| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Review
Volume 15, Number 4, April 2023, pages 187-199
A Rare Pathogen of Bones and Joints: A Systematic Review of Osteoarticular Infections Caused by Gemella morbillorum
Eltaib Saada, d, Mohammed Elamin Farisa, Mohammed S. Abdallaa, Paritosh Prasaia, Elrazi Alib, Jonathan Stakec
aDepartment of Internal Medicine, Ascension Saint Francis Hospital, Evanston, IL, USA
bDepartment of Internal Medicine, One Brooklyn Health, Interfaith Medical Center, Brooklyn, NY, USA
cDepartment of Infectious Diseases and Infection Control, Ascension Saint Francis Hospital, Evanston, IL, USA
dCorresponding Author: Eltaib Saad, Department of Internal Medicine, Ascension Saint Francis Hospital, Evanston, IL, USA
Manuscript submitted February 22, 2023, accepted April 5, 2023, published online April 28, 2023
Short title: Osteoarticular Infections Due to G. morbillorum
doi: https://doi.org/10.14740/jocmr4891
| Abstract | ▴Top |
Osteoarticular infections (OAIs) caused by Gemella morbillorum (G. morbillorum) are a rare clinical entity. This study aimed to review all published cases of OAI due to G. morbillorum. A systematic review of PubMed, Scopus, and Cochrane Library was conducted to report the demographic and clinical characteristics, microbiological data, management, and outcome of OAIs caused by G. morbillorum in the adult population. A total of 16 studies reporting on 16 patients were included in this review. Eight patients had arthritis and eight patients had osteomyelitis/discitis. The most reported risk factors were immunosuppression, poor dental hygiene/dental infections, and recent gastrointestinal (GI) endoscopy. Five cases of arthritis occurred in a native joint while three patients had prostheses. The potential source of G. morbillorum infection was documented in more than half of the cases (56%) (most commonly odontogenic and GI sources (25% and 18%, respectively). The knee and hip joints were the most frequently affected joints in patients with arthritis, while the thoracic vertebrae were the most common sites for osteomyelitis/discitis. The blood cultures were positive in three patients with arthritis (37.5%) and five patients with osteomyelitis/discitis (62.5%). Associated endovascular infection was found in five patients with bacteremia. Contiguous spread (adjacent mediastinitis) was documented in two patients with sternal osteomyelitis and thoracic vertebral osteomyelitis. Surgical interventions were performed for 12 patients (75%). Most strains of G. morbillorum were susceptible to penicillin and cephalosporins. All patients with reported outcomes had achieved complete recovery. G. morbillorum is an emerging pathogen for OAIs in certain susceptible populations with specific risk factors. This review reported the demographic, clinical, and microbiological features of OAIs caused by G. morbillorum. A careful evaluation of an underlying infectious focus is warranted to control the source. When G. morbillorum bacteremia is present, it is also necessary to have a high index of suspicion to rule out an associated endovascular infection.
Keywords: Gemella morbillorum; Osteoarticular infections; Osteomyelitis; Arthritis; Endovascular infections; Bacteremia; Infection source
| Introduction | ▴Top |
Gemella morbillorum (G. morbillorum) is a facultatively anaerobic, non-motile, non-spore-forming, gram-positive coccus that constitutes part of the normal flora of the oropharynx, gastrointestinal (GI) tract, and female gynecological tract in humans [1, 2]. G. morbillorum was first described in 1917 by Tunicliff [1], it was first identified as Streptococcus morbillorum, then Pepto streptococcus morbillorum [1], and it was eventually classified as the current genus in 1988 based on DNA hybridization experiments and biomolecular features [1]. G. morbillorum was previously considered a very rare pathogen in humans [2], however, it has been increasingly determined to be the culprit organism for various clinical syndromes in the recent literature [2-4]. The most frequently reported G. morbillorum infections are infective endocarditis [2, 3], endovascular infections such as mycotic aneurysms [5], pleural empyema [6], deep abscesses such as liver abscess [7], brain abscess [8], and necrotizing soft tissue infections [9, 10]. G. morbillorum bacteremia could lead to septic shock [4], and many of these reported bacteremic infections were associated with poor outcomes [3, 4, 7, 9].
