| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Review
Volume 16, Number 4, April 2024, pages 138-154
What We Do and What We Should Do Against Malnutrition in Spinal Cord Injury: A Position Paper From Italian Spinal Cord Injury Network Rehabilitation Centers
Alessandra Arenia, h , William Capecib
, Adriana Cassinisc
, Luisa De Palmad
, Giulio Del Popoloe
, Florinda Fergnanif
, Laura Pelizzarig
aSpinal Unit, Montecatone Rehabilitation Institute, Imola, Italy
bSpinal Cord Unit, University Hospital of Marche, Ancona, Italy
cSpinal Unit, ASST GOM Niguarda Ca’ Granda, Milan, Italy
dSpinal Unit, Department of Neuroscience and Translational Medicine, Azienda Ospedaliero-Universitaria Policlinico, Bari, Italy
eDepartment of Neuro-Urology and Spinal Unit, Azienda Ospedaliera-Universitaria Careggi, Firenze, Italy
fU.O Complesso Mielolesi, Azienda Ospedaliera CTO-Pini, Milan, Italy
gSpinal Unit and Neurorehabilitation, Department of Rehabilitation Medicine, AUSL Piacenza, Piacenza, Italy
hCorresponding Author: Alessandra Areni, Spinal Unit, Montecatone Rehabilitation Institute, Imola, Italy
Manuscript submitted August 21, 2023, accepted February 6, 2024, published online April 30, 2024
Short title: Malnutrition in SCI
doi: https://doi.org/10.14740/jocmr5015
| Abstract | ▴Top |
Spinal cord injury (SCI) is a traumatic event that significantly impacts body composition and alters energy and nutritional needs. This places patients with SCI at a high risk of malnutrition, which can hinder optimal functional recovery, prolong hospital stays, increase hospital admissions, and contribute to the development of obesity and cardiovascular and metabolic ailments in chronic patients. Consequently, there is an urgent need for clear guidance to support clinicians in managing the nutritional needs of patients with SCI at different stages of the disease, including the acute (0 - 4 months after injury), post-acute (4 - 26 months after injury), and post-discharge phases. This study utilized a cross-sectional survey to assess the strategies employed in seven spinal units across Italy to address the nutritional needs of patients with SCI during the acute, post-acute, and post-discharge phases of the condition. Eight clinicians (five physiatrists, two internists, and one urologist) and one nurse participated in the survey. Following the survey completion, the participants were invited to partake in a round table session to delve deeper into the questionnaire results to gather their opinions and gain insights into clinical practices related to the various challenges surrounding the management of malnutrition in patients with SCI. We here review the available evidence on the energy needs and nutritional requirements of patients with SCI, highlighting the clinical aspects that deserve more attention throughout the distinct phases of the disease. We additionally provide an overview of the scenario regarding the management of malnutrition in patients with SCI across various spinal units in Italy. Through this comprehensive analysis, we aimed to enhance understanding and provide valuable insights for clinicians working with patients with SCI, equipping them with the knowledge and confidence to provide nutritional support to patients with SCI efficiently. By addressing the challenges of defining nutritional needs and presenting a practical guide, we aspire to contribute to the overall management and care of individuals with SCI and the prevention of malnutrition and its associated complications, thereby improving patient outcomes.
Keywords: Spinal cord injury; Malnutrition; Obesity
| Introduction | ▴Top |
Spinal cord injury (SCI) impacts body composition and nutritional needs. Following SCI, there is a decrease in lean body mass and energy expenditure, while fat body mass tends to increase [1]. These factors, combined with a sedentary lifestyle and positive energy balance, contribute to a condition known as “neurogenic obesity”, which, in turn, predisposes to cardiometabolic alterations [1-9]. Additionally, undernutrition may occur, especially in the first period after SCI, partly due to anxiety and depression; it is associated with longer hospital stays and adverse outcomes [10].
In the rehabilitation phase, approximately 50% of SCI patients experience malnutrition [11], which can coexist with obesity [12]. The World Health Organization defines malnutrition as “deficiencies, excesses or imbalances in a person’s intake of energy and/or nutrients” and highlights that the condition includes both undernutrition and overweight and obesity [13]. If untreated, malnutrition can lead to longer hospital stays, increased in-hospital mortality, pressure ulcers, suboptimal functional recovery, obesity and other cardiometabolic issues [14].
Although guidelines are available [2, 15, 16], there is an urgent need for innovative protocols to manage the nutritional status of SCI patients across the different stages of the disease [17]. This work aimed to provide practical guidance for clinicians in spinal units to determine the energy needs and assess the nutritional status of patients with SCI during the acute (0 - 4 months post-injury), post-acute (4 - 26 months post-injury), and post-discharge phases of the disease, with the goal of preventing malnutrition and related complications.
| Materials and Methods | ▴Top |
A cross-sectional survey was used to initiate a discussion. The survey questionnaire was designed by the project coordinator (AA) to assess the strategies employed in seven spinal units across Italy to address the nutritional needs of SCI patients during the acute, post-acute, and post-discharge phases. Eight clinicians (five physiatrists, two internists, and one urologist) and one nurse participated in the survey, which was administered to participants through SurveyMonkey. Qualitative data from the questionnaires were entered into Excel (Microsoft Office) spreadsheets. Following the survey completion, the participants discussed the results during a round table held on March 2023, and provided their opinions on the clinical management of malnutrition in SCI patients.
| Results and Discussion | ▴Top |
Acute phase of SCI
During the acute phase of SCI (0 - 4 weeks after the injury), peculiar metabolic changes occur: lower basal energy expenditure, heightened nitrogen excretion, anorexia, weight loss, and depletion of nutritional markers [1, 2, 18, 19]. Despite nutritional support, patients often experience negative nitrogen balance for up to 2 months after injury, which usually improves without intervention [20].
Attempts to correct this imbalance by increasing caloric intake can lead to overfeeding and subsequent hypercapnia, hyperglycemia, uremia, and hypertriglyceridemia [21]. This contributes to the accumulation of adipose tissue and extended mechanical ventilation due to heightened respiratory effort [22]. On the other hand, underfeeding can exacerbate nitrogen loss [23].
Therefore, proper nutritional support is crucial; conducting a nutritional assessment within the first 48 h post-injury improves outcomes [16]. During hospital admission, a registered dietician (RD) should assess body composition and resting metabolism. Estimating the total daily energy expenditure (TDEE) is essential to provide appropriate energy intake recommendations [3].
Energy needs
Patients with acute SCI typically exhibit an energy expenditure up to 54% lower than those without [1, 2, 18], with a TDEE reduced by > 50% in tetraplegia [4]. However, the Spinalis Foundation proposed to estimate the energy requirements of SCI patients by reducing TDEE by 7.5% for people with paraplegia (estimated energy requirements: 28 kcal/kg) and by 12.5% for those with tetraplegia (23 kcal/kg) compared with able body individuals [19].
Indirect calorimetry (IC) is the gold standard for determining energy expenditure [2]. Nevertheless, IC might not be immediately accessible. In such instances, RDs can utilize simplified formulas to estimate energy requirements. The commonly used 25 - 30 kcal/kg/day formula is not different from that used for non-disabled individuals, which can easily result in overfeeding and shows poor correlation with IC results [24]. The accuracy of predictive equations is 40-75% compared with IC [25]. Predictive equations are even less accurate for obese and underweight patients [26]. Generally, equations developed for non-disabled individuals that incorporate anthropometric measures, including the Harris-Benedict equation despite the proposed activity correction factor of 1.15 for SCI individuals [27], could overestimate resting energy expenditure [28, 29], leading to overfeeding in SCI patients [27]. Therefore, clinicians should carefully monitor for signs and symptoms of overfeeding. Notably, equations that utilize the free fatty mass (FFM) to predict basal metabolic rate (BMR) [29-31] result in lower percentages of error, so they should be preferred when analysis of body composition is available [29]. In the absence of data on body composition, models based on anthropometric measures specifically developed for the SCI population [1, 29] can be used, aligning with the recent recommendations of the Practice-based Evidence in Nutrition document by the Dietitians of Canada [32]. TDEE should be reassessed more than once a week, and proactive strategies should be implemented to optimize energy and protein intake [33].
Only one panelist affirmed using available formulas to calculate the resting energy expenditure of patients and to make corrections based on the level of physical activity and severity of pathology. However, during the discussion, the panel deemed simplistic formulas more suitable for patients with less complex conditions. They emphasized that for complex cases (patients with moderate/severe risk of malnutrition, as determined by the spinal nutrition screening tool (SNST), including individuals with a higher level of injury, dysphagia, age greater than 60 years or less than 18 years, severe pressure injuries, high weight loss, or a need for artificial ventilation), the assistance of a dietitian with expertise in using SCI-specific predictive equations described in the literature [1, 29-31] is necessary. Validated SCI-specific predictive equations are needed, especially for the evaluation of complex patients (Fig. 1).
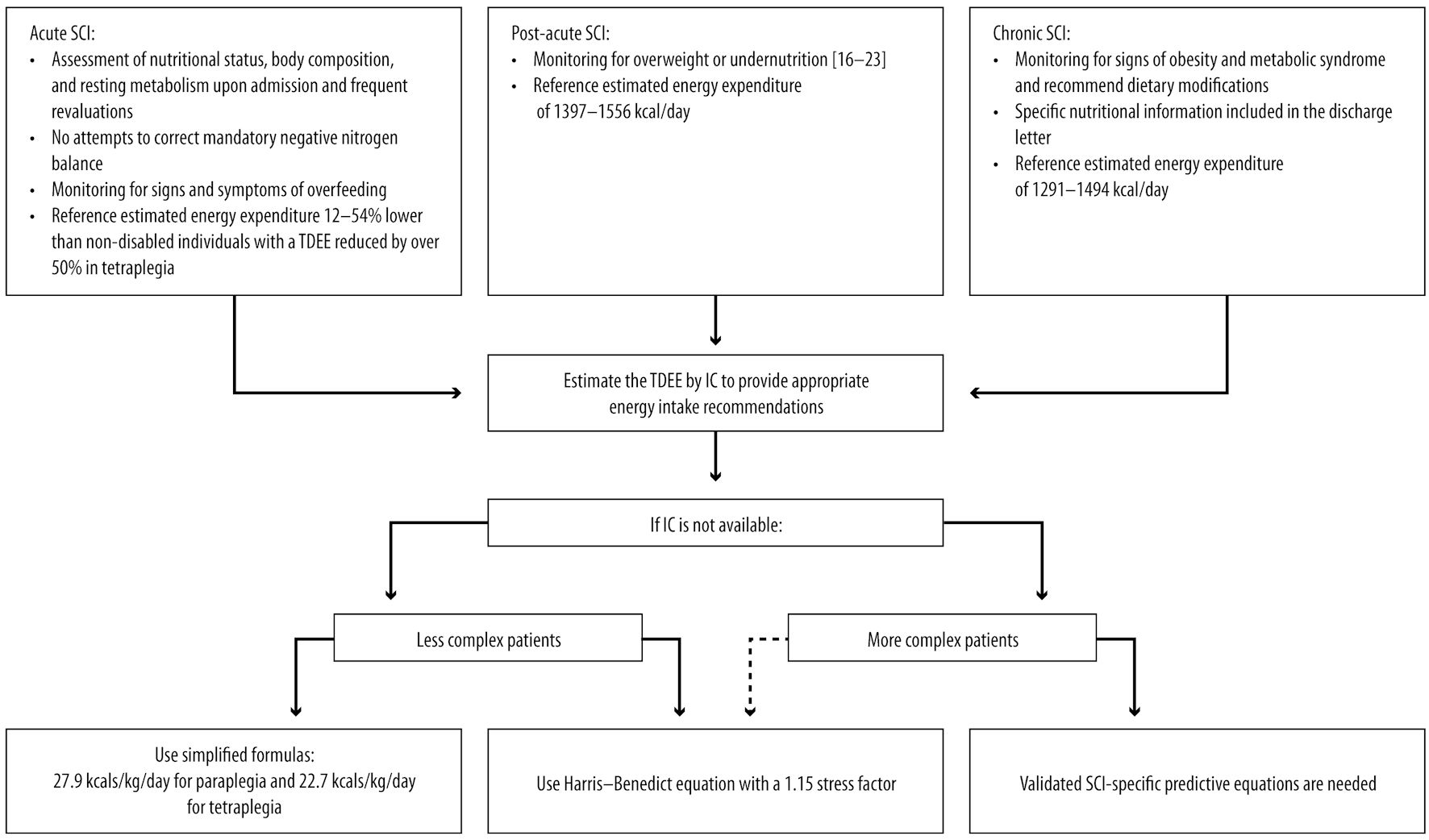 Click for large image | Figure 1. SCI: management considerations for nutritional assessment and energy needs. SCI: spinal cord injury; TDEE: total daily energy expenditure; IC: indirect calorimetry. |
Nutrition assessment
For patients who can consume food voluntarily, RDs should encourage adherence to heart-healthy dietary guidelines [34]. In addition, RDs should address specific vitamin and/or mineral deficiencies through supplementation and consider diet-related ailments, such as neurogenic bowel.
