| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 15, Number 10-11, December 2023, pages 431-437
A Retrospective Study Comparing the Effect of Conventional Coagulation Parameters Vs. Thromboelastography-Guided Blood Product Utilization in Patients With Major Gastrointestinal Bleeding
Chhabindra Nepala, g, Ojbindra KCb, Manisha Koiralab, Ananta Subedic, Rakshya Sharmac, Srinadh Annangid, Suha Jabake, Said Chaabanf
aDepartment of Pulmonology and Critical Care, Faith Regional Health Services, Norfolk, NE, USA
bDepartment of Hospital Medicine, Faith Regional Health Services, Norfolk, NE, USA
cDepartment of Hospital Medicine, Avera McKennan Hospital and University Health Center, Sioux Falls, SD, USA
dDivision of Pulmonary and Critical Care, University of Kentucky, Lexington, KY, USA
eDivision of Gastroenterology, University of Nebraska Medical Center, Omaha, NE, USA
fDivision of Pulmonary and Critical Care Medicine, University of Nebraska Medical Center, Omaha, NE, USA
gCorresponding Author: Chhabindra Nepal, Department of pulmonary and critical care, Faith Regional Health Services, Norfolk, NE, USA
Manuscript submitted September 6, 2023, accepted October 28, 2023, published online December 9, 2023
Short title: TEG-Guided Blood Product Use in GIB
doi: https://doi.org/10.14740/jocmr5022
| Abstract | ▴Top |
Background: The use of thromboelastography (TEG) has demonstrated decreased blood product utilization in patients with specific etiologies of major gastrointestinal bleeding (GIB), such as variceal and non-variceal bleeding in cirrhosis patients; however, in a non-cirrhosis patient with GIB, there is far less evidence in the literature. Our retrospective study compares the effect of TEG-guided blood product utilization in patients with major GIB with all etiologies, including cirrhosis, admitted to medical intensive care unit (MICU).
Methods: A retrospective chart review was conducted on patients admitted to the MICU of a tertiary academic medical center diagnosed with GIB using ICD-9/10 codes from 2014 to 2018. A total of 1,889 patients were identified, and validation criteria such as “GI or hepatology consult note”, type and screen, pantoprazole, or octreotide drip” were used, which resulted in 997 patients, out of which 369 had a diagnosis of cirrhosis. Propensity score matching was done for baseline variables (age, sex, and race), ICU length of stay, hospital length of stay, ventilator days, and vasopressor use. As a result, 88 patients were included in the final analysis, with 44 in TEG and 44 in non-TEG group. A sub-group analysis was done in 46 patients with cirrhosis, 23 in TEG group and 23 in non-TEG group after propensity score matching.
Results: There was significantly higher total blood volume (4,207 mL vs. 2,568 mL, P = 0.04) in the TEG group as compared to the non-TEG group, including total volume of cryoprecipitate (80 mL vs. 55 mL, P = 0.03) and total volume of platelet (543 mL vs. 327 mL, P = 0.03). In the cirrhosis sub-group, there was no significant difference in the amount of blood products transfused between the two groups.
Conclusion: This study revealed that TEG is not superior to conventional coagulation parameters in limiting the volume of blood product transfusion in major GIB patients in ICU settings.
Keywords: Thromboelastography; Gastrointestinal bleeding; Coagulation; Blood products
| Introduction | ▴Top |
Major gastrointestinal bleeding (GIB) is a life-threatening condition that requires aggressive resuscitation with prompt transfusion of blood products. Large-volume transfusions are needed in cases of unstable patients with major GIB [1]. However, large-volume transfusions have been associated with increased mortality, and restrictive transfusion strategies have better clinical outcomes [2-4]. Thromboelastography (TEG) is a point-of-care diagnostic test that measures viscoelastic clot strength in whole blood and provides an assessment of platelet function, clot formation, and fibrinolysis [5]. It has been proven to be more effective than conventional coagulation parameters such as prothrombin time (PT), international normalized ratio (INR), activated partial thromboplastin time (aPTT), and platelets for assessing the need for blood product transfusion in trauma patients, cardiac surgery, liver transplantation and inherited coagulopathies [5-8].
