| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Case Report
Volume 15, Number 4, April 2023, pages 243-249
A Rare Case of New-Onset Crohn’s Disease in a Patient With Chronic Palmoplantar Pustulosis
Satoshi Tanidaa, b, d, Shoichiro Yoshiic, Ryoji Kuboc, Takuya Takahamab, Shun Sasohb, Yoshimasa Kubotab, Tesshin Banb, Tomoaki Andob, Makoto Nakamurab, Takashi Johb
aEducation and Research Center for Community Medicine, Nagoya City University Graduate School of Medical Sciences, Mizuho-ku, Nagoya, Aichi 467-8601, Japan
bDivision of Gastroenterology, Gamagori City Hospital, Gamagori, Aichi 443-8501, Japan
cDivision of Dermatology, Gamagori City Hospital, Gamagori, Aichi 443-8501, Japan
dCorresponding Author: Satoshi Tanida, Education and Research Center for Community Medicine, Nagoya City University Graduate School of Medical Sciences, Mizuho-ku, Nagoya, Aichi 467-8601, Japan
Manuscript submitted February 25, 2023, accepted April 12, 2023, published online April 28, 2023
Short title: CD Associated With Palmoplantar Pustulosis
doi: https://doi.org/10.14740/jocmr4896
| Abstract | ▴Top |
A 44-year-old woman who had been diagnosed with palmoplantar pustulosis (PPP) at 34 years old was diagnosed with moderate Crohn’s disease (CD) based on endoscopic, radiological, and pathological findings. As treatment with corticosteroids, ultraviolet, and cyclosporin had achieved partial response, PPP had been refractory in a chronic continuous state. Oral prednisolone was initially started to treat CD, but clinical remission was not achieved. Intravenous ustekinumab was subsequently started at 260 mg for clinical remission of CD. Eight weeks after starting ustekinumab, clinical remission and mucosal healing were achieved and PPP manifestations on the palms and soles were markedly improved. Ustekinumab appears to offer an effective therapeutic option for patients with PPP but has yet to be approved for this induction in Japan. CD is a rare gastrointestinal involvement in PPP patients that requires attention.
Keywords: Palmoplantar pustulosis; New-onset Crohn’s disease; Generalized pustular psoriasis; Ustekinumab; Clinical remission
| Introduction | ▴Top |
Palmoplantar pustulosis (PPP) is a chronic, relapsing skin disease affecting the palms and/or soles. This pathology is characterized by crops of sterile pustules, erythema and scales occurring on one or both hands and feet symmetrically. A nationwide study in a Japanese population displayed a prevalence of 0.12% for PPP [1]. In Western countries, PPP is classified as a subtype of generalized pustular psoriasis. However, in Japan, PPP is considered a distinct entity from pustular psoriasis because PPP has a lower prevalence of mutations in the gene for interleukin (IL)-36 receptor antagonist, an anti-inflammatory protein, compared with generalized pustular psoriasis [2] and the coexistence of PPP and psoriasis is rare [3]. The current treatment in Japan includes topical therapy [4], ultraviolet (UV) phototherapy [5, 6], oral immunosuppressants including cyclosporin [7]. Biologics can also be selected for patients showing inadequate response to these conventional therapies and a persistently moderate or severe disease. Regarding the pathophysiology of PPP, the IL-23/IL-17 pathway (via proliferation of type 17 helper T cells (Th17)) is proposed to upregulate cytokine production [8-10] and activate monocyte and neutrophil infiltration, leading to pustule formation [11]. The largest body of evidence suggests a positive response to the use of ustekinumab for PPP [12]. Guselkumab, a monoclonal antibody that binds to the p19 subunit of IL-23 and thus blocks the IL-23 signaling pathway [13], was approved in November 2018 in Japan.
Crohn’s disease (CD) often strikes the entire digestive system and organs via chronic, progressive, transmural inflammation of the alimentary tract. The number of CD patients has been increasing in Japan for years. However, secondary CD in a patient with PPP does not appear to have been reported previously. Treatments for moderate-to-severe CD include 5-aminosalicylic acids, corticosteroids, thiopurines, and biologics including tumor necrosis factor (TNF)-α inhibitors. Moreover, IL-12 and IL-23 are important cytokines which are involved in the adaptive immune responses and their common pathway has been found to play an important role in the induction of intestinal inflammation [14]. Based on the outcomes of clinical trials which have assessed the therapeutic effect of an IL-12/IL-23 inhibitor in patients with CD and demonstrated rapid clinical effects with a safety profile [15, 16], ustekinumab, anti-IL-12/23 antibodies, was approved for the treatment of moderate-to-severe CD in Japan in 2017.