Although surveyed hospitals have a dedicated nutrition team, which includes a clinician, a dietitian conducting ward rounds, a nutrition nurse specialist, especially when managing artificial nutrition is necessary, and a gastroenterologist or an equivalent specialist for issues with artificial nutrition and the management of patients with intestinal problems, only five of the eight panelists reported the presence of protocols for nutrition management in SCI patients, which are, in some cases, limited to selected parameters (e.g., nutritional regimen). Only two of the panel members regularly monitor malnutrition risk at hospital admission with designated tools, which vary across different spinal units. A re-evaluation takes place every month or once a week for critically ill patients and every time a change in clinical conditions occurs. The two panelists monitor the nutritional status with blood tests, anthropometric measures, and instrumental tests.
A standardized protocol for assessing nutrition in SCI patients is crucial to address these gaps. The protocol should specify the frequency and duration of assessments and establish a common screening tool for all spinal units. Given the rapid changes in body composition, the panel deems that anthrophometric, biochemical and dietary assessments should be repeated once a week during the acute phase of SCI (Figs. 2, 3).
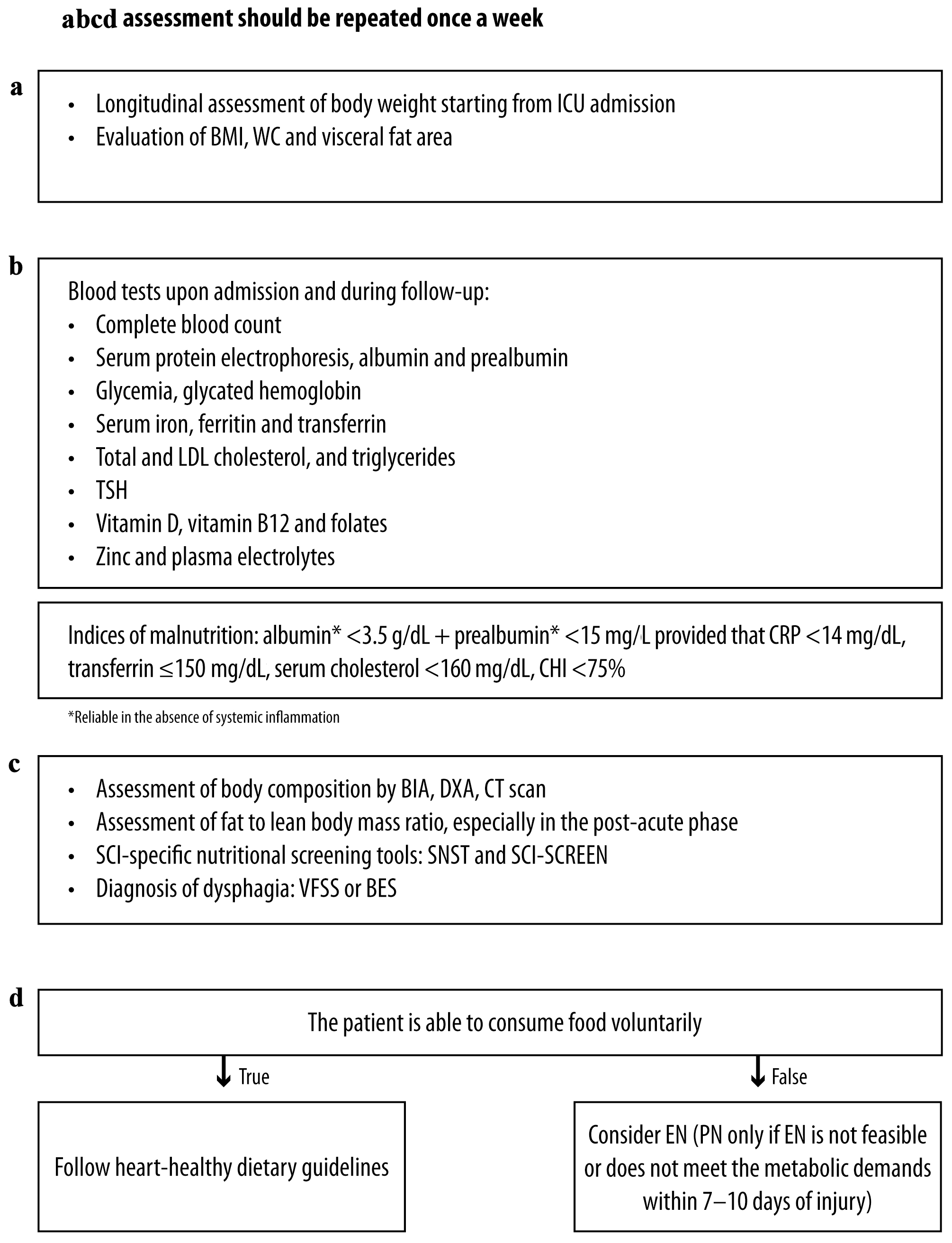 Click for large image | Figure 2. Acute and post-acute SCI: management considerations for (a) anthropometric, (b) biochemical, (c) clinical and (d) dietary assessment. (a-d) Assessment should be repeated once a week. TSH: thyroid-stimulating hormone; BMI: body mass index; ICU: intensive care unit; CRP: C-reactive protein; CHI: creatinine height index; LDL: low-density lipoprotein; DXA: dual X-ray absorptiometry; BIA: bioelectrical impedance analysis; CT: computerized tomography; SNST: spinal nutrition screening tool; BES: bedside swallowing evaluation; VFSS: videofluoroscopic swallow studies; EN: enteral nutrition; PN: parenteral nutrition; WC: waist circumference. |
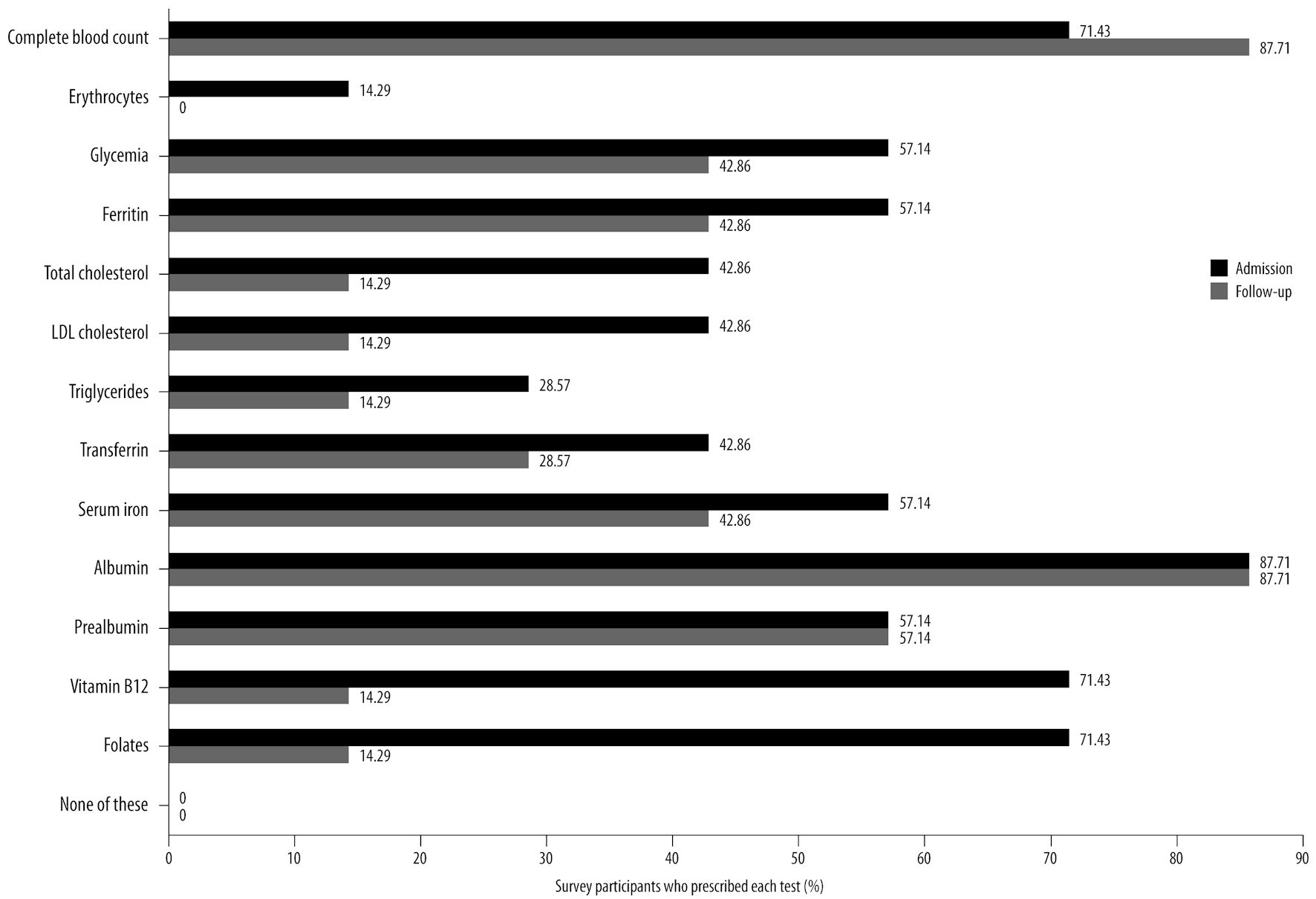 Click for large image | Figure 3. Blood tests commonly prescribed by the panelists upon hospital admission and during follow-up of acute patients with SCI, based on survey results, along with the corresponding percentage of survey participants who prescribe each test. SCI: spinal cord injury; LDL: low-density lipoprotein. |
1) Anthropometric assessment
Body mass index (BMI) is an unreliable method to assess nutritional status in SCI patients, especially in the acute and initial post-acute phases, because of changes in body composition [35]. Similarly, waist circumference (WC) is also not optimal due to paralysis-related variations [36].
However, the panelists emphasized the importance of continuously assessing body weight from the intensive care unit (ICU) using a consistent and reproducible method. Additionally, the patient’s height should be measured, with the patient lying in a bed or on a static plane, then vertically, in order to calculate BMI. The panel also emphasized the need to assess BMI possibly once weekly during the acute phase due to the frequent changes in body composition during this phase, taking into account the weight of clothing and shoes.
