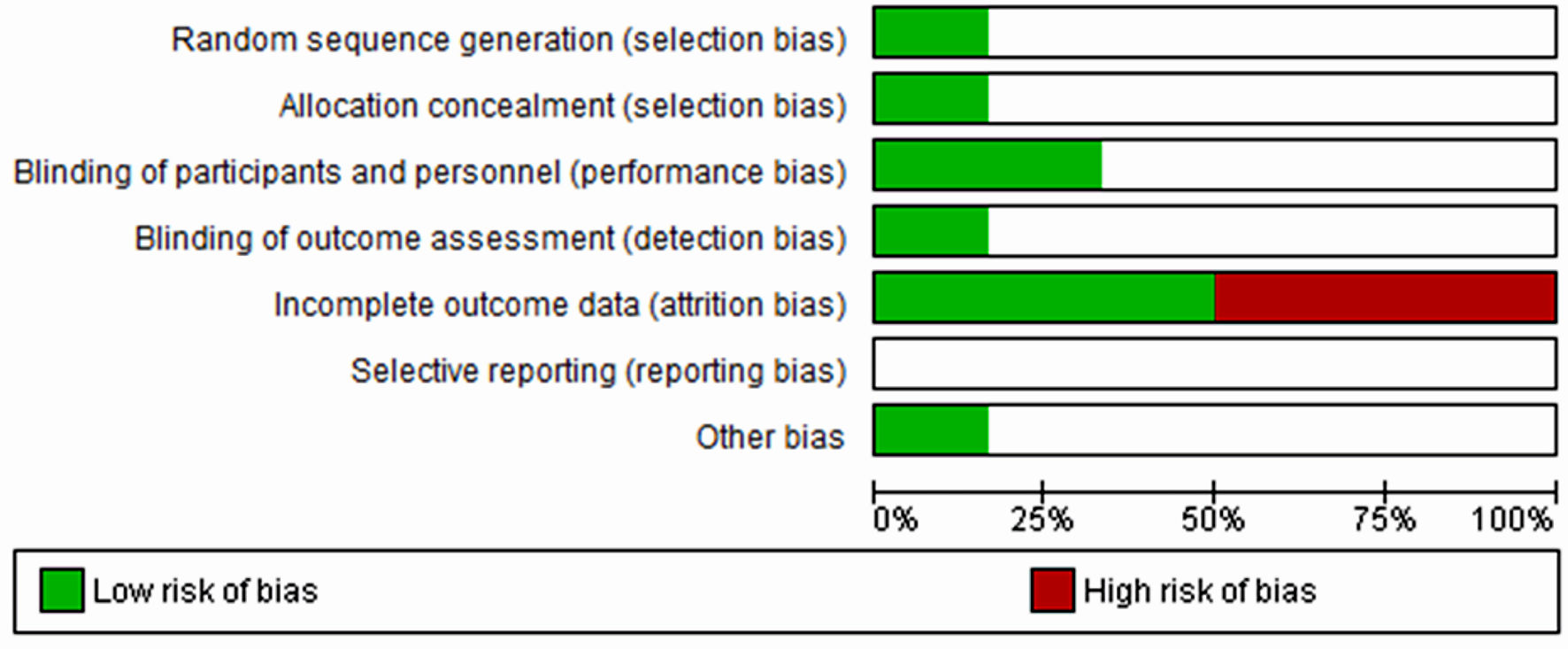
Figures

Table
| Author, year | Study type | Duration and frequency | N | Intervention drug | Assessing psychiatric symptom | Scale(s) used | Mean score change (endline-baseline) | Findings |
|---|---|---|---|---|---|---|---|---|
| N: number; MDD: major depressive disorder; BUP: buprenorphine; SAM: samidorphan; HAM-D: Hamilton Rating Scale for Depression; MADRS: Montgomery-Asberg Depression Rating Scale; CGI-S: Clinical Global Impressions-Severity; ANOVA: analysis of variance; SE: standard error; SSRI: selective serotonin reuptake inhibitor; SNRI: serotonin/norepinephrine reuptake inhibitor. | ||||||||
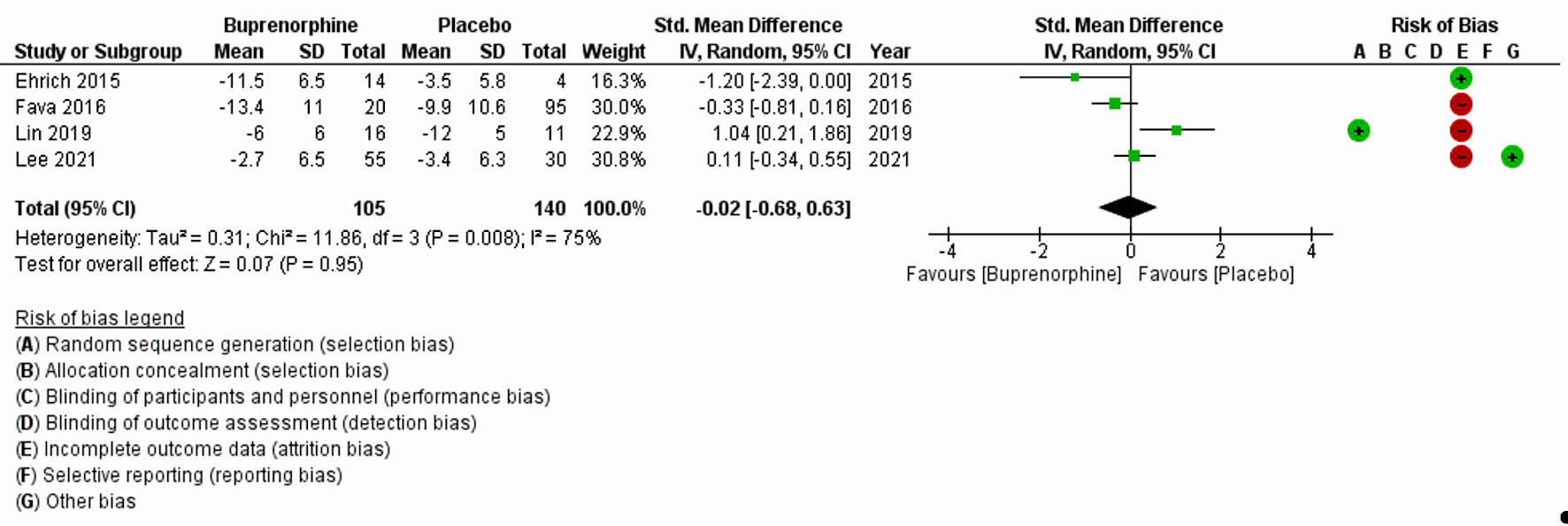
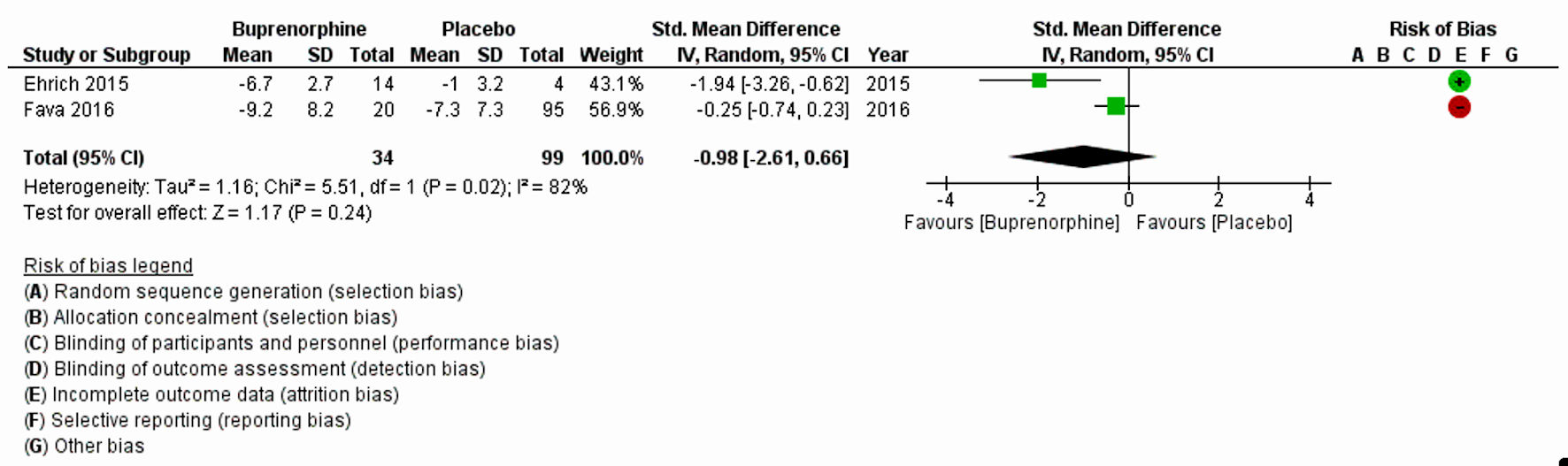
| Ehrich et al, 2015 [11] | Randomized placebo-controlled trial | 7 days, once daily dosing | 45 | BUP, SAM and placebo used: 1) BUP/SAM: 8:1 dose-ratio: 2 mg/0.25 mg, 4 mg/0.5 mg; 2) BUP/SAM 1:1 dose-ratio: 4 mg/4 mg, 8 mg/8 mg; 3) placebo | MDD | HAM-D17 and MADRS | HAM-D17 total score (P = 0.032) and MADRS total score (P = 0.054) | Following 7 days of treatment in subjects with MDD, a 1:1 ratio of