Here, we report a rare case of new-onset CD in a patient with chronic continuous PPP, who had been refractory to treatment with systemic corticosteroid, UV phototherapy and cyclosporin. Induction therapy with ustekinumab for the treatment of PPP and CD rapidly achieved clinical remission and mucosal healing of CD with marked improvement of PPP within 8 weeks.
| Case Report | ▴Top |
Investigations
This patient had been diagnosed with PPP in July 2012, at 34 years old. Oral prednisolone (PSL) was started at 5 mg/day along with topical corticosteroid ointment. Oral PSL and topical corticosteroid ointment were subsequently tapered off in September 2012. She then received psoralen ultraviolet A (PUVA) and narrow-band UVB phototherapy three times a month for the treatment of persistent PPP from September 2012 to November 2017. Phototherapy was continued with a switch to excimer light three times a month from July 2019 to January 2021 (wavelength, 308 ± 2 nm; cumulative exposure dose, 29,952 mJ/cm2). Oral cyclosporin (Neoral®; Novartis, Switzerland) had been administered continually at 100 mg/day since June 2021.
In October 2022, at 44 years old, the patient was admitted to our hospital due to rapid exacerbation of symptoms with fever reaching 39.0 °C, and recurrent episodes of severe abdominal pain and diarrhea (10 times/day) accompanied by bloody stool (CD activity index (CDAI), 359). The patient had no history of lung or joint disease, and no family history of CD. Laboratory investigations showed: white blood cell (WBC) counts, 10,800/µL; red blood cell (RBC) counts, 404 × 104/µL; hemoglobin (Hb), 12.0 g/dL; total protein (TP), 6.9 g/dL; albumin (Alb), 3.5 g/dL; and