Osteoarticular infections (OAIs) remain challenging to diagnose and treat in modern medical practice [11], and their incidence has been increasing, particularly in certain high-risk populations such as elderly patients and those with immunosuppressive conditions [12]. Staphylococcus aureus and coagulase-negative staphylococci remain the leading pathogens in OAIs (up to two-thirds of cases), followed by gram-negative organisms (Enterococcus spp., Pseudomonas aeruginosa, Escherichia coli, and Cutibacterium acnes) [11]. Interestingly, G. morbillorum has been sporadically reported as the causative organism for some OAIs in the last three decades [13-15]. At present, most of the studies on G. morbillorum-associated OAI are isolated case reports with a lack of current evidence from systematic reviews. This study aimed to review all published cases of OAI due to G. morbillorum and report their demographic characteristics, clinical features, and microbiological data.
| Methods | ▴Top |
Data search
The PubMed, Scopus, and Cochrane Library databases were searched through 16 November 2022 for eligible studies following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines [16]. A search was run using MeSH terms which included “Gemella morbillorum” in all text AND “infectious arthritis,” in all text, or “periprosthetic”, or “joint infection” in all text, or “septic arthritis” in all text, or “osteomyelitis”, or “discitis”, or “spondylarthritis”, or “infectious spondylarthritis” in all text or “bone infection” in all text. The search was restricted to articles published in English and limited to studies that involved humans only.
Studies selection
Studies were included in the analysis if they met the following inclusion criteria: 1) involved OAIs (defined as septic arthritis, periprosthetic joint infection (PJI), infectious spondylarthritis, osteomyelitis, and discitis) in adult patients (defined as an age of 18 years or older), which are caused by G. morbillorum (as monomicrobial infection or as part of polymicrobial infection); 2) reported details of patients’ clinical features, microbiology characteristics, management, and outcome. The exclusion criteria were: 1) secondary research papers (e.g., narrative reviews), editorials, and papers that were not associated with primary research; 2) studies that did not report all clinical or microbiological data of study interest or without full text; 3) studies reporting non-OAI caused by G. morbillorum; 4) studies of OAIs caused by Gemella species other than G. morbillorum; 5) studies not published in English; and 6) reports that included pediatric patients. Three investigators (ES, MF, and MA) independently screened the titles and abstracts for inclusion/exclusion criteria. The resulting screened references were then retrieved and rescreened the full-text publications for potentially relevant articles. The final decision of the eligible studies selection was based on consensus. The discrepancies were resolved in conjunction with the fourth author (PP). Reference lists of included studies were searched to identify potential literature that met inclusion criteria.
Outcomes of interests
The primary outcome was to report the demographic and clinical features of patients with OAI caused by G. morbillorum. Secondary outcomes were to record data on: 1) the site of OAI; 2) the possible risk factors; 3) the presumed source of G. morbillorum infection and associated extra-articular infections; 4) the antimicrobial susceptibility profile of G. morbillorum; and 5) the management (including medical antimicrobial therapy and/or operative intervention) and the outcomes of these infections.
Data extraction
Data from each eligible study were extracted by three investigators (ES, MF, and MA). General information included authors, type of study, and year of publication. Demographic data included the patient’s age, gender, and possible risk factors. Clinical characteristics included the clinical syndrome variant (arthritis, PJI, osteomyelitis, and infectious spondylodiscitis or discitis), the site of affected joints and/or bones, presence of prosthesis (and the time interval between the prosthesis placement and infection onset), systemic and local symptoms, the presence of extra-articular infections), the presumed source of G. morbillorum, and microbiological results of cultures of the blood, synovial joint, and bone material/deep tissue and antibiotic susceptibility data. The extracted data also included relevant information regarding the management (operative interventions, administered antibiotics, and the total duration of antimicrobial therapy) and the reported outcome of the OAIs.