The clinical use of TEG has been expanding, and past studies have demonstrated decreased blood product utilization with the use of TEG in patients with specific etiologies of major GIB, such as cirrhosis patients with non-variceal and variceal bleeding [9, 10]. However, in non-cirrhotic patients with major GIB, there is less evidence described in the literature; till now, only one retrospective study demonstrated increased blood product utilization without any clinical benefit [11]. We conducted this retrospective study to compare the effect of TEG-guided blood product utilization in patients with major GIB with all etiologies, including cirrhosis admitted in the medical intensive care unit (MICU).
| Materials and Methods | ▴Top |
Study design and data collection
A retrospective chart review was conducted on patients admitted to the MICU of a single tertiary academic medical center with a diagnosis of GIB (both upper and lower GIB) using ICD-9/10 codes between January 1, 2014 and December 30, 2018. The administrative database was queried for ICU admissions with a final ICD-9 diagnosis code of “gastrointestinal hemorrhage” (587), “hemorrhage of the gastrointestinal tract, unspecified” (578.9), or a final ICD-10 diagnosis code of “hematemesis” (K92.0) and “gastrointestinal hemorrhage” (K92.2). This study was approved by the University of Kentucky IRB number 47751, and conducted in compliance with all the applicable institutional ethical guidelines for the care, welfare and use of animals.
A total of 1,889 patients were identified, and the sample obtained was further validated using criteria such as “patients with GI or hepatology consult note, type and screen, pantoprazole drip or octreotide drip”. A total of 997 patients met the validation criteria, out of which 369 patients had a diagnosis of cirrhosis. IBM-SPSS software version 26 was used for initial calculations, and propensity score matching was done in a 1:1 ratio with the nearest neighbor algorithm using the “Matchit” package in R. Matching was done for baseline variables that included age, sex, race and severity of disease with ICU length of stay (LOS), hospital LOS, ventilator days and vasopressor use. A total of 88 patients were included in the final analysis after propensity score matching, 44 patients in the conventional coagulation-guided group (non-TEG group) and 44 patients in the TEG-guided blood product utilization groups (TEG group) (Fig. 1). A sub-group analysis was conducted on patients with the diagnosis of cirrhosis; a total of 46 patients were identified after propensity score matching with 23 patients in the non-TEG and 23 patients in the TEG group (Fig. 1).
