| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Case Report
Volume 15, Number 6, June 2023, pages 332-335
An Uncommon Presentation of Acute Thoracic Aortic Dissection
MacKenzie Bartona, Hao Wanga, b
aDepartment of Emergency Medicine, John Peter Smith Health Network, Fort Worth, TX 76104, USA
bCorresponding Author: Hao Wang, Department of Emergency Medicine, John Peter Smith Health Network, Fort Worth, TX 76104, USA
Manuscript submitted April 12, 2023, accepted June 17, 2023, published online June 29, 2023
Short title: An Uncommon Case Acute Aortic Dissection
doi: https://doi.org/10.14740/jocmr4921
| Abstract | ▴Top |
We present a case of a 40-year-old Caucasian male with past medical history of polysubstance abuse (cocaine and methamphetamine), who presented to the emergency department (ED) complaining of intermittent cough with associated chest discomfort and shortness of breath for 2 weeks. Initial vital signs demonstrated borderline tachycardia (98 beats per minute), tachypnea (37 times per minutes), and hypoxia (oxygen saturation 89% on room air), and his physical exam was grossly unremarkable. A preliminary workup including a computed tomography angiography (CTA) revealed a type A aortic dissection with both thoracic and abdominal involvement for which the patient was admitted. This patient had resection of the ascending aorta with graft placement, cardiopulmonary bypass, aortic root replacement using composite prosthesis and left and right coronary reconstruction and reimplantation and survived a complicated hospital course. This case demonstrates the classic association known to exist between recreational drug use, specifically stimulants such as cocaine and amphetamines, and acute aortic dissection (AAD). However, such a presentation of borderline subacute, painless dissection in the setting of polysubstance use raises further questions, since uncommon AAD is typically found in higher-risk populations such as those with connective tissue disorders (Marfan syndrome, Ehlers-Danlos syndrome, Loeys-Dietz syndrome), bicuspid aortic valve, chronic hypertension, or previous aortic pathology. We therefore suggest clinicians strongly consider uncommon AAD as part of their differential diagnosis in patients with known or highly suspected polysubstance abuse.
Keywords: Acute aortic dissection; Uncommon; Polysubstance use
| Introduction | ▴Top |
Acute aortic dissection (AAD) occurs approximately 2.9 - 3.5 cases per 100,000 person-years in the general population [1, 2]. Its mortality varies depending on the different types of AAD, which ranged from over 10% to nearly 30% [3, 4]. Patients with delayed diagnosis and treatment usually resulted in poor clinical outcomes [5]. Since AAD has a relatively higher mortality, accurate diagnosis and prompt management become extremely important. Risk factors for AAD include patients with certain clinical conditions like aortic aneurysm, hypertension, giant cell arteritis, bicuspid aortic valve, family history of aortic disease and connective tissue disorders (Marfan syndrome, Ehlers-Danlos syndrome, Loeys-Dietz syndrome, familial thoracic aortic aneurysm, and dissection syndrome) [6]. Patients with AAD typically present with sudden-onset tearing and/or migratory chest/back/abdominal pain, syncope, shortness of breath, and some neurological symptoms such as weakness, paraplegia, etc. The most common clinical presentations are chest pain (70-90%), back pain (20-40%), and syncope/loss of consciousness (10-30%) [7, 8].
In certain cases, patients with AAD might present with uncommon symptoms. These include shortness of breath, cough and a clinical presentation resembling acute congestive heart failure [9], especially among patients with Marfan syndrome. Unlike traditional aortic dissection, painless aortic dissection presents with subtle or no symptoms can result in delayed diagnosis and misdiagnosis, ultimately leading to a higher mortality rate [10].