Study quality assessment
All included studies were evaluated using the methodological index of non-randomized studies (MINOR) [17] (Table 1, [13-15, 18-30]). The quality of each study was categorized according to the total score for each study: poor (0 - 5), moderate (6 - 10), and good (11 - 15) [17].
 Click to view | Table 1. Quality Assessment Scores of Case Reports Using an 8-Point MINORS Scale |
Data analysis
A systematic review was conducted without a meta-analysis due to the lack of comparable data in the included studies. Continuous variables with normal distributions are expressed as the means ± standard deviation and 95% confidence interval (CI). Categorical variables were expressed as sums and percentages. Statistical analyses were conducted in SPSS for Windows (Version 26, IBM, Armonk, NY, USA). Results were considered statistically significant at P < 0.05.
| Results | ▴Top |
Literature search
A total of 68 articles from PubMed, Scopus, and Cochrane Library were screened. 25 duplicates were removed. After reviewing the titles and abstracts, 23 articles were retrieved for full-text review. Of those studies, seven were not further considered based on the exclusion criteria, as three lacked detailed clinical and microbiological data, three reported non-OAIs caused by G. morbillorum, and the full text was not available in English for one study. Finally, 16 studies that met the inclusion criteria were included [13-15, 18-30]. The PRISMA flow diagram detailing the search process is shown in Figure 1.
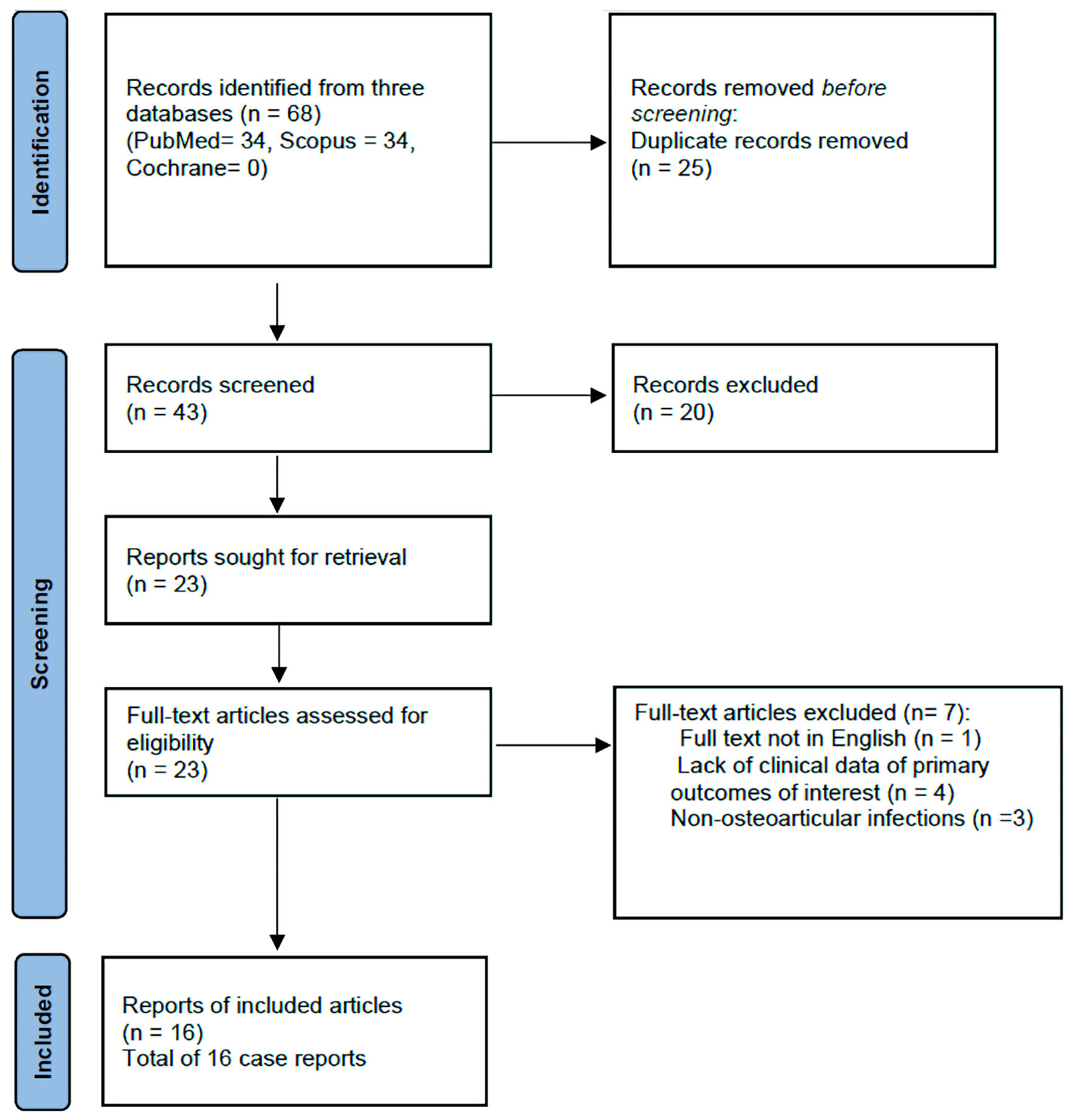 Click for large image | Figure 1. Flow diagram of systematic review search. |
Included studies characteristics
The 16 studies included in this systematic review involved 16 patients in total. Out of these studies, 10 were reported from Europe [14, 15, 18, 20-23, 25, 27, 30], five studies were conducted in Northern America [13, 19, 24, 28, 29], and one study was reported from Asia [26]. The time of the publishing of these studies was recorded (four studies between 1990 - 1999 [13, 18, 21, 25], four cases between 2000 - 2009 [14, 19, 23, 29], five reports between 2010 - 2019 [15, 20, 26, 27, 30], and three studies from 2020 to November 2022 [22, 24, 28].