C-reactive protein, 11.28 mg/dL. A negative result was obtained from an antigenemia (C-7HRP) test for cytomegalovirus. Further, neither the pathogenic microbe Clostridium difficile nor its A or B toxins were detected in stool cultures (Table 1). Abdominal computed tomography revealed thickening and edematous changes in the entire colon wall, particularly in the transverse colon, without fistulae or abscesses, and no inflammatory changes were evident in the small intestine. Colonoscopy on admission in November 2022 showed skipped ulcers fused with the adjacent ulcer and/or longitudinally aligned in the mucosa of the ascending, transverse, and descending colon, and rectum (Fig. 1a). Simple endoscopic score for Crohn’s disease (SES-CD) was 18, presenting severe activity. Simultaneously, contrast-enhanced radiography revealed longitudinal ulcers, edematous changes and stenoses in the transverse colon (Fig. 1b). Histopathological examinations revealed mild infiltration of neutrophils and lymphocyte aggregates in the mucosa and submucosa with erosive epithelium. Immunohistochemical examination of colon biopsy specimens for cytomegalovirus also yielded negative results. In addition, regarding skin findings, a small number of blisters and pustules, and scattered scales on diffuse erythematous skin were seen to be present on both palms (Fig. 2a) and soles (Fig. 2b) on admission. Palmoplantar pustulosis area and severity index (PPPASI) was 15.8 (Fig. 3).
 Click to view | Table 1. Laboratory Findings on Admission |