In recent years, the association between AAD and substance abuse has been investigated. There is a statistically significant connection between amphetamine abuse and AAD [11]. Additionally, cocaine-related aortic dissection has already been reported in the previous study [12]. However, reports of substance abuse related to painless aortic dissection are still limited. Here we report a case of a patient with history of hypertension and polysubstance abuse with no prior cardiac or relevant family history, who presented to the emergency department (ED) with complains of shortness of breath and cough with no active chest pain found to have an acute thoracic aortic dissection. We propose a connection between atypical presentation and polysubstance abuse in the setting of acute thoracic aortic dissection.
| Case Report | ▴Top |
An unemployed, 40-year-old, 5-foot 9-inch, 164-pound Caucasian male (body mass index (BMI) 24) with past medical history of cocaine abuse, methamphetamine abuse, alcohol use disorder and uncontrolled hypertension, presents to an urban level 1 trauma center complaining of cough and shortness of breath worsening over the last 2 weeks. Patient had been seen twice prior to the index ED visit. The first visit was to an urgent care center over the previous 6 days for dysuria/penile discharge and was treated empirically for gonorrhea/chlamydia. The second visit was presented to the ED for a similar chief complaint, he had a chest X-ray performed which was normal and screening labs ordered, including electrocardiogram (ECG) and troponin that were not performed as the patient eloped from the ED.
He then presented to the ED 2 days later complaining of shortness of breath, cough and concerns he may have coronavirus disease 2019 (COVID-19), with symptoms worsening over the last 2 weeks. At the time of presentation, the patient admitted having shortness of breath that was worsening with exertion, dry cough. This patient was pain-free at the time of presentation but noted intermittent chest pain associated with cough only over the last 2 weeks. Of note, the patient admitted to a known history of hypertension but was not taking his prescribed 50 mg atenolol due to financial and social reasons. He also admitted to subjective fever, chills, and fatigue but stated his nausea, vomiting, and testicular pain (which he was seen for at the urgent care) had resolved.
On physical examination, this patient was hypertensive (150/68 mm Hg), hypoxic (87% on room air), tachypneic (37 times per minute) and borderline tachycardic (98 beats per minute). He was placed on 2 L of oxygen via nasal cannula and roomed in the ED as a priority alert. He was noted to have increased work of breathing with tachypnea, accessory muscle use and coarse lung sounds bilaterally. Pulses were equal and there was no evidence of volume overload including lower extremity edema or jugular vein distention (JVD). His heart rate was regular rhythm with normal heart sounds. No neurological deficits were found in the exam. On arrival at the ED, given his presentation and acute hypoxic respiratory failure, the patient was treated empirically with ipratropium-albuterol (DuoNeb) 0.5 mg/3 mg, and 6 mg of Decadron.
Given his clinical presentation, a relatively broad cardiopulmonary and infectious workup was ordered. ECG obtained on presentation showed normal sinus rhythm with suggestion of left ventricular hypertrophy but with no acute emergent pathology (no evidence of right heart strain and no ST segment elevation). Notable labs included a white blood count (WBC) of 19.14 × 109/L (increased from 14.8 × 109/L occurred 2 days ago), stable hemoglobin at 12.3 g/dL, creatinine of 1.56 mg/dL, normal troponin (34 ng/L), C-reactive protein (CRP) of 10.6 mg/dL (elevated), N-terminal pro-brain natriuretic peptide (NT-proBNP) of 5,411 pg/mL (elevated) and a negative COVID PCR. Arterial blood gas showed pH 7.46, arterial partial pressure of oxygen (PaO2) 67.4 mm Hg, partial pressure of carbon dioxide (PCO2) 30.9 mm Hg, HCO3 21.5 mmol/L, and lactate 1.6 mmol/L.
A chest X-ray was ordered immediately which showed cardiomegaly, central vascular congestion with interval development of diffuse perihilar consolidation, and trace effusions (Fig. 1). COVID, which had a known increased risk of coagulopathy and pulmonary embolism (PE) was high on our initial differentials. Given the severity of his presentation, a D-dimer was deferred for a statim CTA PE. This showed “acute thoracic aortic dissection beginning in the aortic root and extending into the descending thoracic aorta at least to the level of the diaphragmatic hiatus”. In addition, pulmonary edema, bilateral pleural effusions (right more than left), and patchy bilateral ground-glass opacities were also observed. There was no PE (Figs. 2, 3).