2) Biochemical assessment and blood tests
Traditional serum protein markers do not accurately reflect the nutritional status in the ICU setting [37]. Albumin and pre-albumin are influenced by acute-phase response and systemic inflammation, so they should not be employed in case of acute stress or increased inflammatory status [38]. The long half-life of albumin (21 days) makes it unsuitable for monitoring the acute response to nutrition therapy [39]. Pre-albumin outperforms albumin as an indicator of recent nutrition because of its shorter half-life (2 - 3 days) [40] and reduced susceptibility to metabolic processes [41]. According to some authors, a pre-albumin level < 11 mg/dL can be considered an index of malnutrition when combined with a C-reactive protein (CRP) level < 15 mg/L, which rules out the presence of an acute inflammatory state [40, 42]. However, a recent American Society for Parenteral and Enteral Nutrition (ASPEN) position paper highlights that both albumin and pre-albumin do not specifically reflect the current nutrition state of a patient [43]. These markers should be used to identify patients likely to be at an increased risk of poor outcomes if adequate nutrition is not provided.
A transferrin level ≤ 200 mg/dL is an additional indicator for adjustment of nutritional support [40]. Serum cholesterol < 160 mg/dL and a creatinine height index (CHI) < 75% (measured by 24-h creatinine excretion) are also considered indices of malnutrition [40].
Figure 3 presents the list of blood serum proteins that the panelists typically assess upon hospital admission and during follow-up for acute patients with SCI.
The panel emphasized the importance of establishing a standardized set of serum protein markers that accurately reflect the nutritional status of acute SCI patients and are suitable for analysis upon hospital admission and periodic monitoring. Additionally, blood tests can assist in developing a nutritional supplementation program.
Furthermore, an evaluation of plasma osmolarity would be beneficial in cases involving parenteral nutrition (PN).
3) Clinical assessment
Regularly monitoring the nutritional status is essential during rehabilitation since medical conditions, nutrient requirements, and other factors change continuously. Complete SCI can lead to 27-56% atrophy at 6 - 24 weeks [44]. The Global Leadership Initiative on Malnutrition (GLIM) identifies reduced muscle mass as an index of fat-free mass < 17 kg/m2 for men or < 15 kg/m2 for women [45].
Presently, there is a lack of SCI-specific recommendations or appropriate tools to effectively differentiate among cachexia, age-related sarcopenia, and malnutrition [46]. Screening tools originally developed for the non-disabled population, such as the cachexia score (CASCO) [47] and short portable sarcopenia measure (SPSM), and SARC-F (strength, assistance in walking, rising from a chair, climbing stairs, and falls) necessitate the transportation of specialized equipment and exhibit low sensitivity, respectively [48, 49]. The study by Dionyssiotis et al [46] suggests that, for research purposes, we could classify paraplegics using the current functional definition of the European Working Group on Sarcopenia in Older People (EWGSOP) for sarcopenia. Nonetheless, the accuracy and precision of these measurements are not yet clearly established. Diagnostic approaches for sarcopenia and cachexia include anthropometric measures, such as BMI or estimated weight loss, as well as dual X-ray absorptiometry (DXA), bioelectrical impedance analysis (BIA), computed tomography (CT) scan, and magnetic resonance imaging (MRI), which are difficult to access in some settings and therefore reserved for selected specific cases or clinical studies [50]. BIA is a rapid, simple, noninvasive, and relatively reliable bedside method [51] that is considered equally valuable as DXA in SCI patients [52]. The panel advises that BIA assessment be repeated once a week or every 2 weeks during the acute phase of SCI. While whole-body DXA is reliable in SCI, its accuracy may be limited in individuals with high-fat mass or very low fat-free mass [53]. MRI plays a crucial role in diagnosing vertebral injury and abnormalities of soft tissues [54]. CT provides precise quantification of skeletal muscle and adipose tissue depots, but it is highly costly [53]. Ultrasound measures muscle mass and changes in muscle tissue at the bedside, making it suitable in the ICU [55]. However, in patients with SCI, ultrasound may be influenced by changes in tissue thickness.
The panel uses ultrasound to assess muscle thickness, BIA with a longitudinal evaluation of angle phase, and DXA, whenever available, to assess the nutritional status of SCI patients. As for DXA, there are no specific cut-off values for SCI exist. However, given the aforementioned potential applicability of the current functional definition of EWGSOP for sarcopenia to paraplegic SCI patients for research purposes [46], the panel suggests that the cutoffs for appendicular lean mass divided by height squared (ALM/height2) proposed by EWGSOP (men B 7.23 kg/m2 and women B 5.67 kg/m2) [56, 57] may be applicable to paraplegic SCI patients. The panel deemed that elastosonography, if conducted for other reasons, can provide information on skeletal muscle fibrosis and the risk of skeletal muscle bleeds.
Individuals with SCI often experience dehydration, edema, and fluid fluctuations, which can impact the body’s hydration, decreasing intracellular and increasing extracellular water [58]. Fluctuations of the hydration status can result in over- or under-estimation of body fat when assessing body composition with BIA [59], ultrasound [60], and DXA [61]. Equations to calculate total body water (TBW) in healthy subjects are not valid in SCI patients [58].
The panel emphasized the importance of accurately monitoring the hydration status of patients, especially those catheterized in the ICU. Patients are often overhydrated, which can improve bowel functions but pose challenges for intermittent catheterization. Water intake can be reduced to minimize the frequency of catheterization, putting patients at risk of dehydration. Assessing water balance becomes difficult when the catheter is removed, but the bladder diary closely monitors it.
Existing tools for the assessment of nutritional status have been developed for the general population and may not accurately capture the nutritional status of patients with SCI [62]. To overcome this limitation, a SCI-specific nutritional screening tool, the SNST, was developed to assess the risk of malnutrition in adult patients with SCI and can predict outcomes [14, 62].
All the panelists agreed on the importance of monitoring nutritional status, since malnutrition impacts prognosis. Despite its current low use, the SNST should be implemented for initial nutritional assessments and follow-up.
The SCI-SCREEN is a screening model designed to identify malnutrition risk and to detect underweight and overweight, offering a cost-effective and user-friendly approach [63]. According to the panel, although SCI-SCREEN has not been validated specifically for individuals with SCI, its specificity is evident and offers a rapid assessment process.
Dysphagia commonly accompanies cervical SCI and can lead to adverse consequences, often requiring the implementation of a texture-modified diet associated with weight loss or malnutrition [64]. Clinical assessments for dysphagia should include bedside swallowing evaluation (BES), videofluoroscopic swallow studies (VFSS) and fiberoptic endoscopic evaluation of swallowing (FEES). VFSS and BSE are suitable and comparable methods for diagnosing dysphagia in SCI patients [65]; however, a meta-analysis failed to establish a correlation between FEES findings and dysphagia [66].
Typically, the initial assessment involves a BES of water intake, followed by a logopedic assessment. If necessary, a FEES is conducted.
4) Dietary assessment
The panel believes that an RD should conduct dietary assessment. However, in severe cases where enteral or PN is planned, the involvement of an expert nutritionist is necessary.
a) Enteral nutrition (EN)
While concerns about ileus and other complications have historically led to delayed enteral feeding in SCI patients, studies have shown that initiating within the first 72 h is safe [67, 68].
Tailoring the nutrition therapy to each patient’s specific needs is important to optimize clinical outcomes. A well-defined protocol is essential to ensure optimal EN in ICU settings. This protocol should incorporate the following: an initial feeding rate determined upon the patient’s tolerance, a gradual increase in the feeding rate, a clear rate goal established in collaboration with an RD, the implementation of a comprehensive bowel management program, and the use of a prokinetic agent if the patient exhibits three consecutive elevated gastric residual volumes [69, 70]. Adherence to this protocol promotes early initiation of EN, increases the volume of feed delivered, optimizes caloric intake, and leads to shorter hospital stays and improved morbidity and mortality outcomes [69, 70].
Despite the absence of SCI-specific studies, EN is preferred over PN following SCI due to its association with reduced infectious morbidity, hyperglycemia, ICU length of stay and mortality in several trauma populations [20, 71]. Nasogastric administration is the recommended initial route for EN, while the nasojejunal route serves if nasogastric feeding is not tolerated [20]. In most critical patients, initiating EN in the stomach is acceptable, but if there is a risk of aspiration or intolerance to gastric EN, the infusion level should be adjusted to a lower position [71]. A percutaneous endoscopic gastrostomy is the preferred approach for feeding tube placement [20]. In cases of neurogenic bowel, prebiotic/probiotic supplementation provided enterally to the patients may be beneficial.
Figure 4 shows the management considerations for EN during acute SCI.
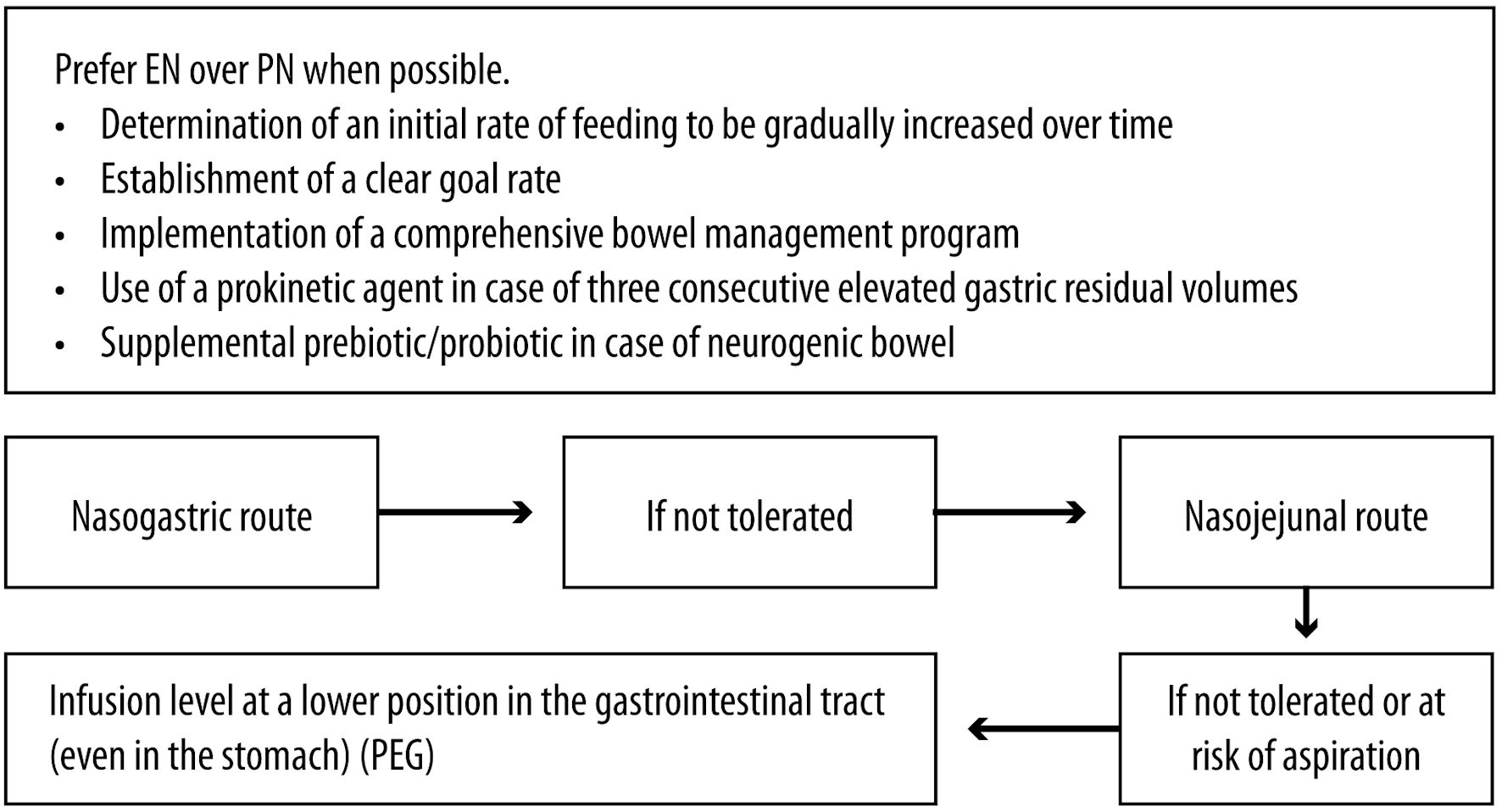 Click for large image | Figure 4. Acute SCI: management considerations for enteral nutrition. SCI: spinal cord injury; EN: enteral nutrition; PN: parenteral nutrition; PEG: percutaneous endoscopic gastrostomy. |
b) PN
If EN does not meet the metabolic demands within 5 - 10 days of injury, total PN should be started [20, 71].