All individual 16 studies were evaluated using the methodological index for non-randomized studies (MINORS) scale [17], 13 studies were rated to have a moderate quality (MINORS score 6 - 10), and three studies were deemed of poor quality (MINORS score 0 - 5) (Table 1, [13-15, 18-30]). As all 16 studies were case reports, the overall quality of the evidence contributing to this systematic review was rated as low to very low using the grading of recommendations assessment, development, and evaluation (GRADE) [31].
Demographic characteristics and underlying risk factors
The age of patients enrolled in the review ranged variably from 41 to 90 years; the mean age was 61 years [9-24]. About 69% (13/16) of patients were males and 31% (5/16) were females. Half of the patients (8/16) had septic arthritis [13, 14, 18, 19, 21-23, 30], while eight patients were diagnosed with osteomyelitis, spondylodiscitis, or discitis) [15, 20, 24-29] (Table 2 [13-15, 18-30]).
 Click to view | Table 2. Patient’s Demographic Characteristics, Clinical Features, Possible Risk Factors, and Extra-Articular Infections of 16 Cases of Osteoarticular Infections Caused by Gemella morbillorum |
In the septic arthritis group, three patients were immunocompromised [13, 19, 22], and three patients had a prosthetic joint [21-23]; the time interval between periprosthetic arthritis and prosthesis placement ranged from 1 year [21] to 10 years [22, 23]. Two patients had poor dental hygiene/recent dental infections as predisposing factors [13, 23], and one patient had an intrinsic joint disease (concurrent acute gout arthritis) [30]. Nevertheless, two patients (2/8, 25%) had no identified possible risks for the occurrence of G. morbillorum arthritis [14, 18].
All eight patients with osteomyelitis/discitis had documented underlying risk factor(s) as follows; host immunosuppression status in three patients [15, 25, 27], recent blunt trauma with bone fractures in two patients [20, 26], recent upper endoscopy examination with interventions [15, 29] and poor dental health/ recent dental infections in two patients [24, 26], respectively, one patient had an underlying joint disease (ankylosing spondylitis) [24], and prior gynecological surgery with a chronic complex pelvic infection was presumed as a possible risk in a patient with sacral osteomyelitis [28] (Table 2 [13-15, 18-30]).