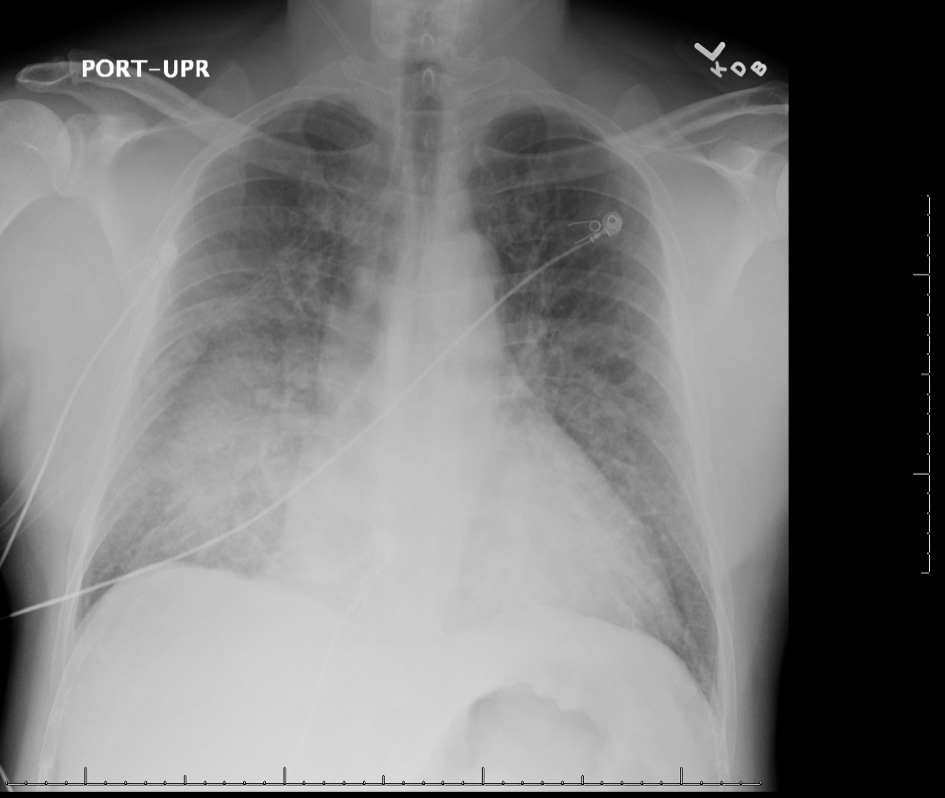 Click for large image | Figure 1. Chest X-ray (anterior-posterior view) showing cardiomegaly, perihilar consolidations, and suspected pulmonary edema with trace effusions (scale on screen 1 cm increments). |
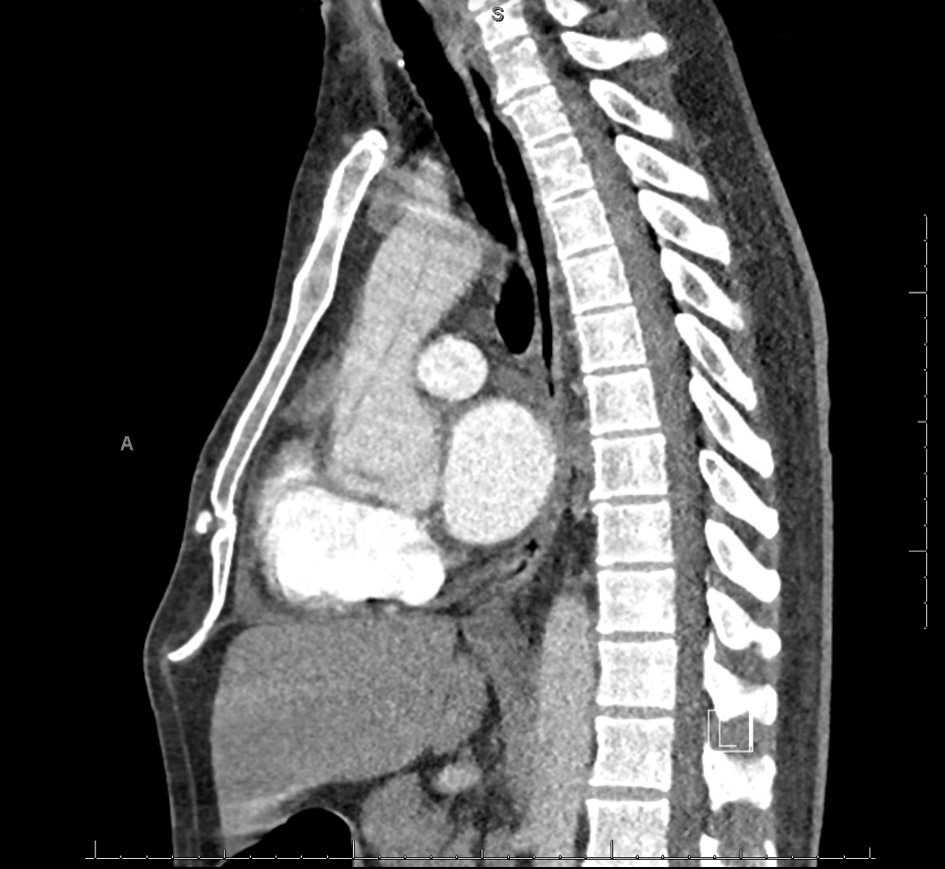 Click for large image | Figure 2. CTA PE protocol (sagittal view) showing descending aorta view with dissection. Note contrast timing in the pulmonary vessels (scale on screen 1 cm increments). CTA: computed tomography angiography; PE: pulmonary embolism. |
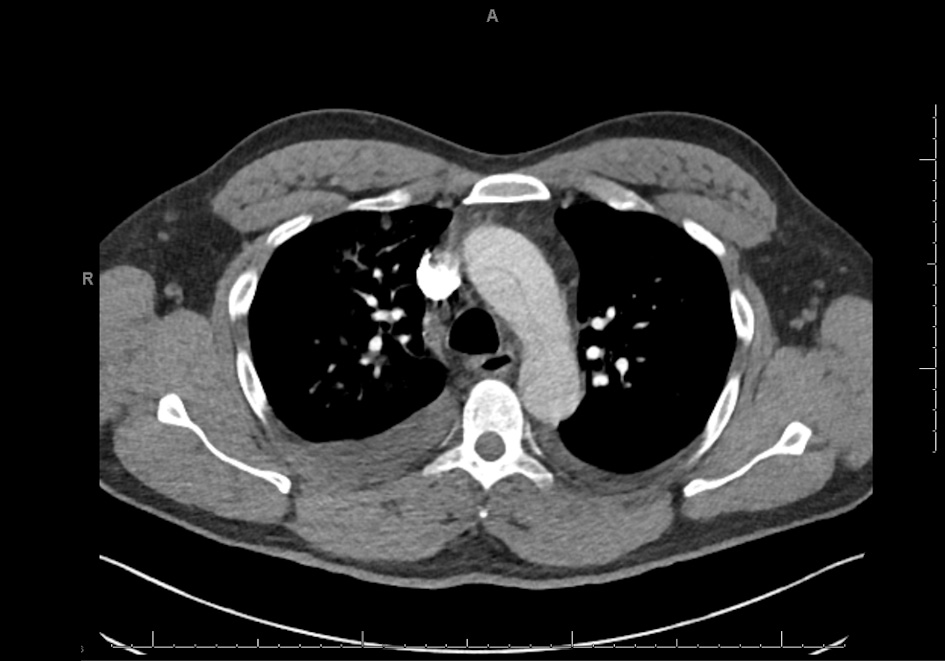 Click for large image | Figure 3. CTA PE protocol (axial view) showing aortic arch with dissection (scale on screen 1 cm increments). CTA: computed tomography angiography; PE: pulmonary embolism. |
Esmolol infusion was started (75 µg/kg/min) with goal systolic blood pressure < 120 mm Hg and heart rate (HR) goal < 70 beats per minute. Cardiothoracic (CT) surgery was consulted, and the patient was taken to the operating room emergently. He was found to have severe aortic insufficiency and underwent resection of the ascending aorta with graft placement, cardiopulmonary bypass, aortic root replacement using composite prosthesis and left and right coronary reconstruction and reimplantation. This patient was noted to have a complicated operative course. Patient was coagulopathic, received multiple blood products intraoperatively and was taken to the intensive care unit (ICU) in critical condition. Patient was extubated on postoperative day 2, transferred out of the ICU on postoperative day 3, developed prolonged ileus starting postoperative day 5, which required total parenteral nutrition but was eventually advanced back to regular diet and discharged on postoperative day 18 with metoprolol and aspirin prescriptions.