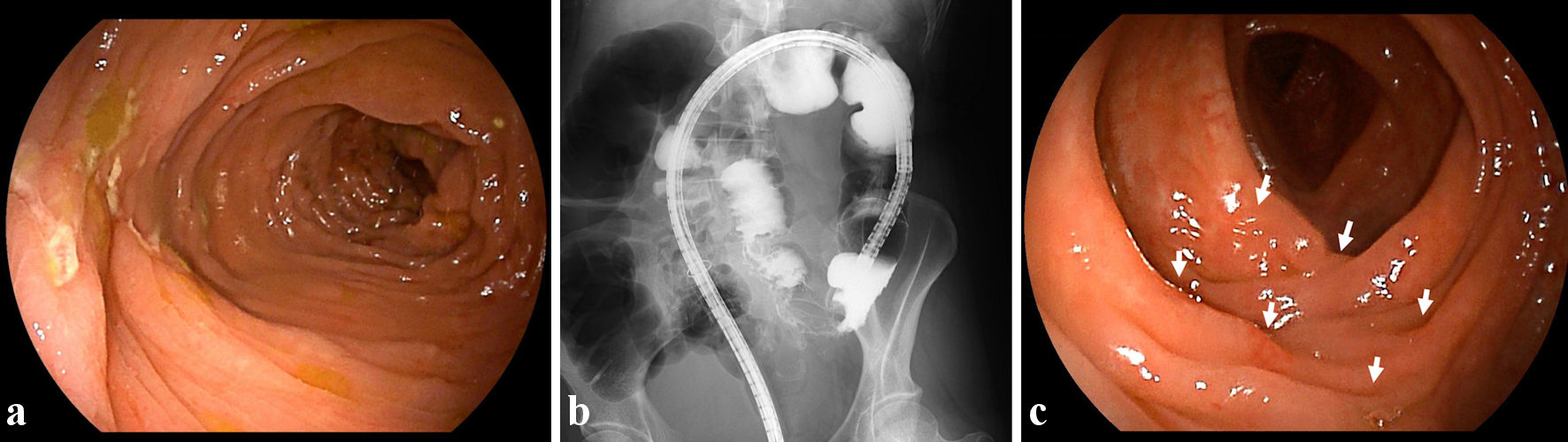 Click for large image | Figure 1. Findings from colonoscopy and radiology. (a) On admission, colonoscopic image shows skipped ulcers fused with the adjacent ulcer and/or longitudinally aligned in the mucosa of the transverse colon. (b) On admission, radiological findings with contrast media reveal longitudinal ulcers as well as edematous changes and stenoses in the transverse colon. (c) At 8 weeks after starting ustekinumab, colonoscopic image shows skipped ulcer scars (white arrows) in the transverse colon. |
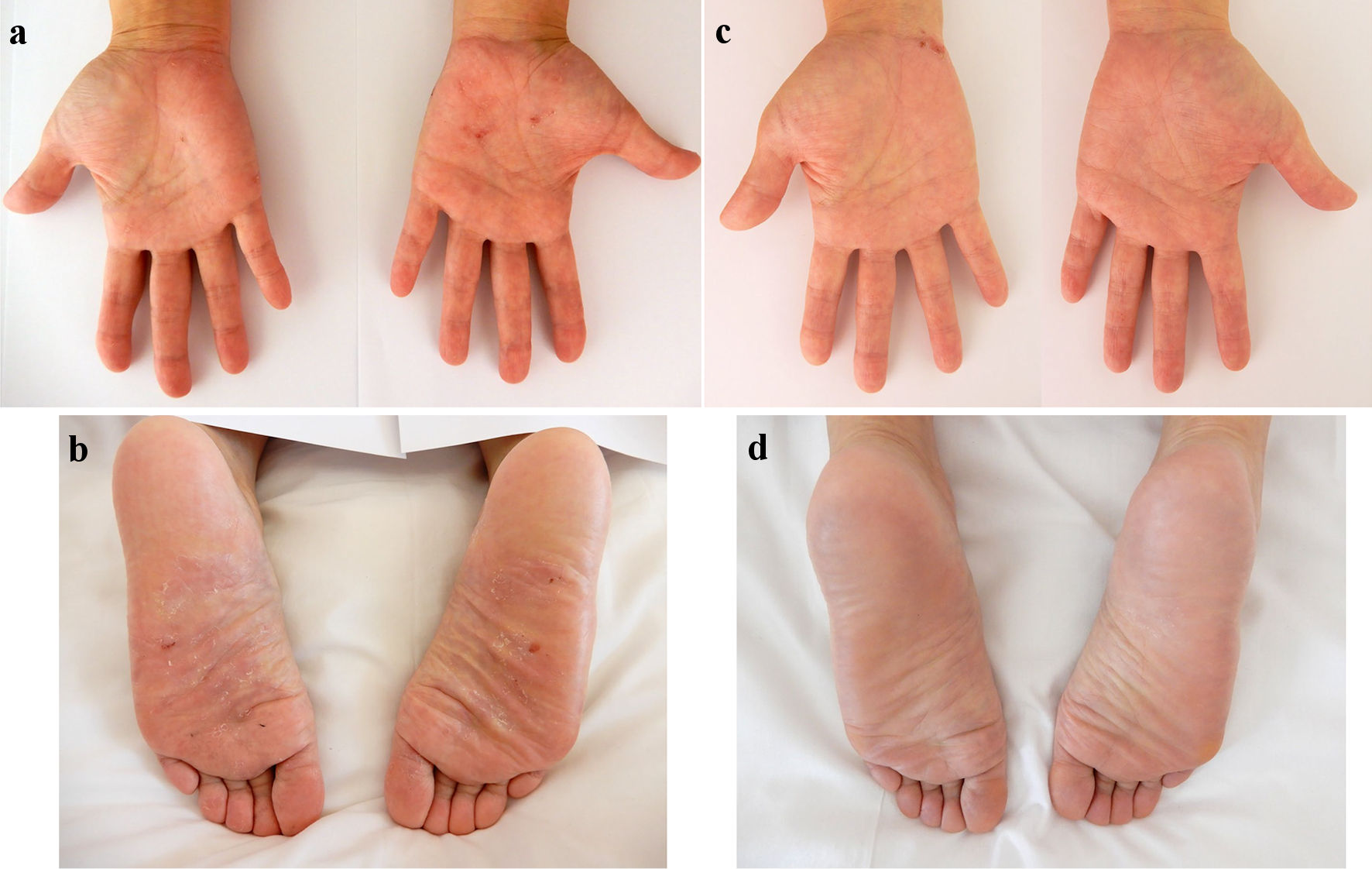 Click for large image | Figure 2. Skin findings of palmoplantar pustulosis on the palms and soles. A small number of blisters and pustules, and scattered scales on diffuse erythematous skin were present on both the palms (a) and soles (b) on admission. At 8 weeks after starting ustekinumab, focal scale is present on the right palm (c) without blisters, pustules, scales, or erythema on the left palm and both soles (c, d). |
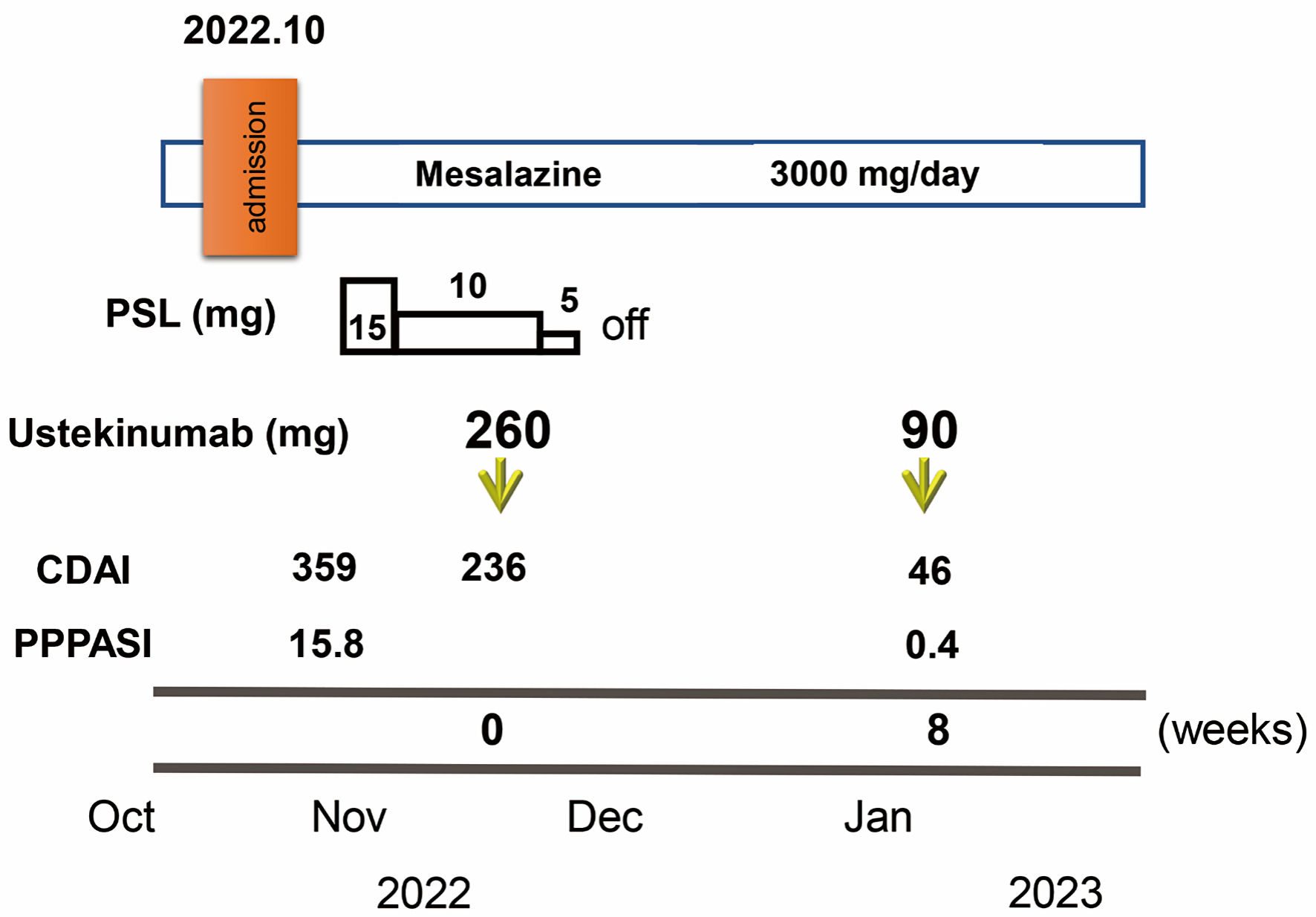 Click for large image | Figure 3. Clinical course through 8 weeks. Crohn’s disease activity index (CDAI) and palmoplantar pustulosis area and severity index (PPPASI) on admission were 359 and 15.8, respectively. Oral prednisolone (PSL) was started at 15 mg/day accompanied by mesalazine at 3,000 mg/day. CDAI decreased to 236. In November 2022, ustekinumab was started at 260 mg based on 6 mg/kg in week 0 with the aim of clinical remission of CD and PPP. The dose of PSL was tapered off in 1 week. At 8 weeks after starting ustekinumab, clinical remission was achieved with a CDAI of 46 and PPP manifestations on the palms and soles were markedly improved (PPPASI: 0.4). |
Diagnosis
Based on all these findings, the patient was diagnosed with new-onset CD associated with chronic continuous PPP.