BUP and SAM, the ratio associated with maximal antagonism of opioid effects, exhibited statistically significant improvement vs. placebo in HAM-D17 total score (P = 0.032) and nearly significant improvement in MADRS total score (P = 0.054). |
| Fava et al., 2016 [19] | Randomized double-blinded, placebo-controlled trial (multicenter) | 10 weeks, once daily dosing | 142 | BUP/SAM at 2 mg/2 mg (the 2/2 dosage group) or 8 mg/8 mg (the 8/8 dosage group) or placebo | MDD | HAM-D, MADRS, and the CGI-S scale | Compared with the placebo group, there were significantly greater improvements in the 2/2 dosage group across the three depression outcome measures (HAM-D: -2.8, 95% CI: -5.1, -0.6; MADRS: -4.9, 95% CI: -8.2, -1.6; CGI-S: -0.5, 95% CI: -0.9, -0.1). | Results of this trial demonstrate clinically meaningful antidepressant effects for the BUP/SAM combination compared with placebo in patients with major depression and an insufficient response to SSRIs or SNRIs. There was also evidence of improvement in the 8/8 dosage group, although it did not achieve statistical significance. |
| Lee et al, 2022 [8] | Randomized placebo-controlled trial (multisite) | 8 weeks, once daily dosing | 85 | 0.2 mg of BUP or placebo | Treatment resistant depression | MADRS | No significant differences between the treatment groups in the MADRS trajectories over time (F3,443 = 0.26, P = 0.85). | There were no significant differences between the BUP and placebo groups in MADRS changes over time or adverse effects. |
| Lin et al, 2019 [17] | Randomized double-blinded, placebo-controlled trial | 8 weeks, once daily dosing | 31 | Low-dose BUP or placebo (0.2 mg/day and increased by 0.2 mg/day each week based on depression severity and tolerability up to a maximum of 1.2 mg/day) | Treatment-resistant MDD | Total score and the dysphoria subscale of the MADRS | No significant group (placebo vs. BUP) difference in improvement of depressive symptoms (with either the total MADRS or dysphoria subscale); the mixed ANOVA on weekly MADRS revealed no significant interaction between group and time (F (8,168) = 0.44, P = 0.898), and no significant group differences (F(1,21) = 0.62, P = 0.439), but there was a significant decrease in MADRS across time independent of group (F(8,168) = 3.46, P < 0.005). | Participants in both the BUP and placebo groups showed similar changes in depressive symptoms |