The patient was seen for follow-up 1 month after presenting to the ED and at that time was recovering as expected with no pain or shortness of breath. A 6-month follow-up CTA was ordered for aortic surveillance. He also followed up with family medicine a week later as well as cardiology 2 months later. Patient was compliant with aspirin and metoprolol at that time but was lost to follow-up after that.
| Discussion | ▴Top |
Here, we reported a case of 40-year-old male, who presented to ED with shortness of breath and cough, no chest pain upon arrival. He was eventually diagnosed with AAD. The patient was taken to the operation room immediately after AAD was diagnosed. The hospital operational findings confirmed AAD and severe aortic insufficiency. Prompt diagnosis and treatment are the key management for patients with AAD. As stated above, AAD can be easily missed if certain patients had uncommon clinical presentations. AAD without the three most common presentations (i.e., chest pain, back pain, and syncope) is uncommon, especially among patients without any risk factors that attributed to the uncommon clinical presentations (such as elderly patients, Marfan syndrome). Our patient had multiple ED/urgent care visits in the past with diverse complaints, which may mislead the healthcare providers to make appropriate differential diagnoses.
Numerous studies have identified the intensity of the onset of pain and description of the pain as the most reliable factor predicting aortic dissection [13]. The pain is typically sudden at onset, reaches maximal severity quickly and is typically described as tearing. The presentation of AAD depends on the extent of the dissection flap and the specific area of the aorta involved. After reviewing the literature, we found that there were several reports of painless aortic dissection. Most of these patients were elderly “(> 65 years old)” with comorbidities (such as hypertension, diabetes) [6], or with typically associated predisposing genetic conditions including Marfan syndrome and related disorders. Our patient reported no active chest pain at the time of presentation other than with cough, nor had any of the aforementioned high-risk factors which often lead to AAD.
In addition, our patient’s painless presentation with no other high-risk exam features is atypical given the extent of this patient’s dissection from the aortic root to the diaphragm. Typical exam features include wide pulse pressure, aortic insufficiency, diastolic murmur, muffled heart sounds (if large effusion/tamponade), syncope, altered mental status, loss of peripheral pulses, neurological deficit, or Horner syndrome [13]. This patient had none of these presenting features. He also did not have high-risk conditions such as older age, Marfan syndrome, family history of aortic disease, known aortic valvular disease, and recent aortic manipulation.
Though this patient had an atypical story and uncommon physical exam for AAD, he had other known risk factors including hypertension, smoking, and substance abuse. Substance abuse can cause abrupt transient and severe increase in blood pressure (sympathomimetic use), increased sympathetic tone leading to vasoconstriction (cocaine use), and potential development of vasculitis (cocaine use), which have been reported in the literature [14-18]. One study reported by Bhogal et al found that middle-aged male (43 years old) with history of intravenous drug abuse (IVDA) had painless AAD [19]. A study from the international registry of AAD reported more than 7,300 cases of AAD and found that cocaine use was implicated in 1.8% of patients [20]. However, there is limited research reported to address the potential mechanism(s) on the association between atypical/painless AAD and drug use.
In summary, though we have already realized that AAD may present without characteristic chest pain features, its mechanisms are still unclear. This case reported substance abuse especially stimulants use (such as cocaine and methamphetamine) can result in painless AAD. We thus suggest that special attention should be paid among younger persons with recent use of stimulants, as this could be one of the potential mechanisms attributing to the painless AAD presentations. Future study focusing on substance use and AAD is warranted for such validations.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
Authors claimed that there is no conflict of interest.
Informed Consent
Informed consent was given verbally to discuss educational purposes during initial conversation after diagnosis in the emergency department.