The clinical features and the site of infection
Constitutional symptoms (fevers, rigors, and malaise) were recorded in seven patients [13, 22, 24, 25, 27-29], and most of the patients (87.5%) had localized symptoms (joint pain, joint swelling, reduced joint function, and acute back pain) (Table 2 [13-15, 18-30]). No significant differences were noted regarding the pattern of presentation between the two clinical syndromes (P > 0.05). Five cases of arthritis occurred in a native joint [13, 14, 18, 19, 30], while three patients had a PJI [21-23]. The knee [14, 19, 30] and hip [18, 22, 23] joints were the most frequently affected joints in the arthritis group (three patients for each), the wrist was involved in one patient with an infected ipsilateral hemodialysis access site [13], and one patient had an infected elbow joint [21].
The thoracic vertebrae were the most common anatomical sites for the occurrence of osteomyelitis/discitis (three patients [15, 25, 29], followed by the lumbar vertebrae (two patients [26, 27]). One patient had a combined involvement of both thoracic and lumbar segments [24]. The sacrum was affected in one patient with chronic pelvic infection status post complex gynecological surgery [28], and one patient had sternal osteomyelitis following blunt chest wall trauma [20] (Table 2 [13-15, 18-30]).
The presumed source of G. morbillorum infection and the presence of extra-articular infections
A presumed source of infection for G. morbillorum was only identified in three cases with arthritis [13, 22, 23], and five patients had no identified source of G. morbillorum [14, 18, 19, 21, 30]. An odontogenic source was presumed in two out of the three patients with an identified source of G. morbillorum [13, 23], the latter patients had poor oral hygiene with concurrent or recent dental infections that might have predisposed to G. morbillorum bacteremia [13, 23]. A GI source was suggested in the third patient who had a decompensated chronic liver disease and ascites with a possible G. morbillorum gut translocation [22]. An extra-articular infection (infected hemodialysis access site [13] and infective endocarditis [23]) was found in the two cases with an odontogenic source of G. morbillorum (Table 2 [13-15, 18-30]).
In contrast to patients with arthritis, those with osteomyelitis/discitis were more likely to have a possible source of infection identified. A potential source was found in six cases (75%) of G. morbillorum infection as follows; an odontogenic source in two patients with recent dental infections [24, 26], a GI source in two patients who underwent a recent upper endoscopic examination with interventions [15, 29], a gynecological tract source was presumed in one patient with a prior vaginal hysterectomy with persistent sacrovaginal fistula [28], and a hematogenous spread from a distant infectious source (cardiac vegetations) was suggested in the six patients [25]. Two patients in the osteomyelitis/discitis group lacked an identified source for G. morbillorum infection [20, 27] (Table 2 [13-15, 18-30]). Half of the cases in this group (50%, 4/8) were associated with extra-articular infections [20, 24, 25, 29]. Contiguous spread (adjacent mediastinitis) was documented in two patients with sternal osteomyelitis and thoracic vertebral osteomyelitis [20, 29], while an endovascular infection (infective endocarditis) was found in two patients [24, 25] (Table 2 [13-15, 18-30]).
Microbiology data and antibiotic susceptibility patterns
The blood cultures were positive in three patients with arthritis (37.5%) [13, 19, 23] while five patients with osteomyelitis/discitis had a positive blood culture (62.5%) [15, 24, 25, 28, 29]. The synovial fluid culture was positive in five patients in the arthritis group (62.5%), while the bone material/deep tissue cultures isolated G. morbillorum in seven cases with osteomyelitis/discitis (87.5%). Notably, all patients with arthritis and G. morbillorum bacteremia were found to have an associated endovascular infection [13, 19, 23]. Antibiotic susceptibility data were unavailable in three patients [19, 21, 29]. Most isolated G. morbillorum strains were penicillin and cephalosporins-susceptible except for one strain resistant to penicillin [27]. Additionally, one case reported resistance to aminoglycosides (gentamicin) [18]. One study did not report the details of antimicrobial therapy [21]. The most frequently used antibiotics were penicillin (60%, 9/15), cephalosporins (53.5%, 8/15), vancomycin (26%, 4/15), clindamycin (26%, 4/15), and aminoglycosides (3/15). Table 3 [13-15, 18-30] summarizes antibiotic susceptibility data and resistance patterns.