The panel suggests that PN should be limited to severe cases, given the risk of altered osmolality, infection, thrombosis and refeeding syndrome. In the survey, five out of seven panelists reported a higher prevalence of patients receiving EN than PN, with one panelist not being aware of this information.
Commercially available standardized PN provides no benefit in clinical outcomes versus compounded PN admixtures in ICU patients [71]. However, during discussion, the panel emphasized the importance of supplementing PN formulas with vitamins and trace minerals.
Safe prescribing of PN involves ensuring appropriate intravenous access, maintaining sterility, and clearly identifying and documenting the therapeutic goals of PN therapy. Reliable vascular access is crucial, and in-line filters should be used [72]. Monitoring for central line-associated bloodstream infection (CLABSI) is necessary, and blood sampling through vascular access devices should be avoided to minimize its risk [73]. Implementing a PN infusion schedule over 10 - 14 h can provide benefits in selected circumstances [74]. It is advisable to prevent unplanned infusion interruptions to avoid potential metabolic disruptions and suboptimal nutrient delivery and to refrain from interrupting PN administration for medication purposes [75]. However, PN should be discontinued before discharge [75].
Close monitoring of fluid requirements, blood electrolytes, triglyceride and glucose, liver and kidney function, and any complication is vital during PN, especially in patients with preexisting electrolyte imbalances, at risk of refeeding syndrome, or in unstable clinical condition [71]. Blood glucose levels should be maintained within 140 - 180 mg/dL for the general ICU population [71]. Blood glucose monitoring should align with the PN infusion schedule and the patient’s clinical status [76]. Careful consideration is necessary when administering subcutaneous insulin before planned PN interruptions [71]. The panel deems that blood glucose and electrolytes should be monitored every other day or at least twice a week during PN. Some panelists extend the frequency of monitoring to three times a day for acute-phase patients who receive PN for 24 h and reduce the frequency when PN is integrated with voluntary feeding.
Survey results indicate that six out of eight panelists can monitor patients under PN with blood tests, including amylase and lipase tests, glycemia, and blood gas analysis (medium consensus among participants). The panelists also suggested considering liver transaminases, lipid profiles, blood electrolytes, and cholestasis indices. Peripheral access should be favored over central access to reduce the risk of infection and thrombosis. Some strategies to reduce the risk associated with peripheral access in surgical patients, such as cyclical infusion through the 18 G cannula or using silicone or polyurethane cannulas in addition to anti-phlebitis solutions, have been recently proposed and may also be applicable to SCI patients [77]. When positioning vascular access, limb mobilization should be allowed. If central access is used, it should be promptly removed when inflammation occurs.
Figure 5 shows the the management considerations for PN during acute SCI.
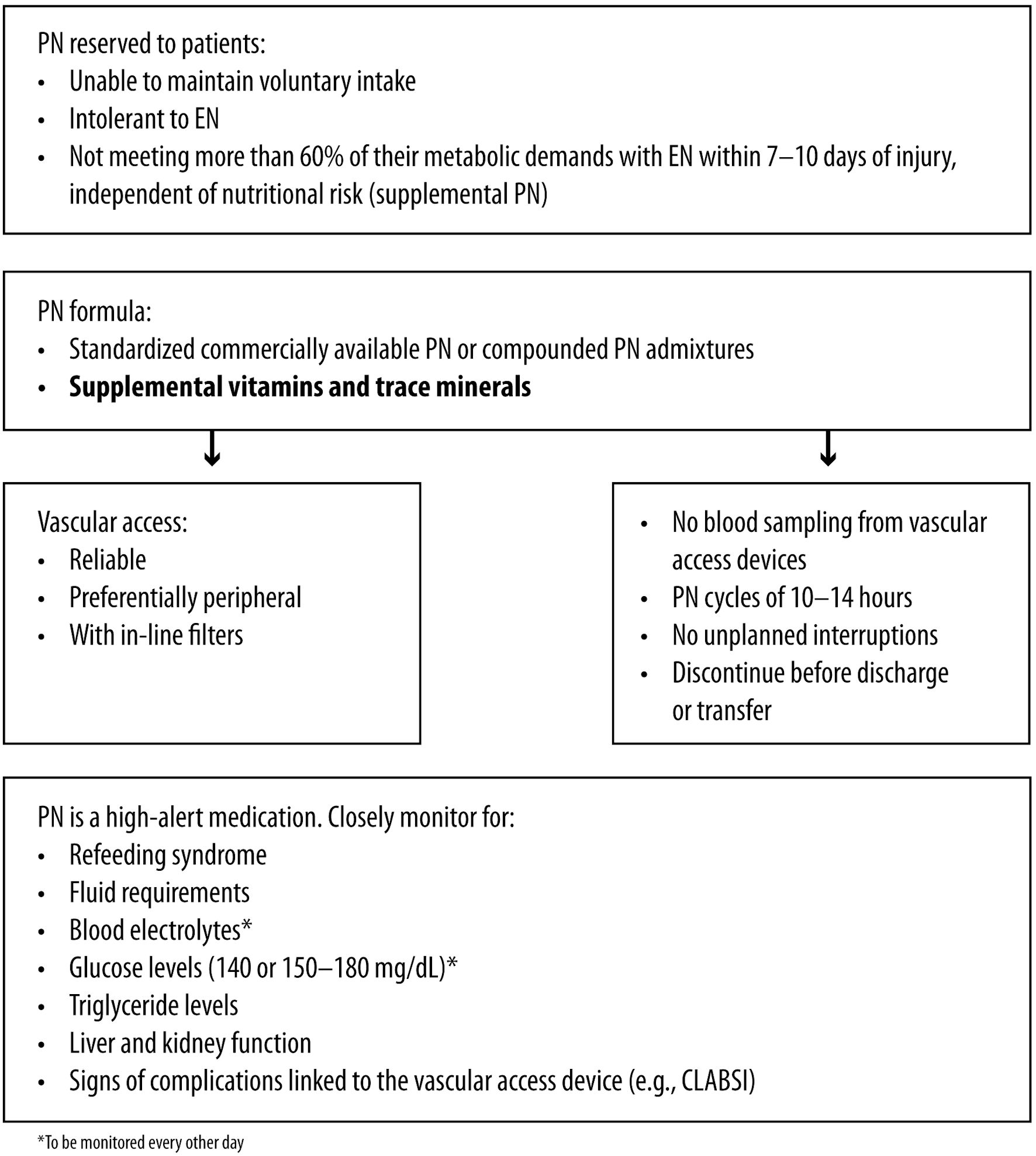 Click for large image | Figure 5. Acute SCI: management considerations for parenteral nutrition. SCI: spinal cord injury; EN: enteral nutrition; PN: parenteral nutrition; CLABSI: central line-associated bloodstream infection. |
Post-acute phase of SCI
The post-acute phase occurs 4 - 26 weeks after trauma, aligning with the rehabilitation program [18]. It is a critical period, with an overall risk of malnutrition of 40-60% in patients with SCI [78]. The risk of undernutrition during the post-acute phase of SCI is up to 47%, while the risk of being overweight can reach 80% [79, 80]. A recent Swiss longitudinal study reported a significant and 2 - 3-fold higher malnutrition risk in individuals aged > 65 years compared to those aged ≤ 65 years during rehabilitation [78].
Patients with tetraplegia tend to have a higher risk of malnutrition than those with paraplegia [37]. Additionally, comorbidities such as dysphagia, ventilator dependence or pressure injuries increase the risk of malnutrition [78].
Energy needs
While resting energy expenditure decreases during the acute phase, it remains steady during the post-acute phase (1,400 - 15,00 KCal/day) [18, 81]. The Academy of Nutrition and Dietetics (AND) guidelines recommend estimating energy needs at 22.7 kcal/kg for individuals with quadriplegia and 27.9 kcal/kg for those with paraplegia [16]. However, these formulas have not been widely replicated nor considered other factors that may affect TDEE [81, 82] and are not deemed accurate in the recent recommendation of Dietitians of Canada [32] (Fig. 1).
Nutrition assessment
The anthropometric, biochemical, clinical, and dietary assessments employed during the post-acute phase of the disease are similar to those described for the acute phase of SCI (Fig. 2). The panel deems they should be repeated with comparable frequency, especially if the patient experiences sudden significant weight loss or has pressure injuries, dysphagia, or is subjected to artificial nutrition. Specific obesity cutoffs have been proposed for established post-acute and chronic patients, ranging from a BMI of 20.2 kg/m2 (with a WC of 81.3 cm) [83] to 22 kg/m2 to 25.3 kg/m2 [84]. Overall, BMI cutoffs of 22 for overweight and 27 for obesity seem adequate to identify patients at risk of overnutrition [78, 85].
Correlations exist between obesity indicators, such as a visceral fat area > 100 cm2, a WC > 81.3 cm, a BMI > 22.5 kg/m2, and increased cardiometabolic risk [86, 87]. Notably, even a BMI < 22 kg/m2 can be associated with a percentage of body fat considered obese [82]. WC may be a better indicator of obesity and cardiovascular risk in SCI patients than BMI and waist-to-hip ratio [9, 88].
As for dietary habits, a study found similarities between patients in rehabilitation and those with chronic SCI, with excessive fat intake, inadequate consumption of carbohydrates, and low intakes of vitamins C, D, E, biotin, folic acid, potassium and iron [89].
It is advisable to regularly assess fat mass and lean body mass in SCI individuals in the post-acute phase. In particular, the ratio of fat body mass/lean body mass may serve as a more reliable metric than body fat percentage, especially considering the substantial reduction in lean body mass in patients with SCI, particularly in cases of tetraplegia [90]. However, assessing body fat in individuals with SCI poses challenges because of the need for specialized equipment, technical expertise, and time commitment. Therefore, surrogate measures like BMI and WC are commonly employed [7].
Obesity can have both favorable and unfavorable effects on individuals with SCI. Those with higher body weight and BMI upon initiation of rehabilitation tend to show greater improvements in activities of daily living [91]. However, obesity also increases the risk of complications [92]. The panel highlighted the need to monitor the lipid profile and to provide regular electrocardiographic assessments during rehabilitation for the reduction of cardiovascular risk.
The panel emphasized the challenge of accurately measuring the volitional food intake of inpatients. Indeed, since the food provided by the hospital can be unappetizing, patients may rely on food brought by visitors, the amount of which is difficult to quantify. Only four out of eight panelists reported monitoring the amount of food intake in their spinal unit. Keeping a nutritional diary during hospitalization may help address this issue.
Post-discharge phase of SCI
Metabolic syndrome is highly prevalent in individuals with chronic SCI, with higher rates among those with tetraplegia [6]. Dietary and nutritional modifications targeting the energy mismatch are recommended to prevent and treat cardiometabolic syndrome [92].