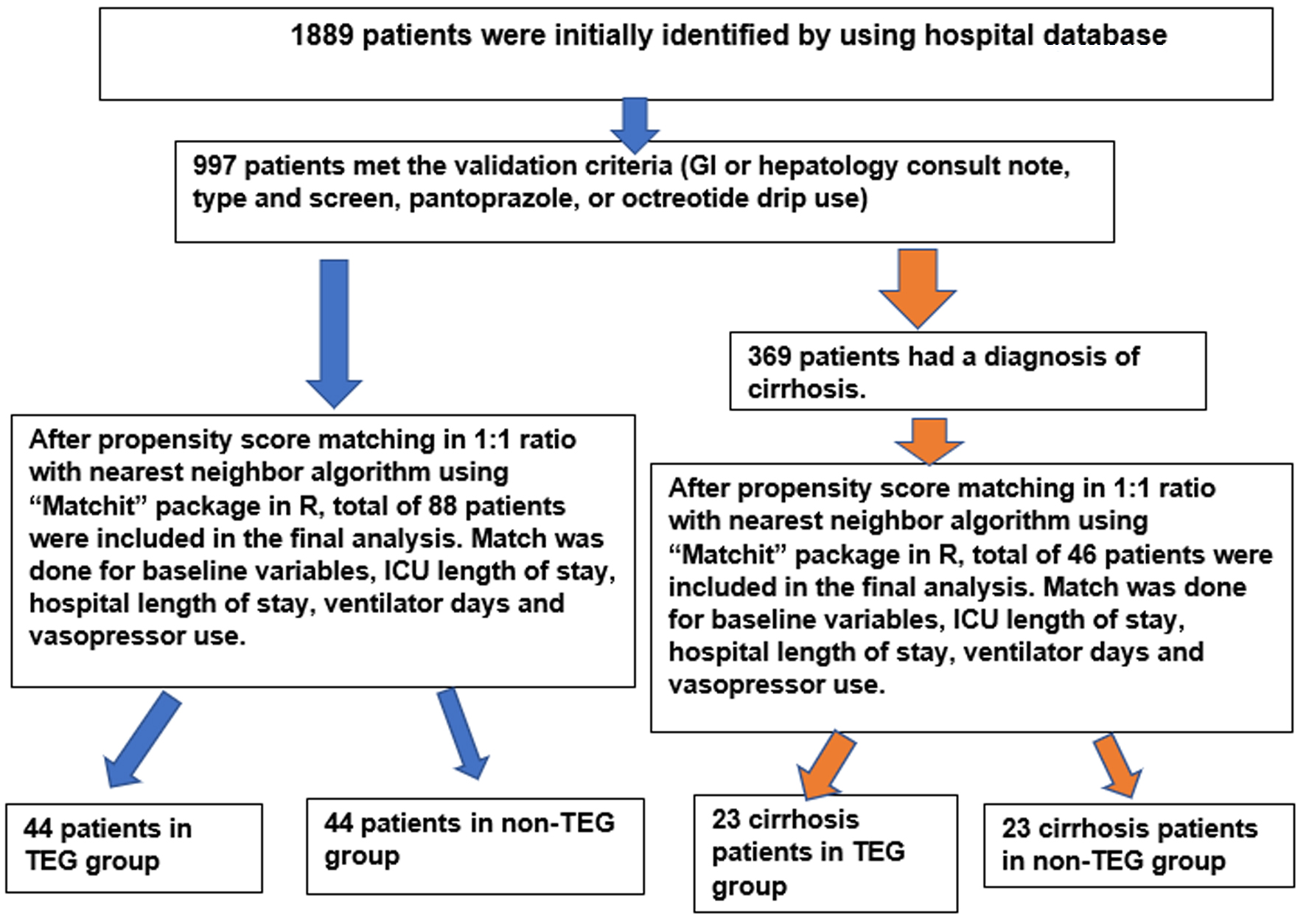 Click for large image | Figure 1. The patient inclusion in study flow chart. GI: gastrointestinal; ICU: intensive care unit; TEG: thromboelastography. |
In both conventional and TEG group, packed red blood cells (PRBCs) were transfused to keep hemoglobin more than 7 g/dL or in hemodynamically unstable patients with more than 2 units drop in hemoglobin from the baseline. In conventional group, platelets were transfused to target platelet count > 50,000/µL and in TEG group platelets were transfused to target kinetics time (K-time) 1 - 3 min, alpha angle (55 - 78°) and maximum amplitude (MA) 51 - 69 mm. In conventional group, cryoprecipitate was transfused to keep fibrinogen level > 150 mg/dL and in TEG group cryoprecipitate was transfused to target K-time 1 - 3 min and alpha angle 55 - 78°. In conventional group, fresh frozen plasma (FFP) was transfused to keep INR above 1.5 and in TEG group FFP was transfused to target reaction time (R-time) 3 - 8 min. TEG in this study was performed by the critical care fellows, ICU residents and the ICU attendings.
The primary outcome was the total amount of blood products (PRBCs, platelets, FFP, and cryoprecipitate) transfused between the two groups during hospitalization. The secondary outcomes were the total amount and volume of blood products transfused from admission to interventions (endoscopy or interventional radiology (IR)-guided embolization) between the two groups and the number of interventions such as upper gastrointestinal (UGI) endoscopies, colonoscopies, embolization and transjugular intrahepatic portosystemic shunt (TIPS) needed for GIB during the hospital admission between the two groups.
In addition, the baseline variables including age, gender, race, comorbid conditions, admission laboratory values (sodium, blood urea nitrogen, creatinine, hemoglobin, platelet, INR, aPTT, and albumin), home antiplatelets use, home anticoagulation use, home beta-blockers (propranolol and nadolol) use, hospital anticoagulation use, sequential organ failure assessment (SOFA) score in admission, oral and intravenous pantoprazole use in hospital, octreotide drip use in hospital, ICU LOS, ventilator days, and vasopressor use were examined between the TEG and non-TEG groups (Table 1) as well as TEG and non-TEG sub-groups in patients with cirrhosis (Table 1).