Treatment, follow-up and outcomes
Oral PSL was started at 15 mg/day along with 3,000 mg/day of mesalazine. PSL was subsequently decreased to 10 mg/day after 7 days of PSL. Symptoms including diarrhea and abdominal pain attributed to CD improved without achieving clinical remission (CDAI, 236). PPP manifestations also showed partial improvement, but, particularly on both soles, did not resolve. In November 2022, the patient received intravenous ustekinumab at 260 mg at week 0, based on 6 mg/kg with the aim of achieving clinical remission of CD. The dose of PSL was tapered off 1 week after initiating this therapy. At 8 weeks after starting ustekinumab, clinical remission was achieved (CDAI, 46) and focal scale remained present on the right palm (Fig. 2c) without blisters, pustules, scales, or erythema on the left palm or both soles (Fig. 2c, d), resulting in marked improvement (PPPASI, 0.4) (Fig. 3). Follow-up endoscopy showed no ulcerated surfaces or disease lesions affecting the colon, although a narrowed lumen at Bauhin’s valve prevented passage of the endoscope (SES-CD, 3) (Fig. 1c). Mucosal healing [16] was consequently achieved. Maintenance therapy with ustekinumab at 90 mg has since been continued. As of 3 months later, the patient remained in stable CD remission under a treatment regimen of scheduled ustekinumab maintenance therapy at 90 mg and mesalazine at 3,000 mg/day. No adverse events were observed.
| Discussion | ▴Top |
We have reported here a rare case of new-onset CD in a patient with refractory PPP. Induction therapy with ustekinumab was initiated to treat both PPP and CD, rapidly achieving clinical remission and mucosal healing of CD with marked improvement of PPP within 8 weeks.
PPP is a refractory disease affecting the hands and/or feet that can cause recurrent blisters and aseptic pustules. The prevalence of psoriasis among patients with inflammatory bowel disease (IBD), including ulcerative colitis and CD, has increased compared with the background population. Similarly, patients with psoriasis show an increased risk of developing IBD [17]. PPP and CD are immune-mediated refractory diseases presenting with a relapsing-remitting clinical course. Many patients with CD develop extraintestinal inflammation with skin inflammation, including erythema nodosum, pyoderma gangrenosum, epidermolysis bullosa acquisita and psoriasis [18, 19]. A database analysis based on 93 studies reporting data on psoriasis among patients with IBD and vice versa showed that the presence of CD was significantly associated with psoriasis (odds ratio: 2.0; 95% confidence interval (CI): 1.4 - 2.9), and that the presence of psoriasis was significantly associated with CD (odds ratio: 2.2; 95% CI: 1.6 - 3.1) [20]. Moreover, bidirectional two-sample randomization analyses based on summary statistics from genome-wide association studies investigating associations with IBD, particularly CD, and psoriasis and including 5,956 CD cases versus 14,927 control cases, and 5,621 psoriasis cases versus 251,323 control cases in Western countries showed that genetically predicted CD was associated with psoriasis (pooled odds ratio: 1.16; 95% CI: 1.12 - 1.20; P < 0.001), whereas no significant association was observed in the reverse direction [21]. These results suggested that psoriasis and CD share several genetic susceptibility loci and that the pathogenetic mechanisms underlying both diseases overlap to some degree [22], although the details remain unknown. A small number of reports have described paradoxical PPP induced by anti-TNF-α antibodies in patients with CD [23, 24] and paradoxical hidradenitis suppurativa during biologics [25]. To the best of our knowledge, this presents the first case of new-onset CD in a patient with PPP.