The panel suggested to provide specific nutritional information in the discharge letter, including details of nutritional supplementation during the hospital stay, and the patient’s nutritional status in relation to anthropometric measures and BMI. Six out of eight panelists reported including blood test results and nutritional recommendations; two included anthropometric data, and only one provided estimated energy needs.
Energy needs
A further decrease in resting energy expenditure was reported among patients after they returned home and at 2.5 years post-injury [18]. This decrease may be attributed to reduced activity after rehabilitation and a return to pre-trauma nutritional habits. The resting energy expenditure is 1,291 - 1,494 kcal/day in patients with chronic SCI and is influenced by the severity of the injury and patients’ characteristics [18] (Fig. 1). However, energy expenditure in SCI individuals is lower than in the non-disabled population due to decreased metabolically active tissue and reduced physical activity [93]. The panel highlights that energy needs have to be assessed whenever possible during visits and also in this phase; in particular, a re-evaluation is strongly advised in case of a change in clinical conditions (e.g., need for artificial ventilation, the appearance of a pressure lesion, multiple septic states). Farkas et al [27] assessed the impact of the level of SCI on TDEE. They found that individuals with paraplegia exhibited a significantly higher BMR and TDEE compared to those with tetraplegia. Accordingly, other studies have reported a 54% reduction in TDEE for individuals with tetraplegia [94] and around 20% for those with paraplegia [95]. As regards the influence of sex on energy expenditure, Buchholz et al reported a lower RMR in women compared to men [95]; in contrast, Gorgey et al did not observe significant differences in BMR in the two sexes [96].
Regarding energy intake, studies have reported values of 1,250 - 2,100 kcal/day in SCI patients, but the reliability of estimates depends on the accuracy of the dietary questionnaires; therefore, the actual values may be higher, and the difference in energy intake between individuals with chronic tetraplegia and paraplegia remains uncertain [97, 98]. However, the metabolic rate and total caloric intake are increased by 150 - 400 kcal/day in individuals with chronic SCI, contrasting with the 15-20% deficit recommended [97].
Nutritional assessment
The nutritional assessment in chronic SCI patients should be repeated at each follow-up visit, at least every year, to identify signs of malnutrition as early as possible and immediately if a change in the clinical condition occurs (Fig. 6).
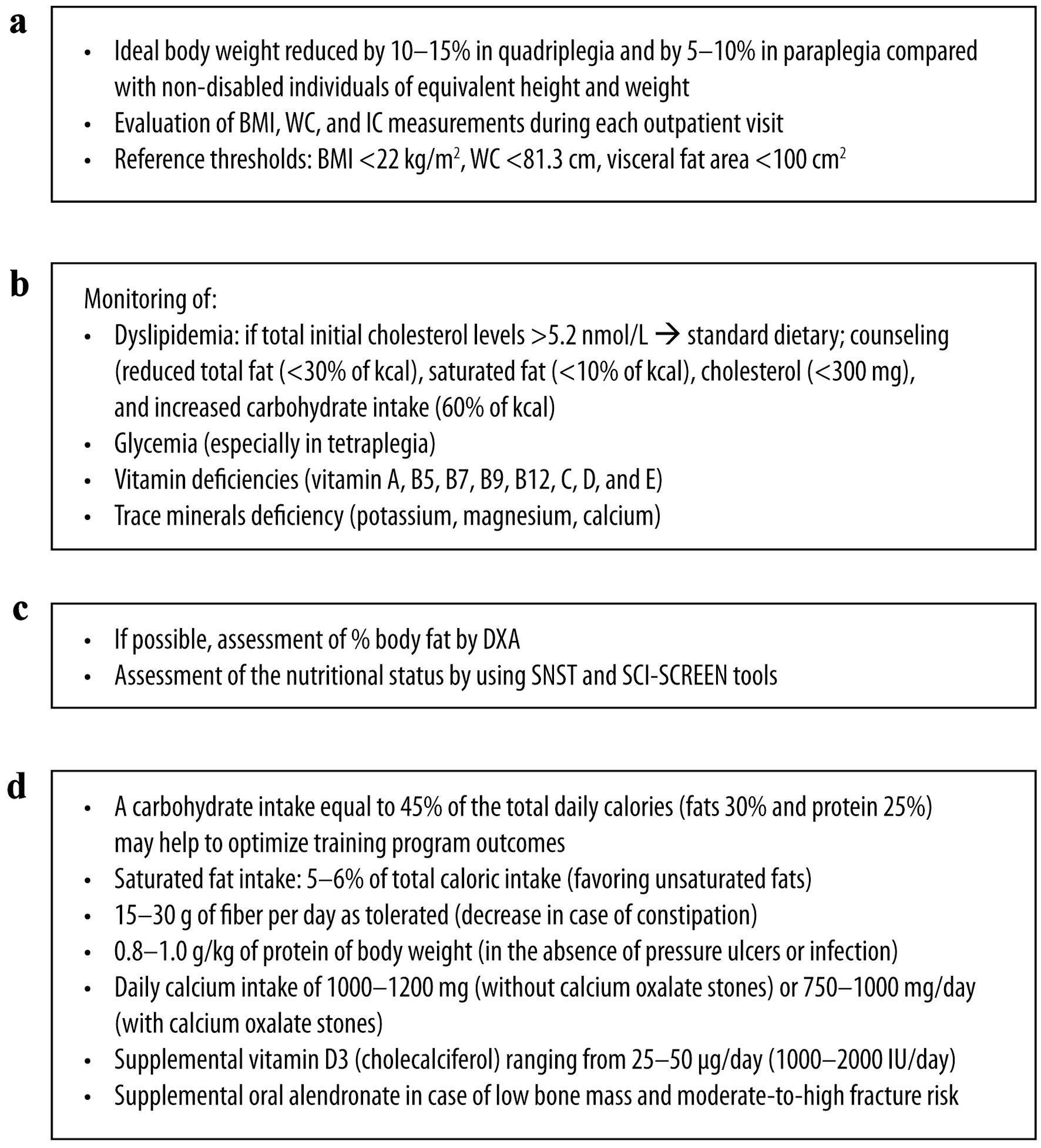 Click for large image | Figure 6. Post-discharge SCI: management considerations for (a) anthropometric, (b) biochemical, (c) clinical and (d) dietary assessment. SCI: spinal cord injury; IC: indirect calorimetry; BMI: body mass index; DXA: dual X-ray absorptiometry; SNST: spinal nutrition screening tool; WC: waist circumference. |
1) Anthropometric assessment
A BMI ≥ 25 kg/m2 is associated with metabolic syndrome in SCI [6]. A lower BMI was observed in individuals with tetraplegia compared with paraplegic ones but with higher total body fat percentage, lower lean body mass, and increased visceral fat area and volume [99].
According to the AND guidelines, the ideal body weight for SCI patients should be reduced by 10-15% in the case of quadriplegia and 5-10% in the case of paraplegia compared with non-disabled individuals [16].
Measuring BMI, WC and IC during each outpatient visit is recommended. However, relying solely on these tools may not comprehensively assess nutritional status. The panelists unanimously agreed that patients must maintain an optimal weight, determined based on the individual characteristics of each patient (e.g., age, sex, height, thoracic, abdominal and thigh circumference, percentage of fat mass), residual activity and the impact on the quality of life, especially when dealing with comorbidities, such as diabetes, cardiovascular disease, and hypertension, in order to reduce cardiovascular risk and complications from malnutrition. The authors suggest that formulas, such as that developed by Lorenz et al [100], and comparison with percentiles of healthy people [101] may be used to increase the accuracy of the estimate.
2) Biochemical assessment and blood tests
The panel recommends repeating biochemical assessments and blood tests at each follow-up visit during the post-discharge phase or more frequently if a change in clinical conditions occurs. SCI patients have a higher risk of dyslipidemia than the general population, and this risk is higher in those with paraplegia [5]. Furthermore, the injury level affects the degree of dyslipidemia, with higher serum triglycerides and low-density lipoprotein (LDL) cholesterol in people with SCI below T5 - 6 compared with those with SCI above T4 [102, 103]. The severity of dyslipidemia appears closely linked to the duration of SCI and with both visceral and subcutaneous abdominal fat [104]. Men with SCI generally exhibit a higher cardiometabolic risk and lower high-density lipoprotein (HDL) cholesterol than women [105]. Furthermore, a correlation exists between low HDL cholesterol and low testosterone [106].
The panel recommends an evaluation of neurogenic obesity and cardiometabolic risk to be performed at least annually, with second-level tests, such as cardiac echography, myocardial scintigraphy, cardiac MRI or cardiac CT scan, and stress tests performed when deemed necessary.
Diet, pharmacology, and exercise can effectively address adiposity and dyslipidemia in SCI individuals [107]. If total initial cholesterol levels are > 5.2 nmol/L, standard dietary counseling should be provided (total fat: < 30% of kcal; saturated fat: < 10%; cholesterol: < 300 mg; carbohydrate intake: 60%) [108].
About 50-60% of SCI patients show impaired glucose tolerance, with a higher prevalence in those with tetraplegia than in those with paraplegia [109]. SCI patients are also more prone to insulin resistance and have a higher incidence of diabetes [110]. Differences in hemoglobin, white blood cell count, albumin, prealbumin, serum iron, and percentage saturation levels have been reported in SCI patients compared with controls; however, all values are usually within the normal range [111].
The most common deficiencies in individuals with SCI include vitamins A, B5, B7, B9, B12, C, D, and E, and potassium, magnesium, and calcium [112, 113].
3) Clinical assessment
An increased body fat percentage of ≥ 30% was reported, with a lower increase in younger patients and those with tetraplegia [114]. Unfortunately, based on the panel’s experience, time constraints during outpatient visits pose challenges in conducting precise body composition assessments. Therefore, using fast and reliable tools such as the SNST and the SCI-SCREEN to assess nutritional status is preferable. However, the general criteria for diagnosing malnutrition remain applicable. In this regard, both the AND and the ASPEN define malnutrition as the presence of at least two of the following characteristics: insufficient caloric intake, weight loss, loss of muscle mass, loss of subcutaneous tissue, localized or generalized fluid accumulation (which may sometimes obscure weight loss), and reduced functional status as indicated by handgrip strength [38]. The GLIM criteria adopt a comprehensive approach that combines at least one phenotypic indicator (such as nonvolitional weight loss, low BMI, or decreased muscle mass) with one etiologic indicator (such as reduced food intake, impaired absorption or disease burden, or inflammatory condition) [45]. Despite the fact that SCI patients experience sarcopenia secondary to the injury, the panel highlights that these criteria remain applicable. Muscle loss due to SCI needs to be acknowledged and treated along all the phases of the disease: acute-phase patients require monitoring to maintain an adequate caloric intake, and post-acute and chronic patients need longitudinal follow-up of the considered parameters, comparing each set of data with observations from the previous 6 months.
4) Dietary assessment
Carbohydrate and fat intake are usually above the recommended values in patients with chronic SCI, while fiber intake is too low [89] and associated with an increased risk of fat storage [115], insulin resistance, and type 2 diabetes [3, 82, 116]. A standardized dietary protocol for individuals with SCI can comprise carbohydrates for 45% of the total daily calories, fats for 30% and proteins for 25% [117]. The American Heart Association limits the recommended saturated fat intake for persons with chronic SCI to 5-6% of total caloric intake [118]. In the panel’s opinion, this recommendation should be implemented as soon as possible, starting from the rehabilitation phase, to prevent fat accumulation. A higher consumption of carbohydrates has been reported among men compared to women with SCI [2]. Additionally, women with SCI were reported to consume more n-3 linolenic acid than men [2]. Groah et al [119] observed that females with SCI had a lower total calorie intake compared to males. Males with paraplegia and tetraplegia, as well as the only women with tetraplegia included in the study, exhibited a higher-than-recommended intake of fat and carbohydrates when compared to women with paraplegia [119]. Although the AND recommends consuming 15 g/day of fiber and increasing it to 20 g/day depending on tolerance following SCI and not for people at risk of gut ischemia in ICU, this recommendation is based on weak and conditional evidence [16]. High-fiber consumption without sufficient fluid intake can lead to constipation. Furthermore, fruit and vegetable consumption falls below the recommended intake for persons with SCI [50].