 Click to view | Table 1. Baseline Variables of Study Population and Cirrhosis Sub-Group and Evaluation of Differences Between TEG and Non-TEG Group |
Data analysis
The baseline characteristics of the TEG and non-TEG groups were compared using the Chi-square test for categorical variables and the t-test for continuous variables. In addition, the Mann-Whitney U test was used for the significance of continuous dependent variables, which were not normally distributed. We consider P-values less than 0.05 to be significant.
| Results | ▴Top |
There were 997 patients who met the validation criteria for GIB, out of which 369 patients had a diagnosis of cirrhosis. A total of 88 patients were included in the final analysis after propensity score matching with 46 patients in the cirrhosis sub-group. There were 44 patients in the TEG group and 44 patients in the non-TEG group, and in the cirrhosis sub-group, there were 23 patients in TEG cirrhosis-subgroup and 23 patients in the non-TEG cirrhosis sub-group.
The study’s primary endpoint to evaluate the total volume of blood product transfused during the hospitalizations revealed significantly higher total blood volume (4,207 mL vs. 2,568 mL, P = 0.04) in the TEG group as compared to the non-TEG group. In addition, the total volume of cryoprecipitate transfused (80 mL vs. 55 mL, P = 0.03) and total volume of platelet transfused (543 mL vs. 327 mL, P = 0.03) were significantly higher in the TEG group vs. non-TEG group; however, the total RBC transfused (2,549 mL vs. 1,274 mL, P = 0.21) and total plasma transfused (1,036 mL vs. 913 mL, P = 0.13) were noted to be higher but without statistical significance in TEG and non-TEG groups (Table 2). In the cirrhosis sub-group, there was no significant difference in the amount of blood products transfused between the two groups (Table 2).
 Click to view | Table 2. Primary and Secondary Outcomes of TEG and Non-TEG Group Along With Cirrhosis Sub-Group |
The study’s secondary endpoint to evaluate the total amount and volume of blood products transfused from admission to interventions revealed higher total blood volume (807 mL vs. 246 mL, P = 0.62) but without statistical significance between TEG vs. non-TEG group. The total GI procedures for bleeding, such as GI endoscopies, colonoscopies, embolization, and TIPS needed, were similar without statistical significance (Table 2). In the cirrhosis sub-group, there was no significant difference in the amount of blood products transfused between the two groups and the total number of procedures for bleeding between the two groups (Table 2). The patient outcomes variables such as ICU LOS, ventilator days, vasopressor use, and need for continuous renal replacement therapy (CRRT) revealed no significant difference between the TEG and non-TEG groups as well as the sub-groups of TEG and non-TEG cirrhosis patients (Table 1), respectively.
| Discussion | ▴Top |
Any GIB that results in hemodynamic instability with signs of poor perfusion (altered mental status, syncope, and pallor) and transfusion of more than two units of PRBCs during the initial resuscitation is considered major GIB and is a life-threatening condition [1]. Major GIB requires prompt diagnosis and skilled resuscitations; hence it is managed in MICUs. Transfusion of blood products and correction of coagulopathy is a significant aspect of effective resuscitations, and large-volume transfusions are often needed in unstable major GIB patients [1]. However, large-volume transfusions have been associated with complications such as dilutional coagulopathy, hypothermia, hypocalcemia, hyperkalemia, transfusion-associated circulatory overload (TACO), and transfusion-related lung injury (TRALI), which has led to increased mortality; hence, evidence-based conservative blood products transfusions have shown to have better clinical outcomes [2, 3].
TEG is a point-of-care diagnostic test that measures viscoelastic clot strength in whole blood and provides an assessment of platelet function, clot formation, and fibrinolysis [5]. It was first developed in 1948 and subsequently used to guide blood product transfusion in trauma, liver transplant surgery, cardiac surgery, and inherited coagulopathies. Some studies showed that the use of TEG in resuscitating surgical and trauma patients decreased blood product transfusion and decreased mortality with better clinical outcomes [5-8], while others showed no superiority to control [12-19]. A systematic review by Zhu et al that included two randomized controlled trials (RCTs) and eight observational trials revealed a probable benefit and the need for more robust evidence to conclude a clear-cut benefit from TEG-guided resuscitation [20].