Regarding the treatment of PPP, topical corticosteroids, vitamin D analogues, and UV phototherapy (topical PUVA therapy, narrow-band UVB phototherapy, excimer light and excimer laser (308 nm)) have been widely used [4, 5, 26, 27]. Non-pharmacological therapy with granulocyte and monocyte adsorption apheresis is another effective and safe therapeutic option [28]. However, as these treatments sometimes fail, systemic drugs including oral retinoids, methotrexate and cyclosporin are subsequently applied, often achieving inadequate response [7]. In addition, methotrexate, cyclosporin and granulocyte and monocyte adsorption apheresis are currently not approved for the treatment of PPP. Recent studies of proposed mechanisms in psoriasis have revealed presentation of antigens from pathogens such as Streptococcus pneumoniae and Haemophilus influenzae from dendritic cells to naive T cells in the tonsil and local lymph nodes, where Th17 lymphocytes are differentiated from naive T cells activated by IL-23, which is produced from dendritic cells. Activated Th17 lymphocytes induce chronic inflammation in PPP [29, 30]. Antagonists against IL-23 are considered efficacious for PPP. Guselkumab blocks IL-23 signals by targeting the 19-protein subunit of IL-23. The efficacy of guselkumab in patients with psoriasis [31, 32] and PPP [13, 33] has been demonstrated very recently. Moreover, CD and psoriasis share the common IL-23/Th17/IL-17 pathway in the disease pathogenesis, as demonstrated by the efficacy of biologic treatments targeting this pathway in CD [16]. The IL-12/23 subunit p40 antagonist (ustekinumab) has also been shown to be effective for PPP in addition to hidradenitis suppurativa [12, 34]. On the other hand, a small randomized-control trial did not find ustekinumab at 45 mg to be superior to placebo after 16 weeks of therapy (P = 1.00) [35], although an open label study demonstrated greater efficacy with subcutaneous ustekinumab at 90 mg than at 45 mg (P = 0.02) in terms of the percentage of subjects achieving clinical clearance by week 16 [36]. Data on the efficacy of ustekinumab against PPP can be conflicting. In the present case, PPP manifestations were markedly improved after 8 weeks of ustekinumab at 260 mg as the starting induction dose, based on a dose of 6 mg/kg. This suggested that 45 mg ustekinumab for induction therapy might be too little to achieve clinical clearance. An amount of ustekinumab ≥ 90 mg may be required to achieve clinical clearance.
Regarding the efficacy of ustekinumab in clinical response and endoscopic healing, a randomized, placebo-controlled study investigated the efficacy and safety in patients with moderate-to-severe CD, in whom conventional therapy had failed or unacceptable side effects occurred (the UNITI 2 study) [15]. That study showed an 8-week clinical response rate of 40.2% in patients receiving approximately 6 mg/kg, significantly higher than the rates (19.6%) among patients receiving placebo (P < 0.001). The 13.5% rate of 8-week mucosal healing (complete absence of ulcers in patients with ulcers at baseline) in patients receiving approximately 6 mg/kg was not superior to the 7.1% rate among patients receiving placebo (P = 0.286) [16]. In addition, successful induction of deep remission in early-stage (median duration, 0.2 years; interquartile range, 0.1 - 0.5 years), moderate-to-severe CD was reportedly associated with a decreased risk of disease progression over a median time of 3 years [37]. In the present case, the patient with ulcers at baseline showed complete resolution of ulcers, because the patient had early-stage CD with short disease duration (0.17 years). No previous reports have described new-onset CD in a patient with refractory PPP, induction therapy with ustekinumab for the treatment of both PPP and CD, and rapid achievement of clinical remission and mucosal healing of CD with marked improvements in PPP. Further prospective investigation is needed to determine the doses of ustekinumab adequate to achieve clinical remission of CD and clinical clearance of PPP in patients with CD associated with refractory PPP.
Learning points
High-dose ustekinumab appears effective as a therapeutic option for patients with PPP, although approval for treatment of PPP has not yet been given in Japan. CD is a rare gastrointestinal involvement that requires attention in PPP patients.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
Satoshi Tanida has received research grants from AbbVie.
Informed Consent
Informed consent was obtained in the form of opt-out on the website during enrollment.
Author Contributions
ST designed and performed the study. ST, SY and RK drafted the manuscript. TT, SS, YK, TB, and TA performed critical editing of the manuscript. ST, SY and RK assisted with and supported sample collection and subsequent analysis with statistics. ST prepared and wrote manuscript. MN and TJ carefully supervised this study.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
PPP: palmoplantar pustulosis; CD: Crohn’s disease; UV: ultraviolet; IL: interleukin; Th17: type 17 helper T cell; TNF: tumor necrosis factor; PSL: prednisolone; CDAI: Crohn’s disease activity index; WBC: white blood cell; RBC: red blood cell; Hb: hemoglobin; TP: total protein; Alb: albumin; SES-CD: simple endoscopic score for Crohn’s disease; PPPASI: palmoplantar pustulosis area and severity index; IBD: inflammatory bowel disease; CI: confidence interval; PUVA: psoralen ultraviolet A
| References | ▴Top |
- Kubota K, Kamijima Y, Sato T, Ooba N, Koide D, Iizuka H, Nakagawa H. Epidemiology of psoriasis and palmoplantar pustulosis: a nationwide study using the Japanese national claims database. BMJ Open. 2015;5(1):e006450.