The protein intake in individuals with SCI typically meets or surpasses recommended values [89, 120]. However, insufficient consumption of protein-rich foods may result in inadequate intake of essential amino acids [91]. In particular, branched-chain amino acids can benefit muscle protein synthesis [121]. Notably, a protein intake within the 1.2 - 2 g/kg/day range is safe for ICU patients with renal function [122]. Protein recommendations during the chronic phase are 0.8 - 1.0 g/kg/body weight in the absence of pressure ulcers or infections [16].
Calcium intake in individuals with SCI is generally low [123]. Given the high prevalence of osteoporosis in SCI, guidelines recommended a daily calcium intake of 1,000 - 1,200 mg (sex and age-dependent) for individuals without calcium oxalate stones and 750 - 1,000 mg/day for individuals with oxalate stones, preferably by dietary intake [124]. A maintenance dose of vitamin D3 between 25 and 50 µg/day (1,000 - 2,000 IU/day) is also suggested [124]. Education plays a crucial role in promoting diets rich in high-calcium foods, vitamin D, and phosphorus to prevent metabolic dysfunction and osteoporosis [125]. The panel does not recommend periodic X-ray scans aimed at monitoring osteoporosis, as they could lead to dangerous cumulative radiation for people with SCI and a relatively young age. They are not useful for monitoring the acute phase of osteoporosis. The panel deems that people with SCI should adhere to a healthy diet with calcium and vitamin D3 supplementation and try to reduce the risk of fractures. Blood vitamin D levels and immobilization hypercalcemia should be monitored. Low-quality evidence exists for the use of alendronate, and unfortunately, no therapy for the prevention of osteoporosis in people with SCI has been devised so far. For non-pharmacologic interventions, very low-quality evidence exists for the effectiveness of standing with or without treadmill walking in acute SCI [126].
Some studies reported high alcohol consumption among SCI patients, with men consuming more alcohol than women [2]. Alcohol consumption should be investigated during dietary recalls, as it represents a risk factor for malnutrition.
| Conclusions | ▴Top |
Patients with SCI face a significant risk of malnutrition. Therefore, it is crucial to ensure adequate monitoring of their nutritional status over time, considering the changes in individual TDEE associated with different phases of the disease and identifying suitable tools for nutritional assessment. Since most of the currently available studies predominantly focus on males, further research on the impact of gender on the nutritional status of SCI patients would help clarify the needs of this population. Although the risk of obesity is higher in the chronic phase compared with undernutrition, it is important to address both ends of the nutritional spectrum. Patients should be followed by a healthcare team that includes an RD, who can regularly assess the patient’s nutritional status and implement lifestyle and dietary modifications. This comprehensive approach aims to prevent undernutrition, obesity, and associated complications, which can adversely affect prognosis and quality of life and increase the risk of hospital readmissions.
Acknowledgments
Editorial assistance was provided by Valeria Benedusi, PhD, Valentina Attanasio, Massimiliano Pianta and Aashni Shah (Polistudium SRL, Milan, Italy). Coordination was provided by Francesca Cappellini, PhD (Polistudium SRL, Milan, Italy).
Financial Disclosure
This study was conducted with the non-conditioning assistance of B. Braun SpA.
Conflict of Interest
None to declare.
Author Contributions
Study conception and design: AA, WC, AC, LDP, GDP, FF, and LP. Collection and interpretation of data: AA, WC, AC, LDP, GDP, FF, and LP. Manuscript drafting: AA, WC, AC, LDP, GDP, FF, and LP. Manuscript editing: AA, WC, AC, LDP, GDP, FF, and LP. Approval to submit: AA, WC, AC, LDP, GDP, FF, and LP.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
SCI: spinal cord injury; RD: registered dietitian; IC: indirect calorimetry; BMI: body mass index; AND: Academy of Nutrition and Dietetics; TDEE: total daily energy expenditure; TBW: total body water; ICU: intensive care unit; CRP: C-reactive protein; CHI: creatinine height index; LDL: low-density lipoprotein; GLIM: Global Leadership Initiative on Malnutrition; DXA: dual X-ray absorptiometry; BIA: bioelectrical impedance analysis; MRI: magnetic resonance imaging; SARC-F: strength, assistance with walking, rise from a chair, climb stairs and fall; CASCO: cachexia score; SPSM: short portable sarcopenia measure; CT scan: computerized tomography scan; SNST: spinal nutrition screening tool; BES: bedside swallowing evaluation; VFSS: videofluoroscopic swallow studies; FEES: fiberoptic endoscopic evaluation of swallowing; EN: enteral nutrition; PN: parenteral nutrition; SCCM: Society of Critical Care Medicine; ASPEN: American Society for Parenteral and Enteral Nutrition; CLABSI: central line-associated bloodstream infection; WC: waist circumference
| References | ▴Top |
- Farkas GJ, Sneij A, Gater DR, Jr. Energy expenditure following spinal cord injury: a delicate balance. Top Spinal Cord Inj Rehabil. 2021;27(1):92-99.
doi pubmed pmc - Farkas GJ, Sneij A, McMillan DW, Tiozzo E, Nash MS, Gater DR, Jr. Energy expenditure and nutrient intake after spinal cord injury: a comprehensive review and practical recommendations. Br J Nutr. 2022;128(5):863-887.
doi pubmed pmc - Farkas GJ, Gater DR. Neurogenic obesity and systemic inflammation following spinal cord injury: A review. J Spinal Cord Med. 2018;41(4):378-387.
doi pubmed pmc - Farkas GJ, Gater DR. Energy expenditure and nutrition in neurogenic obesity following spinal cord injury. J Phys Med Rehabil. 2020;2(1):11-13.
pubmed pmc - Peterson MD, Berri M, Lin P, Kamdar N, Rodriguez G, Mahmoudi E, Tate D. Cardiovascular and metabolic morbidity following spinal cord injury. Spine J. 2021;21(9):1520-1527.
doi pubmed pmc - Gater DR, Jr., Farkas GJ, Berg AS, Castillo C. Prevalence of metabolic syndrome in veterans with spinal cord injury. J Spinal Cord Med. 2019;42(1):86-93.
doi pubmed pmc - Farkas GJ, Burton AM, McMillan DW, Sneij A, Gater DR, Jr. The diagnosis and management of cardiometabolic risk and cardiometabolic syndrome after spinal cord injury. J Pers Med. 2022;12(7):1088.
doi pubmed pmc - Allen KJ, Leslie SW. Autonomic dysreflexia. In: StatPearls. Treasure Island (FL) ineligible companies. 2024.
pubmed - Yahiro AM, Wingo BC, Kunwor S, Parton J, Ellis AC. Classification of obesity, cardiometabolic risk, and metabolic syndrome in adults with spinal cord injury. J Spinal Cord Med. 2020;43(4):485-496.
doi pubmed pmc - Zeng X, Xiang J, Dong L, et al. Advance in nutritional status and intervention after spinal cord injury. Journal of Clinical and Nursing Research. 2021;5:7-14.
doi - Shin JC, Chang SH, Hwang SW, Lee JJ. The nutritional status and the clinical outcomes of patients with a spinal cord injury using nutritional screening tools. Ann Rehabil Med. 2018;42(4):591-600.
doi pubmed pmc - Barazzoni R, Gortan Cappellari G. Double burden of malnutrition in persons with obesity. Rev Endocr Metab Disord. 2020;21(3):307-313.
doi pubmed pmc - https://www.who.int/news-room/questions-and-answers/item/malnutrition. Last access January 11, 2024.
- Wong S, Derry F, Jamous A, Hirani SP, Forbes A. Is undernutrition risk associated with an adverse clinical outcome in spinal cord-injured patients admitted to a spinal centre? Eur J Clin Nutr. 2014;68(1):125-130.
doi pubmed - Nash MS, Groah SL, Gater DR, et al. The clinical practice guidelines for spinal cord medicine on the identification and management of cardiometabolic risk after spinal cord injury; Paralyzed Veterans of America: Washington, DC, USA. 2018.
- Academy of Nutrition and Dietetics. Spinal Cord Injury (SCI) Guidelines Chicago, Illinois. 2009. https://andeal.org/topic.cfm? menu=5292&pcat=3487&cat=5448. Last Access May 25, 2023.
- Pellegrini CA, Burkhart L, Jones K, LaVela SL. Health provider identified barriers and facilitators to weight management for individuals with spinal cord injury. Spinal Cord. 2021;59(10):1061-1071.
doi pubmed pmc - Felleiter P, Krebs J, Haeberli Y, Schmid W, Tesini S, Perret C. Post-traumatic changes in energy expenditure and body composition in patients with acute spinal cord injury. J Rehabil Med. 2017;49(7):579-584.
doi pubmed - https://spinalis.se/wp-content/uploads/2019/05/Spinalis_broschyr_eng_2019_web-1.pdf.
- Thibault-Halman G, Casha S, Singer S, Christie S. Acute management of nutritional demands after spinal cord injury. J Neurotrauma. 2011;28(8):1497-1507.
doi pubmed pmc - Todd SR, Gonzalez EA, Turner K, Kozar RA. Update on postinjury nutrition. Curr Opin Crit Care. 2008;14(6):690-695.
doi pubmed pmc - Klein CJ, Stanek GS, Wiles CE, 3rd. Overfeeding macronutrients to critically ill adults: metabolic complications. J Am Diet Assoc. 1998;98(7):795-806.
doi pubmed - Kreymann G, DeLegge MH, Luft G, Hise ME, Zaloga GP. The ratio of energy expenditure to nitrogen loss in diverse patient groups—a systematic review. Clin Nutr. 2012;31(2):168-175.
doi pubmed - Pelekhaty SL, Ramirez CL, Massetti JM, Gaetani D, Riggin K, Schwartzbauer G, Stein DM. Measured vs predicted energy expenditure in mechanically ventilated adults with acute, traumatic spinal cord injuries. Nutr Clin Pract. 2021;36(2):464-471.
doi pubmed - Ramirez CL, Pelekhaty S, Massetti JM, Galvagno S, Harmon L, Botwinick I, Scalea TM, et al. Validation of predictive equations to assess energy expenditure in acute spinal cord injury. J Trauma Acute Care Surg. 2018;85(5):984-991.
doi pubmed - Frankenfield DC, Ashcraft CM, Galvan DA. Prediction of resting metabolic rate in critically ill patients at the extremes of body mass index. JPEN J Parenter Enteral Nutr. 2013;37(3):361-367.
doi pubmed - Farkas GJ, Gorgey AS, Dolbow DR, Berg AS, Gater DR. Caloric intake relative to total daily energy expenditure using a spinal cord injury-specific correction factor: an analysis by level of injury. Am J Phys Med Rehabil. 2019;98(11):947-952.
doi pubmed pmc - Bauman WA, Cirnigliaro CM, La Fountaine MF, Jensen AM, Wecht JM, Kirshblum SC, Spungen AM. A small-scale clinical trial to determine the safety and efficacy of testosterone replacement therapy in hypogonadal men with spinal cord injury. Horm Metab Res. 2011;43(8):574-579.
doi pubmed - Nightingale TE, Gorgey AS. Predicting basal metabolic rate in men with motor complete spinal cord injury. Med Sci Sports Exerc. 2018;50(6):1305-1312.
doi pubmed - Buchholz AC, McGillivray CF, Pencharz PB. Differences in resting metabolic rate between paraplegic and able-bodied subjects are explained by differences in body composition. Am J Clin Nutr. 2003;77(2):371-378.
doi pubmed - Chun SM, Kim HR, Shin HI. Estimating the Basal metabolic rate from fat free mass in individuals with motor complete spinal cord injury. Spinal Cord. 2017;55(9):844-847.
doi pubmed - Dietitians of Canada. Spinal cord injury summary of recommendations and evidence. In: Practice-based Evidence in Nutrition® [PEN]. 2023.