Author Contributions
MB and HW did the literature search and review, wrote the initial manuscript, reviewed and revised the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Meszaros I, Morocz J, Szlavi J, Schmidt J, Tornoci L, Nagy L, Szep L. Epidemiology and clinicopathology of aortic dissection. Chest. 2000;117(5):1271-1278.
doi pubmed - Clouse WD, Hallett JW, Jr., Schaff HV, Spittell PC, Rowland CM, Ilstrup DM, Melton LJ, 3rd. Acute aortic dissection: population-based incidence compared with degenerative aortic aneurysm rupture. Mayo Clin Proc. 2004;79(2):176-180.
doi pubmed - Harris KM, Nienaber CA, Peterson MD, Woznicki EM, Braverman AC, Trimarchi S, Myrmel T, et al. Early mortality in type A acute aortic dissection: insights from the international registry of acute aortic dissection. JAMA Cardiol. 2022;7(10):1009-1015.
doi pubmed pmc - Pape LA, Awais M, Woznicki EM, Suzuki T, Trimarchi S, Evangelista A, Myrmel T, et al. Presentation, diagnosis, and outcomes of acute aortic dissection: 17-year trends from the international registry of acute aortic dissection. J Am Coll Cardiol. 2015;66(4):350-358.
doi pubmed - Ince H, Nienaber CA. Diagnosis and management of patients with aortic dissection. Heart. 2007;93(2):266-270.
doi pubmed pmc - Fatima S, Sharma K. Painless aortic dissection-diagnostic dilemma with fatal outcomes: what do we learn? J Investig Med High Impact Case Rep. 2017;5(3):2324709617721252.
doi pubmed pmc - Levy D, Goyal A, Grigorova Y, Farci F, Le JK. Aortic dissection. In: StatPearls. Treasure Island (FL): Ineligible companies. 2023.
pubmed pmc - Tsai TT, Nienaber CA, Eagle KA. Acute aortic syndromes. Circulation. 2005;112(24):3802-3813.
doi pubmed - Ali U, Cheung W. Painless aortic dissection presenting with congestive heart failure. BJMP. 2011;4(1):a401.
- Agrawal S, Longfield S, George G. Congestive cardiac failure and aortic dissection in a young man with Marfan's syndrome. J Accid Emerg Med. 1994;11(4):259-260.
doi pubmed pmc - Westover AN, Nakonezny PA. Aortic dissection in young adults who abuse amphetamines. Am Heart J. 2010;160(2):315-321.
doi pubmed pmc - Greve D, Funke J, Khairi T, Montagner M, Starck C, Falk V, Sa M, et al. Cocaine-related aortic dissection: what do we know? Braz J Cardiovasc Surg. 2020;35(5):764-769.
doi pubmed pmc - von Kodolitsch Y, Schwartz AG, Nienaber CA. Clinical prediction of acute aortic dissection. Arch Intern Med. 2000;160(19):2977-2982.
doi pubmed - Eagle KA, Isselbacher EM, DeSanctis RW, International Registry for Aortic Dissection Investigators. Cocaine-related aortic dissection in perspective. Circulation. 2002;105(13):1529-1530.
doi pubmed - Hsue PY, Salinas CL, Bolger AF, Benowitz NL, Waters DD. Acute aortic dissection related to crack cocaine. Circulation. 2002;105(13):1592-1595.
doi pubmed - Nusair M, Abuzetun JY, Khaja A, Dohrmann M. A case of aortic dissection in a cocaine abuser: a case report and review of literature. Cases J. 2008;1(1):369.
doi pubmed pmc - Swalwell CI, Davis GG. Methamphetamine as a risk factor for acute aortic dissection. J Forensic Sci. 1999;44(1):23-26.
pubmed - Pena RCF, Hofmann Bowman MA, Ahmad M, Pham J, Kline-Rogers E, Case MJ, Lee J, et al. An assessment of the current medical management of thoracic aortic disease: A patient-centered scoping literature review. Semin Vasc Surg. 2022;35(1):16-34.
doi pubmed - Bhogal S, Khalid M, Murtaza G, Bhandari T, Summers J. Nearly missed: painless aortic dissection masquerading as infective endocarditis. Cureus. 2018;10(5):e2587.
doi pubmed pmc - Evangelista A, Isselbacher EM, Bossone E, Gleason TG, Eusanio MD, Sechtem U, Ehrlich MP, et al. Insights from the international registry of acute aortic dissection: a 20-year experience of collaborative clinical research. Circulation. 2018;137(17):1846-1860.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