doi pubmed pmc - Takahashi T, Fujimoto N, Kabuto M, Nakanishi T, Tanaka T. Mutation analysis of IL36RN gene in Japanese patients with palmoplantar pustulosis. J Dermatol. 2017;44(1):80-83.
doi pubmed - Yamamoto T. Similarity and difference between palmoplantar pustulosis and pustular psoriasis. J Dermatol. 2021;48(6):750-760.
doi pubmed - Muro M, Kawakami H, Matsumoto Y, Abe N, Tsuboi R, Okubo Y. Topical combination therapy with vitamin D3 and corticosteroid ointment for palmoplantar pustulosis: A prospective, randomized, left-right comparison study. J Dermatolog Treat. 2016;27(1):51-53.
doi pubmed - Su LN, Ren J, Cheng SM, Liu JL, Ding YF, Zhu NW. UVA1 vs. narrowband UVB phototherapy in the treatment of palmoplantar pustulosis: a pilot randomized controlled study. Lasers Med Sci. 2017;32(8):1819-1823.
doi pubmed - Fumimori T, Tsuruta D, Kawakami T, Ohata C, Furumura M, Hashimoto T. Effect of monochromatic excimer light on palmoplantar pustulosis: a clinical study performed in a private clinic by a dermatological specialist. J Dermatol. 2013;40(12):1004-1007.
doi pubmed - Shah S, Nikam B, Kale M, Jamale V, Chavan D. Safety and efficacy profile of oral cyclosporine vs oral methotrexate vs oral acitretin in palmoplantar psoriasis: A hospital based prospective investigator blind randomized controlled comparative study. Dermatol Ther. 2021;34(1):e14650.
doi pubmed - Moschen AR, Tilg H, Raine T. IL-12, IL-23 and IL-17 in IBD: immunobiology and therapeutic targeting. Nat Rev Gastroenterol Hepatol. 2019;16(3):185-196.
doi pubmed - Bissonnette R, Fuentes-Duculan J, Mashiko S, Li X, Bonifacio KM, Cueto I, Suarez-Farinas M, et al. Palmoplantar pustular psoriasis (PPPP) is characterized by activation of the IL-17A pathway. J Dermatol Sci. 2017;85(1):20-26.
doi pubmed - Murakami M, Hagforsen E, Morhenn V, Ishida-Yamamoto A, Iizuka H. Patients with palmoplantar pustulosis have increased IL-17 and IL-22 levels both in the lesion and serum. Exp Dermatol. 2011;20(10):845-847.
doi pubmed - Croxford AL, Karbach S, Kurschus FC, Wortge S, Nikolaev A, Yogev N, Klebow S, et al. IL-6 regulates neutrophil microabscess formation in IL-17A-driven psoriasiform lesions. J Invest Dermatol. 2014;134(3):728-735.
doi pubmed - Morales-Munera C, Vilarrasa E, Puig L. Efficacy of ustekinumab in refractory palmoplantar pustular psoriasis. Br J Dermatol. 2013;168(4):820-824.
doi pubmed - Okubo Y, Morishima H, Zheng R, Terui T. Sustained efficacy and safety of guselkumab in patients with palmoplantar pustulosis through 1.5 years in a randomized phase 3 study. J Dermatol. 2021;48(12):1838-1853.
doi pubmed pmc - Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448(7152):427-434.
doi pubmed - Feagan BG, Sandborn WJ, Gasink C, Jacobstein D, Lang Y, Friedman JR, Blank MA, et al. Ustekinumab as induction and maintenance therapy for Crohn's disease. N Engl J Med. 2016;375(20):1946-1960.
doi pubmed - Rutgeerts P, Gasink C, Chan D, Lang Y, Pollack P, Colombel JF, Wolf DC, et al. Efficacy of Ustekinumab for inducing endoscopic healing in patients with Crohn's disease. Gastroenterology. 2018;155(4):1045-1058.
doi pubmed - Hedin CRH, Sonkoly E, Eberhardson M, Stahle M. Inflammatory bowel disease and psoriasis: modernizing the multidisciplinary approach. J Intern Med. 2021;290(2):257-278.
doi pubmed - Antonelli E, Bassotti G, Tramontana M, Hansel K, Stingeni L, Ardizzone S, Genovese G, et al. Dermatological manifestations in inflammatory bowel diseases. J Clin Med. 2021;10(2):364.
doi pubmed pmc - Harbord M, Annese V, Vavricka SR, Allez M, Barreiro-de Acosta M, Boberg KM, Burisch J, et al. The first European evidence-based consensus on extra-intestinal manifestations in inflammatory bowel disease. J Crohns Colitis. 2016;10(3):239-254.
doi pubmed pmc - Alinaghi F, Tekin HG, Burisch J, Wu JJ, Thyssen JP, Egeberg A. Global prevalence and bidirectional association between psoriasis and inflammatory bowel disease-a systematic review and meta-analysis. J Crohns Colitis. 2020;14(3):351-360.