- Frankenfield DC, Ashcraft CM. Description and prediction of resting metabolic rate after stroke and traumatic brain injury. Nutrition. 2012;28(9):906-911.
doi pubmed - Gardner CD, Vadiveloo MK, Petersen KS, Anderson CAM, Springfield S, Van Horn L, Khera A, et al. Popular dietary patterns: alignment with American Heart Association 2021 dietary guidance: a scientific statement from the American Heart Association. Circulation. 2023;147(22):1715-1730.
doi pubmed - Eriks-Hoogland I, Hilfiker R, Baumberger M, Balk S, Stucki G, Perret C. Clinical assessment of obesity in persons with spinal cord injury: validity of waist circumference, body mass index, and anthropometric index. J Spinal Cord Med. 2011;34(4):416-422.
doi pubmed pmc - Cragg JJ, Ravensbergen HJ, Borisoff JF, Claydon VE. Optimal scaling of weight and waist circumference to height for adiposity and cardiovascular disease risk in individuals with spinal cord injury. Spinal Cord. 2015;53(1):64-68.
doi pubmed - Davis CJ, Sowa D, Keim KS, Kinnare K, Peterson S. The use of prealbumin and C-reactive protein for monitoring nutrition support in adult patients receiving enteral nutrition in an urban medical center. JPEN J Parenter Enteral Nutr. 2012;36(2):197-204.
doi pubmed - White JV, Guenter P, Jensen G, Malone A, Schofield M, Academy of N, Dietetics Malnutrition Work G, et al. Consensus statement of the Academy of Nutrition and Dietetics/American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). J Acad Nutr Diet. 2012;112(5):730-738.
doi pubmed - Levitt DG, Levitt MD. Human serum albumin homeostasis: a new look at the roles of synthesis, catabolism, renal and gastrointestinal excretion, and the clinical value of serum albumin measurements. Int J Gen Med. 2016;9:229-255.
doi pubmed pmc - Dionyssiotis Y. Malnutrition in spinal cord injury: more than nutritional deficiency. J Clin Med Res. 2012;4(4):227-236.
doi pubmed pmc - Dempsey DT, Mullen JL, Buzby GP. The link between nutritional status and clinical outcome: can nutritional intervention modify it? Am J Clin Nutr. 1988;47(2 Suppl):352-356.
doi pubmed - Keller U. Nutritional laboratory markers in malnutrition. J Clin Med. 2019;8(6):775.
doi pubmed pmc - Evans DC, Corkins MR, Malone A, Miller S, Mogensen KM, Guenter P, Jensen GL, et al. The use of visceral proteins as nutrition markers: an ASPEN position paper. Nutr Clin Pract. 2021;36(1):22-28.
doi pubmed - Castro MJ, Apple DF, Jr., Hillegass EA, Dudley GA. Influence of complete spinal cord injury on skeletal muscle cross-sectional area within the first 6 months of injury. Eur J Appl Physiol Occup Physiol. 1999;80(4):373-378.
doi pubmed - Cederholm T, Jensen GL, Correia M, Gonzalez MC, Fukushima R, Higashiguchi T, Baptista G, et al. GLIM criteria for the diagnosis of malnutrition - A consensus report from the global clinical nutrition community. J Cachexia Sarcopenia Muscle. 2019;10(1):207-217.
doi pubmed pmc - Dionyssiotis Y, Skarantavos G, Petropoulou K, Galanos A, Rapidi CA, Lyritis GP. Application of current sarcopenia definitions in spinal cord injury. J Musculoskelet Neuronal Interact. 2019;19(1):21-29.
pubmed pmc - Miller J, Wells L, Nwulu U, Currow D, Johnson MJ, Skipworth RJE. Validated screening tools for the assessment of cachexia, sarcopenia, and malnutrition: a systematic review. Am J Clin Nutr. 2018;108(6):1196-1208.
doi pubmed - Rubbieri G, Mossello E, Di Bari M. Techniques for the diagnosis of sarcopenia. Clin Cases Miner Bone Metab. 2014;11(3):181-184.
pubmed pmc - Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, Ross RR, Garry PJ, et al. Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol. 1998;147(8):755-763.
doi pubmed - Silveira SL, Ledoux TA, Robinson-Whelen S, Stough R, Nosek MA. Methods for classifying obesity in spinal cord injury: a review. Spinal Cord. 2017;55(9):812-817.
doi pubmed - Aldobali M, Pal K, Chhabra HS, Sharawat R. Bioelectrical impedance analysis for historical evaluation in people with spinal cord injury: a systematic review. Materials Proceedings. 2022;10:3.
doi - Spungen AM, Bauman WA, Wang J, Pierson RN, Jr. Measurement of body fat in individuals with tetraplegia: a comparison of eight clinical methods. Paraplegia. 1995;33(7):402-408.
doi pubmed - van der Scheer JW, Totosy de Zepetnek JO, Blauwet C, Brooke-Wavell K, Graham-Paulson T, Leonard AN, Webborn N, et al. Assessment of body composition in spinal cord injury: A scoping review. PLoS One. 2021;16(5):e0251142.
doi pubmed pmc - Freund P, Seif M, Weiskopf N, Friston K, Fehlings MG, Thompson AJ, Curt A. MRI in traumatic spinal cord injury: from clinical assessment to neuroimaging biomarkers. Lancet Neurol. 2019;18(12):1123-1135.
doi pubmed - Hwang BY, Mampre D, Ahmed AK, Suk I, Anderson WS, Manbachi A, Theodore N. Ultrasound in traumatic spinal cord injury: a wide-open field. Neurosurgery. 2021;89(3):372-382.
doi pubmed pmc - Shaw SC, Dennison EM, Cooper C. Epidemiology of sarcopenia: determinants throughout the lifecourse. Calcif Tissue Int. 2017;101(3):229-247.
doi pubmed pmc - Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, Kritchevsky SB, et al. Sarcopenia: alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51(11):1602-1609.
doi pubmed - Aldobali M, Pal K, Chhabra HS. Validation and predicting total body water in people with spinal cord injury using bioelectrical impedance analysis. Journal of Information and Optimization Sciences. 2022;43:25-36.
doi - Schierbauer J, Gunther S, Haupt S, Zimmer RT, Herz D, Voit T, Zimmermann P, et al. Acute fluid intake impacts assessment of body composition via bioelectrical impedance analysis. a randomized, controlled crossover pilot trial. Metabolites. 2023;13(4):473.
doi pubmed pmc - Sarvazyan A, Tatarinov A, Sarvazyan N. Ultrasonic assessment of tissue hydration status. Ultrasonics. 2005;43(8):661-671.
doi pubmed - Carrero JJ, Johansen KL, Lindholm B, Stenvinkel P, Cuppari L, Avesani CM. Screening for muscle wasting and dysfunction in patients with chronic kidney disease. Kidney Int. 2016;90(1):53-66.
doi pubmed - Wong S, Derry F, Jamous A, Hirani SP, Grimble G, Forbes A. Validation of the spinal nutrition screening tool (SNST) in patients with spinal cord injuries (SCI): result from a multicentre study. Eur J Clin Nutr. 2012;66(3):382-387.
doi pubmed - Steensgaard R, Bonne S, Wojke P, Kasch H. SCI-SCREEN: a more targeted nutrition screening model to detect spinal cord-injured patients at risk of malnutrition. Rehabil Nurs. 2019;44(1):11-19.
doi pubmed - Wu XS, Miles A, Braakhuis AJ. Texture-Modified Diets, Nutritional Status and Mealtime Satisfaction: A Systematic Review. Healthcare (Basel). 2021;9(6):624.
doi pubmed pmc - Chaw E, Shem K, Castillo K, Wong SL, Chang J. Dysphagia and associated respiratory considerations in cervical spinal cord injury. Top Spinal Cord Inj Rehabil. 2012;18(4):291-299.
doi pubmed pmc - Giraldo-Cadavid LF, Bastidas AR, Maldonado-Lancheros J, Gasca-Zuluaga DA, Aguilar-Farias MJ, Bohorquez-Tibavisco L. Pneumonia, mortality, and other outcomes associated with unsafe swallowing detected via fiberoptic endoscopic evaluation of swallowing (FEES) in patients with functional oropharyngeal dysphagia: a systematic review and meta-analysis. Dysphagia. 2022;37(6):1662-1672.
doi pubmed - Dvorak MF, Noonan VK, Belanger L, Bruun B, Wing PC, Boyd MC, Fisher C. Early versus late enteral feeding in patients with acute cervical spinal cord injury: a pilot study. Spine (Phila Pa 1976). 2004;29(9):E175-180.
doi pubmed - Rowan CJ, Gillanders LK, Paice RL, Judson JA. Is early enteral feeding safe in patients who have suffered spinal cord injury? Injury. 2004;35(3):238-242.
doi pubmed - Bourgault AM, Ipe L, Weaver J, Swartz S, O'Dea P J. Development of evidence-based guidelines and critical care nurses ' knowledge of enteral feeding. Crit Care Nurse. 2007;27(4):17-22, 25-19; quiz 30.
pubmed - Racco M. An enteral nutrition protocol to improve efficiency in achieving nutritional goals. Crit Care Nurse. 2012;32(4):72-75.
doi pubmed - McClave SA, Taylor BE, Martindale RG, Warren MM, Johnson DR, Braunschweig C, McCarthy MS, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN J Parenter Enteral Nutr. 2016;40(2):159-211.
doi pubmed - Boullata JI. Overview of the parenteral nutrition use process. JPEN J Parenter Enteral Nutr. 2012;36(2 Suppl):10S-13S.
doi pubmed - Buchman AL, Opilla M, Kwasny M, Diamantidis TG, Okamoto R. Risk factors for the development of catheter-related bloodstream infections in patients receiving home parenteral nutrition. JPEN J Parenter Enteral Nutr. 2014;38(6):744-749.
doi pubmed - Suryadevara S, Celestin J, DeChicco R, Austhof S, Corrigan M, Speerhas R, Steiger E. Type and prevalence of adverse events during the parenteral nutrition cycling process in patients being prepared for discharge. Nutr Clin Pract. 2012;27(2):268-273.
doi pubmed - Ayers P, Adams S, Boullata J, Gervasio J, Holcombe B, Kraft MD, Marshall N, et al. A.S.P.E.N. parenteral nutrition safety consensus recommendations. JPEN J Parenter Enteral Nutr. 2014;38(3):296-333.
doi pubmed - Schonenberger KA, Reber E, Durig C, Baumgartner A, Efthymiou A, Huwiler VV, Laimer M, et al. Management of hyperglycemia in hospitalized patients receiving parenteral nutrition. Front Clin Diabetes Healthc. 2022;3:829412.
doi pubmed pmc - Khan A, Laing E, Beaumont A, Wong J, Warrier S, Heriot A. Peripheral parenteral nutrition in surgery - a systematic review and meta-analysis. Clin Nutr ESPEN. 2023;54:337-348.