The use of TEG has been expanding to the non-trauma medical patient population; however, there is less evidence in the literature to suggest a role for TEG in resuscitating major GIB in MICU patients. There have been two RCTs reported in GIB patients. One looked at TEG-guided blood product component use in GIB patients with cirrhosis with non-variceal bleeding [9]. The other looked at TEG-guided blood transfusion in cirrhosis patients with variceal bleeding [10]. In both trials, the TEG-guided transfusion strategy led to a significantly lower use of blood components without compromising hemostasis and with no increase in mortality [9, 10]. On the contrary, a retrospective study with a total of 225 patients with all causes of major GIB noted that TEG-guided transfusion led to increased blood product utilization without any clinical benefits in terms of ICU LOS, respiratory failure, use of renal replacement therapy and mortality rate [11]. In comparison to the RCTs that included cirrhotic patients with variceal and non-variceal bleeding, the retrospective study included patients with all causes of major GIB: patient’s requiring vasopressor use, prior antiplatelets and anticoagulant agents use and septic patients with higher sequential organ failure assessment (SOFA) scores who were critically ill [9-11]. In this retrospective study, TEG patients were noted to be more critically ill than non-TEG patients noted by higher shock index, higher rate of vasopressor utilization, and higher SOFA scores on admission, which might have confounded the primary outcome of blood product utilization [11]. Hence, we aimed in our study to look at all causes of GIB and to match our cohorts to elucidate if there is any overall benefit from TEG-guided blood transfusion resuscitation.
In our retrospective study, 88 patients with all causes of major GIB were included: critically ill patients requiring vasopressors, septic patients with higher SOFA scores, and patients with prior antiplatelets and anticoagulants use. Comparing the patients in the TEG group (N = 44) vs. patients in the non-TEG group (N = 44), there were no statistical differences noted in baseline characteristics of the patients, including the severity of critical illness (use of vasopressors, SOFA scores), or use of antiplatelets and anticoagulants agents (Table 1). There was a statistically significant increased utilization of blood products in the TEG group vs. non-TEG group in terms of total blood volume transfused (4,207 mL vs. 2,568 mL; P = 0.04), total cryoprecipitate volume (80 mL vs. 55 mL; P = 0.03) and total platelet volume transfused (543 mL vs. 327 mL; P = 0.02). These findings echo Rizvi et al’s retrospective study on TEG-guided transfusion in GIB patients, despite matching the severity of patients in both groups [11].
Furthermore, in our subgroup analysis limited to cirrhosis patients, the patients in the TEG cirrhosis sub-group (N = 23) vs. patients in non-TEG cirrhosis sub-group (N = 23) had no statistical differences noted in baseline characteristics that included the severity of critical illness (use of vasopressors, SOFA scores), or use of antiplatelets and anticoagulants agents (Table 1). In this sub-group, the total volume of blood product transfused was higher than the non-TEG group (5,144 mL vs. 2,248 mL; P = 0.06); however, it was not statistically significant. This finding showed a trend towards a higher blood transfusion despite not reaching statistical significance and it contrasted with much of the literature on TEG-guided transfusion in cirrhosis patients that demonstrated a decrease in blood product utilization [9, 10]. It is important to note that the increased blood product utilization may be due to the inclusion of patients with increased severity in our study. While no effect on clinical outcome was noted in either of our groups and this could have been confounded by the retrospective nature of this study, the increase in volume of blood product transfusion does have an impact on hospital resources and adds another layer of financial burden [21-23]. A cross-sectional survey of a randomized sample of hospital-based blood bank and transfusion service directors to determine the average price paid by hospitals to suppliers for blood products showed that the average cost for RBC was $US 343.63 ± 135, FFP $US 60.70 ± 20 and apheresis platelets to be $US 533.90 ± 69 [24]. These costs also do not include economic cost of ordering a TEG. Few recent studies have demonstrated worse inpatient mortality associated with over-transfusion in patients with variceal bleeding than non-variceal bleeding [4]. More prospective studies studying the utility of TEG in blood transfusion resuscitation are needed to help better define its role as it impacts health care utilization of limited resources and costs on the health care system overall.