doi pubmed - Freuer D, Linseisen J, Meisinger C. Association between inflammatory bowel disease and both psoriasis and psoriatic arthritis: a bidirectional 2-sample mendelian randomization study. JAMA Dermatol. 2022;158(11):1262-1268.
doi pubmed pmc - Furiati SC, Catarino JS, Silva MV, Silva RF, Estevam RB, Teodoro RB, Pereira SL, et al. Th1, Th17, and Treg responses are differently modulated by TNF-alpha inhibitors and methotrexate in psoriasis patients. Sci Rep. 2019;9(1):7526.
doi pubmed pmc - Ortiz Salvador JM, Cubells Sanchez L, Subiabre Ferrer D. Palmoplantar pustulosis by adalimumab in a patient with Crohn disease. Med Clin (Barc). 2016;147(12):565.
doi pubmed - Wermuth J, Kind F, Steuerwald M. Palmoplantar pustulosis and acrodermatitis in a patient treated with infliximab for Crohn's sacroiliitis. Clin Gastroenterol Hepatol. 2009;7(2):A28.
doi pubmed - Ruggiero A, Martora F, Picone V, Marano L, Fabbrocini G, Marasca C. Paradoxical hidradenitis suppurativa during biologic therapy, an emerging challenge: a systematic review. Biomedicines. 2022;10(2):455.
doi pubmed pmc - Furuhashi T, Torii K, Kato H, Nishida E, Saito C, Morita A. Efficacy of excimer light therapy (308 nm) for palmoplantar pustulosis with the induction of circulating regulatory T cells. Exp Dermatol. 2011;20(9):768-770.
doi pubmed - Elmets CA, Lim HW, Stoff B, Connor C, Cordoro KM, Lebwohl M, Armstrong AW, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81(3):775-804.
doi pubmed - Fujisawa T, Tawada C, Mizutani Y, Doi T, Yoshida S, Ogura S, Seishima M. Efficacy of granulocyte and monocyte adsorption apheresis for treatment of palmoplantar pustulosis. Ther Apher Dial. 2014;18(3):238-243.
doi pubmed - Neuhauser R, Eyerich K, Boehner A. Generalized pustular psoriasis-Dawn of a new era in targeted immunotherapy. Exp Dermatol. 2020;29(11):1088-1096.
doi pubmed - Ouyang W, Kolls JK, Zheng Y. The biological functions of T helper 17 cell effector cytokines in inflammation. Immunity. 2008;28(4):454-467.
doi pubmed pmc - Ruggiero A, Picone V, Martora F, Fabbrocini G, Megna M. Guselkumab, risankizumab, and tildrakizumab in the management of psoriasis: a review of the real-world evidence. Clin Cosmet Investig Dermatol. 2022;15:1649-1658.
doi pubmed pmc - Ruggiero A, Fabbrocini G, Cinelli E, Ocampo Garza SS, Camela E, Megna M. Anti-interleukin-23 for psoriasis in elderly patients: guselkumab, risankizumab and tildrakizumab in real-world practice. Clin Exp Dermatol. 2022;47(3):561-567.
doi pubmed pmc - Terui T, Kobayashi S, Okubo Y, Murakami M, Zheng R, Morishima H, Goto R, et al. Efficacy and safety of guselkumab in Japanese patients with palmoplantar pustulosis: a phase 3 randomized clinical trial. JAMA Dermatol. 2019;155(10):1153-1161.
doi pubmed pmc - Martora F, Megna M, Battista T, Potestio L, Annunziata MC, Marasca C, Villani A, et al. Adalimumab, ustekinumab, and secukinumab in the management of hidradenitis suppurativa: a review of the real-life experience. Clin Cosmet Investig Dermatol. 2023;16:135-148.
doi pubmed pmc - Bissonnette R, Nigen S, Langley RG, Lynde CW, Tan J, Fuentes-Duculan J, Krueger JG. Increased expression of IL-17A and limited involvement of IL-23 in patients with palmo-plantar (PP) pustular psoriasis or PP pustulosis; results from a randomised controlled trial. J Eur Acad Dermatol Venereol. 2014;28(10):1298-1305.
doi pubmed - Au SC, Goldminz AM, Kim N, Dumont N, Michelon M, Volf E, Hession M, et al. Investigator-initiated, open-label trial of ustekinumab for the treatment of moderate-to-severe palmoplantar psoriasis. J Dermatolog Treat. 2013;24(3):179-187.
doi pubmed - Ungaro RC, Yzet C, Bossuyt P, Baert FJ, Vanasek T, D'Haens GR, Joustra VW, et al. Deep remission at 1 year prevents progression of early Crohn's disease. Gastroenterology. 2020;159(1):139-147.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