doi pubmed - Flury I, Mueller G, Perret C. The risk of malnutrition in patients with spinal cord injury during inpatient rehabilitation-A longitudinal cohort study. Front Nutr. 2023;10:1085638.
doi pubmed pmc - Wong S, Kenssous N, Hillier C, Pollmer S, Jackson P, Lewis S, Saif M. Detecting malnutrition risk and obesity after spinal cord injury: a quality improvement project and systematic review. Eur J Clin Nutr. 2018;72(11):1555-1560.
doi pubmed - Wong S, Derry F, Grimble G, Forbes A. How do spinal cord injury centres manage malnutrition? A cross-sectional survey of 12 regional centres in the United Kingdom and Ireland. Spinal Cord. 2012;50(2):132-135.
doi pubmed - Cox SA, Weiss SM, Posuniak EA, Worthington P, Prioleau M, Heffley G. Energy expenditure after spinal cord injury: an evaluation of stable rehabilitating patients. J Trauma. 1985;25(5):419-423.
pubmed - Gater DR, Jr. Obesity after spinal cord injury. Phys Med Rehabil Clin N Am. 2007;18(2):333-351.
doi pubmed - Yun JH, Chun SM, Kim JC, Shin HI. Obesity cutoff values in Korean men with motor complete spinal cord injury: body mass index and waist circumference. Spinal Cord. 2019;57(2):110-116.
doi pubmed - Ayas NT, Epstein LJ, Lieberman SL, Tun CG, Larkin EK, Brown R, Garshick E. Predictors of loud snoring in persons with spinal cord injury. J Spinal Cord Med. 2001;24(1):30-34.
doi pubmed - Laughton GE, Buchholz AC, Martin Ginis KA, Goy RE, The SHAPE SCI Research Group. Lowering body mass index cutoffs better identifies obese persons with spinal cord injury. Spinal Cord. 2009;47(10):757-762.
doi pubmed - Sumrell RM, Nightingale TE, McCauley LS, Gorgey AS. Anthropometric cutoffs and associations with visceral adiposity and metabolic biomarkers after spinal cord injury. PLoS One. 2018;13(8):e0203049.
doi pubmed pmc - Shin JW, Kim T, Lee BS, Kim O. Factors affecting metabolic syndrome in individuals with chronic spinal cord injury. Ann Rehabil Med. 2022;46(1):24-32.
doi pubmed pmc - Dorton MC, Lucci VM, de Groot S, Loughin TM, Cragg JJ, Kramer JK, Post MWM, et al. Evaluation of cardiovascular disease risk in individuals with chronic spinal cord injury. Spinal Cord. 2021;59(7):716-729.
doi pubmed - Perret C, Stoffel-Kurt N. Comparison of nutritional intake between individuals with acute and chronic spinal cord injury. J Spinal Cord Med. 2011;34(6):569-575.
doi pubmed pmc - Farkas GJ, Gorgey AS, Dolbow DR, Berg AS, Gater DR. The influence of level of spinal cord injury on adipose tissue and its relationship to inflammatory adipokines and cardiometabolic profiles. J Spinal Cord Med. 2018;41(4):407-415.
doi pubmed pmc - Tanaka M, Momosaki R, Wakabayashi H, Kikura T, Maeda K. Relationship between nutritional status and improved ADL in individuals with cervical spinal cord injury in a convalescent rehabilitation ward. Spinal Cord. 2019;57(6):501-508.
doi pubmed - Bigford G, Nash MS. Nutritional health considerations for persons with spinal cord injury. Top Spinal Cord Inj Rehabil. 2017;23(3):188-206.
doi pubmed pmc - Price M. Energy expenditure and metabolism during exercise in persons with a spinal cord injury. Sports Med. 2010;40(8):681-696.
doi pubmed - Mollinger LA, Spurr GB, el Ghatit AZ, Barboriak JJ, Rooney CB, Davidoff DD, Bongard RD. Daily energy expenditure and basal metabolic rates of patients with spinal cord injury. Arch Phys Med Rehabil. 1985;66(7):420-426.
pubmed - Buchholz AC, McGillivray CF, Pencharz PB. Physical activity levels are low in free-living adults with chronic paraplegia. Obes Res. 2003;11(4):563-570.
doi pubmed - Gorgey AS, Farkas GJ, Dolbow DR, Khalil RE, Gater DR. Gender dimorphism in central adiposity may explain metabolic dysfunction after spinal cord injury. PM R. 2018;10(4):338-348.
doi pubmed - Farkas GJ, Pitot MA, Berg AS, Gater DR. Nutritional status in chronic spinal cord injury: a systematic review and meta-analysis. Spinal Cord. 2019;57(1):3-17.
doi pubmed - Farkas GJ, Sneij A, Gater DR, Jr. Dietetics after spinal cord injury: current evidence and future perspectives. Top Spinal Cord Inj Rehabil. 2021;27(1):100-108.
doi pubmed pmc - Raguindin PF, Bertolo A, Zeh RM, Frankl G, Itodo OA, Capossela S, Bally L, et al. Body composition according to spinal cord injury level: a systematic review and meta-analysis. J Clin Med. 2021;10(17):3911.
doi pubmed pmc - Lorenz MW, Graf M, Henke C, Hermans M, Ziemann U, Sitzer M, Foerch C. Anthropometric approximation of body weight in unresponsive stroke patients. J Neurol Neurosurg Psychiatry. 2007;78(12):1331-1336.
doi pubmed pmc - Gray GE, Gray LK. Anthropometric measurements and their interpretation: principles, practices, and problems. J Am Diet Assoc. 1980;77(5):534-539.
pubmed - La Fountaine MF, Cirnigliaro CM, Kirshblum SC, McKenna C, Bauman WA. Effect of functional sympathetic nervous system impairment of the liver and abdominal visceral adipose tissue on circulating triglyceride-rich lipoproteins. PLoS One. 2017;12(3):e0173934.
doi pubmed pmc - La Fountaine MF, Cirnigliaro CM, Hobson JC, Dyson-Hudson TA, Mc Kenna C, Kirshblum SC, Spungen AM, et al. Establishing a threshold to predict risk of cardiovascular disease from the serum triglyceride and high-density lipoprotein concentrations in persons with spinal cord injury. Spinal Cord. 2018;56(11):1051-1058.
doi pubmed pmc - Rankin KC, O'Brien LC, Segal L, Khan MR, Gorgey AS. Liver adiposity and metabolic profile in individuals with chronic spinal cord injury. Biomed Res Int. 2017;2017:1364818.
doi pubmed pmc - Sabour H, Latifi S, Soltani Z, Shakeri H, Norouzi Javidan A, Ghodsi SM, Hadian MR, et al. C-reactive protein as an available biomarker determining mental component of health-related quality of life among individuals with spinal cord injury. J Spinal Cord Med. 2017;40(3):329-337.
doi pubmed pmc - Abilmona SM, Sumrell RM, Gill RS, Adler RA, Gorgey AS. Serum testosterone levels may influence body composition and cardiometabolic health in men with spinal cord injury. Spinal Cord. 2019;57(3):229-239.
doi pubmed pmc - Gater DR. Point: Counterpoint synopsis of cardiometabolic risk after spinal cord injury. Spinal Cord Ser Cases. 2019;5:98.
doi pubmed pmc - https://scireproject.com/wp-content/uploads/2022/03/Nutrition_FINAL_V7.pdf.
- Bauman WA, Spungen AM. Disorders of carbohydrate and lipid metabolism in veterans with paraplegia or quadriplegia: a model of premature aging. Metabolism. 1994;43(6):749-756.
doi pubmed - Elder CP, Apple DF, Bickel CS, Meyer RA, Dudley GA. Intramuscular fat and glucose tolerance after spinal cord injury—a cross-sectional study. Spinal Cord. 2004;42(12):711-716.
doi pubmed - Lynch AC, Palmer C, Lynch AC, Anthony A, Roake JA, Frye J, Frizelle FA. Nutritional and immune status following spinal cord injury: a case controlled study. Spinal Cord. 2002;40(12):627-630.
doi pubmed - Moussavi RM, Garza HM, Eisele SG, Rodriguez G, Rintala DH. Serum levels of vitamins A, C, and E in persons with chronic spinal cord injury living in the community. Arch Phys Med Rehabil. 2003;84(7):1061-1067.
doi pubmed - Petchkrua W, Little JW, Burns SP, Stiens SA, James JJ. Vitamin B12 deficiency in spinal cord injury: a retrospective study. J Spinal Cord Med. 2003;26(2):116-121.
doi pubmed - Gorgey AS, Caudill C, Sistrun S, Khalil RE, Gill R, Castillo T, Lavis T, et al. Frequency of dietary recalls, nutritional assessment, and body composition assessment in men with chronic spinal cord injury. Arch Phys Med Rehabil. 2015;96(9):1646-1653.
doi pubmed - Khalil RE, Gorgey AS, Janisko M, Dolbow DR, Moore JR, Gater DR. The role of nutrition in health status after spinal cord injury. Aging Dis. 2013;4(1):14-22.
pubmed pmc - Gorgey AS, Dolbow DR, Dolbow JD, Khalil RK, Castillo C, Gater DR. Effects of spinal cord injury on body composition and metabolic profile - part I. J Spinal Cord Med. 2014;37(6):693-702.
doi pubmed pmc - Gorgey AS, Mather KJ, Cupp HR, Gater DR. Effects of resistance training on adiposity and metabolism after spinal cord injury. Med Sci Sports Exerc. 2012;44(1):165-174.
doi pubmed - The American Heart Association Diet and Lifestyle Recommendations. Available from: https://www.heart.org/en/healthy-living/healthy-eating/eat-smart/nutrition-basics/aha-diet-and-lifestyle-recommendations. Accessed May 24, 2023.
- Groah SL, Nash MS, Ljungberg IH, Libin A, Hamm LF, Ward E, Burns PA, et al. Nutrient intake and body habitus after spinal cord injury: an analysis by sex and level of injury. J Spinal Cord Med. 2009;32(1):25-33.
doi pubmed pmc - Silveira SL, Winter LL, Clark R, Ledoux T, Robinson-Whelen S. Baseline dietary intake of individuals with spinal cord injury who are overweight or obese. J Acad Nutr Diet. 2019;119(2):301-309.
doi pubmed - Shimomura Y, Murakami T, Nakai N, Nagasaki M, Harris RA. Exercise promotes BCAA catabolism: effects of BCAA supplementation on skeletal muscle during exercise. J Nutr. 2004;134(6 Suppl):1583S-1587S.
doi pubmed - Danielis M, Lorenzoni G, Azzolina D, Iacobucci A, Trombini O, De Monte A, Gregori D, et al. Effect of protein-fortified diet on nitrogen balance in critically ill patients: results from the OPINiB trial. Nutrients. 2019;11(5):972.
doi pubmed pmc - Sneij A, Farkas GJ, Carino Mason MR, Gater DR. Nutrition education to reduce metabolic dysfunction for spinal cord injury: a module-based nutrition education guide for healthcare providers and consumers. J Pers Med. 2022;12(12):2029.
doi pubmed pmc - Consortium for Spinal Cord Medicine. Clinical practice guidelines on bone health and osteoporosis management in individuals with spinal cord injury. Paralyzed Veterans of America: Washington, DC, USA, 2022.
- Weaver CM, Alexander DD, Boushey CJ, Dawson-Hughes B, Lappe JM, LeBoff MS, Liu S, et al. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int. 2016;27(1):367-376.
doi pubmed pmc - Soleyman-Jahi S, Yousefian A, Maheronnaghsh R, Shokraneh F, Zadegan SA, Soltani A, Hosseini SM, et al. Evidence-based prevention and treatment of osteoporosis after spinal cord injury: a systematic review. Eur Spine J. 2018;27(8):1798-1814.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