The major limitation of our study is the retrospective nature of the study, with a higher propensity for confounding bias. The body mass index was not considered in propensity score matching which is one of the limitations of the study. Given the retrospective nature of the study, the sample size calculation was not done for the study which is another limitation of the study. There might have been variations in physician experience with TEG use and the amount of blood product transfused for given TEG abnormalities. In addition, this study could not examine whether TEG was repeated after the transfusion of appropriate blood products. Inpatient mortality and rebleeding rate were not evaluated in this study which is another limitation of the study.
Conclusion
The patients in the MICU with major GIB are critically ill with multiple medical comorbidities. This study revealed that TEG is not superior to conventional coagulation parameters in terms of limiting the volume of blood product transfusion in major GIB patients in ICU settings. This study showed a statistically significant increase in the total blood volume transfused, including the total volume of cryoprecipitate and platelet transfusion. Given the study’s retrospective nature, a well-designed RCT to assess the utility of TEG in major GIB patients would be more appropriate to examine the benefit of TEG vs. conventional parameter-guided transfusions in this patient population. The importance of a prospective RCT would be to help define the role of TEG in resuscitative measures in the MICU patient population as it impacts health care utilization of limited resources and health care costs.
Acknowledgments
None to declare.
Financial Disclosure
There was no specific funding source to be mentioned.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
The consent was not obtained from patients as data were analyzed anonymously.
Author Contributions
Chhabindra Nepal (CN): conceptualization, data curation, formal analysis, investigation, methodology, resources, writing-original draft, writing-review and editing. Ojbindra KC (OK): data curation, formal analysis, resources, writing-original draft, writing-review, and editing. Manisha Koirala (MK): resources, writing-review and editing. Ananta Subedi (AS): formal analysis, resources, writing-review and editing. Rakshya Sharma (RS): resources, writing-review and editing. Srinadh Annangi (SA): formal analysis, supervision, writing-review and editing. Suha Jabak (SJ): formal analysis, resources, writing-review and editing. Said Chaaban (SC): investigation, methodology, resources, formal analysis, supervision, writing-review and editing.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- D'Amore K, Swaminathan A. Massive Gastrointestinal Hemorrhage. Emerg Med Clin North Am. 2020;38(4):871-889.
doi pubmed - Yang JC, Sun Y, Xu CX, Dang QL, Li L, Xu YG, Song YJ, et al. Correlation between red blood cell transfusion volume and mortality in patients with massive blood transfusion: A large multicenter retrospective study. Exp Ther Med. 2015;9(1):137-142.
doi pubmed pmc - Villanueva C, Colomo A, Bosch A, Concepcion M, Hernandez-Gea V, Aracil C, Graupera I, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368(1):11-21.
doi pubmed - Radadiya D, Devani K, Rockey DC. The impact of red blood cell transfusion practices on inpatient mortality in variceal and non-variceal gastrointestinal bleeding patients: a 20-year US nationwide retrospective analysis. Aliment Pharmacol Ther. 2022;56(1):41-55.
doi pubmed - Subramanian M, Kaplan LJ, Cannon JW. Thromboelastography-Guided Resuscitation of the Trauma Patient. JAMA Surg. 2019;154(12):1152-1153.
doi pubmed - Shore-Lesserson L, Manspeizer HE, DePerio M, Francis S, Vela-Cantos F, Ergin MA. Thromboelastography-guided transfusion algorithm reduces transfusions in complex cardiac surgery. Anesth Analg. 1999;88(2):312-319.
doi pubmed - Wang SC, Shieh JF, Chang KY, Chu YC, Liu CS, Loong CC, Chan KH, et al. Thromboelastography-guided transfusion decreases intraoperative blood transfusion during orthotopic liver transplantation: randomized clinical trial. Transplant Proc. 2010;42(7):2590-2593.
doi pubmed - Nogami K. The utility of thromboelastography in inherited and acquired bleeding disorders. Br J Haematol. 2016;174(4):503-514.
doi pubmed - Kumar M, Ahmad J, Maiwall R, Choudhury A, Bajpai M, Mitra LG, Saluja V, et al. Thromboelastography-guided blood component use in patients with cirrhosis with nonvariceal bleeding: a randomized controlled trial. Hepatology. 2020;71(1):235-246.