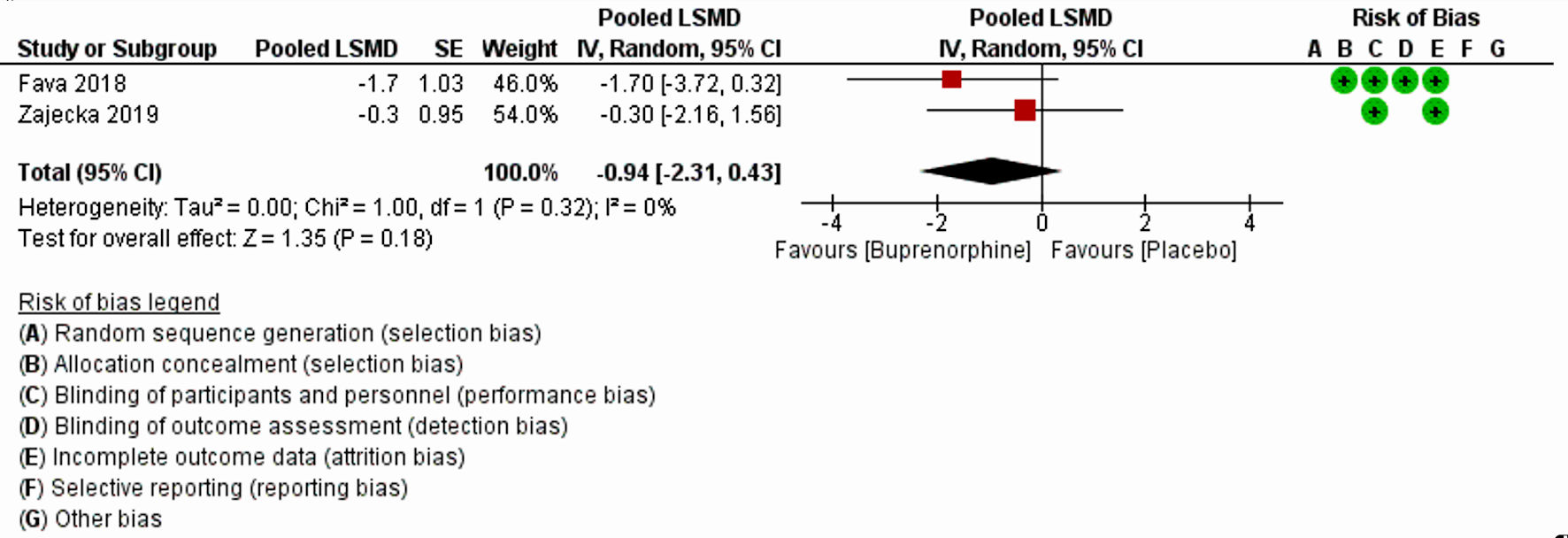
| Fava et al, 2018 [20] | Randomized placebo-controlled trial | 5 weeks, 6 weeks, once daily dosing | 122 | BUP/SAM (2 mg/2 mg) + antidepressant or placebo + antidepressant for 5 weeks | MDD | MADRS least square mean difference | No change in MADRS-10 at week 5 versus placebo: -1.8, P = 0.109 | MADRS-10 LSMD score indicated BUP was more effective than placebo but was not statistically significant |
| Zajecka et.al, 2019 [18] | Randomized double-blind placebo- controlled trial | 6 weeks, once daily dosing | 295 | BUP/SAM 2 mg/2 mg or placebo for 6 weeks | MDD | MADRS least square mean difference | Least-squares mean change in MADRS-10 score at end of treatment was -4.8 (SE: 0.67) in the BUP/SAM 2 mg/2 mg group and -4.6 (SE: 0.66) in the placebo group (mean difference -0.3 (SE 0.95), P = 0.782). | MADRS-10 score did not meet the primary end point. Postbaseline improvement in MADRS-10 in the BUP/SAM 2 mg/2 mg group was noted but was not statistically significant. |