doi pubmed - Rout G, Shalimar, Gunjan D, Mahapatra SJ, Kedia S, Garg PK, Nayak B. Thromboelastography-guided blood product transfusion in cirrhosis patients with variceal bleeding: a randomized controlled trial. J Clin Gastroenterol. 2020;54(3):255-262.
doi pubmed - Rizvi G, Marcinkowski B, Srinivasa N, Jett A, Benjenk I, Davison D, Yamane D. Impact on blood product utilization with thromboelastography guided resuscitation for gastrointestinal hemorrhage. J Intensive Care Med. 2023;38(4):368-374.
doi pubmed - Cochrane C, Chinna S, Um JY, Dias JD, Hartmann J, Bradley J, Brooks A. Site-of-care viscoelastic assay in major trauma improves outcomes and is cost neutral compared with standard coagulation tests. Diagnostics (Basel). 2020;10(7):486.
doi pubmed pmc - Campbell D, Wake E, Walters K, Ho D, Keijzers G, Wullschleger M, Winearls J. Implementation of point-of-care ROTEM® into a trauma major haemorrhage protocol: A before and after study. Emerg Med Australas. 2021;33(3):457-464.
- Unruh M, Reyes J, Helmer SD, Haan JM. An evaluation of blood product utilization rates with massive transfusion protocol: Before and after thromboelastography (TEG) use in trauma. Am J Surg. 2019;218(6):1175-1180.
doi pubmed - Wang H, Robinson RD, Phillips JL, Ryon A, Simpson S, Ford JR, Umejiego J, et al. Traumatic abdominal solid organ injury patients might benefit from thromboelastography-guided blood component therapy. J Clin Med Res. 2017;9(5):433-438.
doi pubmed pmc - Mohamed M, Majeske K, Sachwani GR, Kennedy K, Salib M, McCann M. The impact of early thromboelastography directed therapy in trauma resuscitation. Scand J Trauma Resusc Emerg Med. 2017;25(1):99.
doi pubmed pmc - Yin J, Zhao Z, Li Y, Wang J, Yao D, Zhang S, Yu W, et al. Goal-directed transfusion protocol via thrombelastography in patients with abdominal trauma: a retrospective study. World J Emerg Surg. 2014;9:28.
doi pubmed pmc - Tapia NM, Chang A, Norman M, Welsh F, Scott B, Wall MJ, Jr., Mattox KL, et al. TEG-guided resuscitation is superior to standardized MTP resuscitation in massively transfused penetrating trauma patients. J Trauma Acute Care Surg. 2013;74(2):378-385; discussion 385-376.
doi pubmed - Kashuk JL, Moore EE, Wohlauer M, Johnson JL, Pezold M, Lawrence J, Biffl WL, et al. Initial experiences with point-of-care rapid thrombelastography for management of life-threatening postinjury coagulopathy. Transfusion. 2012;52(1):23-33.
doi pubmed - Zhu Z, Yu Y, Hong K, Luo M, Ke Y. Utility of viscoelastic hemostatic assay to guide hemostatic resuscitation in trauma patients: a systematic review. World J Emerg Surg. 2022;17(1):48.
doi pubmed pmc - Stokes EA, Wordsworth S, Staves J, Mundy N, Skelly J, Radford K, Stanworth SJ. Accurate costs of blood transfusion: a microcosting of administering blood products in the United Kingdom National Health Service. Transfusion. 2018;58(4):846-853.
doi pubmed - Oge T, Kilic CH, Kilic GS. Economic impact of blood transfusions: balancing cost and benefits. Eurasian J Med. 2014;46(1):47-49.
doi pubmed pmc - Indelen C, Uygun Kizmaz Y, Kar A, Shander A, Kirali K. The cost of one unit blood transfusion components and cost-effectiveness analysis results of transfusion improvement program. Turk Gogus Kalp Damar Cerrahisi Derg. 2021;29(2):150-157.
doi pubmed pmc - Toner RW, Pizzi L, Leas B, Ballas SK, Quigley A, Goldfarb NI. Costs to hospitals of acquiring and processing blood in the US: a survey of hospital-based blood banks and transfusion services. Appl Health Econ Health Policy. 2011;9(1):29-37.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