 Click to view | Table 3. Medical and Surgical Management, Antibiotics Susceptibility, Duration of Antibiotic Therapy, and Outcome of 16 Cases of Osteoarticular Infections Caused by Gemella morbillorum |
Management and outcome
Empiric antimicrobial therapy was provided for all 16 patients. Susceptibility-guided antibiotics selection was employed for all patients with reported susceptibility data [13-15, 18, 20, 22-28, 30]. Operative interventions were performed in seven patients with arthritis (87.5%). Prothesis revision or removal with debridement was carried out in all three patients with PJI [21-23], arthroscopic or open arthrotomy with washout was performed in three cases [14, 18, 30], and percutaneous needle aspiration with irrigation was done for one patient [19]. Surgical interventions were performed for five patients (62.5%) with osteomyelitis/discitis [15, 20, 26-28]. The details of surgical procedures were summarized in Table 3 [13-15, 18-30]. The total duration of antibiotics treatment ranged from 6 to 15 weeks (mean of 7 weeks) for the arthritis group and 6 to 12 weeks (mean of 8 weeks) for the osteomyelitis/discitis group. While the outcome was not documented for one case [21], all reported patients achieved full recovery. Table 3 [13-15, 18-30] records the outcome for the patients at varied periods of follow-up.
| Discussion | ▴Top |
This systematic review described the demographic characteristics, clinical features, microbiology, treatment, and outcome of OAI caused by G. morbillorum. A total of 16 cases were summarized in this review. The first case of OAI due to G. morbillorum was described by Omran et al in 1993 in a patient with G. morbillorum bacteremia and wrist joint arthritis [13]. The clinical syndromes of OAI included arthritis, osteomyelitis, spondylitis, and discitis. The mean age in this study was 61 years which agreed with recent studies from Europe that described an epidemiological trend of a raising incidence of OAI in older populations (above 65 years) [32, 33]. This observation was attributed to the increasing prevalence of medical comorbidities with longer life expectancy (mainly diabetes mellitus) and implant-related complications [32, 33]. A higher prevalence of OAI among males observed in our study (69%) is also in keeping with the global literature on OAI [34]. Our demographic findings of a higher frequency of G. morbillorum-associated OAI among older male patients are also in line with other non-OAI caused by Gemella species (such as infective endocarditis) as per a review conducted by Youssef et al [35].
The most common risk factors for G. morbillorum OAI in our study were host immunosuppression (diabetes mellitus and immunosuppressive therapy), presence of medical devices (prosthesis), poor dental health with dental infections, recent endoscopic interventions, and underlying intrinsic joint disease (rheumatoid arthritis). Many of the above-mentioned predisposing factors are shared with OAI due to other pathogens such as Staphylococcus aureus and coagulase-negative staphylococci [11, 34, 36]. Immunomodulators and cytotoxic therapy were strongly associated with a higher incidence of OAI [11, 36]. The presence of an implant can be a crucial factor for acquiring infections with opportunistic organisms, or those less likely to be pathogenic, particularly in immunocompromised patients [35], as exemplified by our study that reported three cases of PJI due to G. morbillorum [21-23].
The literature reported a particular predilection for G. morbillorum OAI to occur following recent dental infections [13, 15, 24, 26] and endoscopic interventions [23-25]. Being an opportunistic organism [24], G. morbillorum usually requires a disruption of colonized mucosae (by a procedural intervention such as GI endoscopy [15, 29] or poor dental hygiene with dental caries [13]), which presumably triggers transient bacteremia and eventually results in the occurrence of distant hematogenous infections (i.e., septic arthritis [13, 15], osteomyelitis [23, 24] and infective endocarditis [23, 35]). The risk of serious hematogenous dissemination is higher in immunocompromised hosts and those with pre-existing conditions, for instance, medical devices or cardiac valves [13, 15, 23, 24, 35]. It has also been postulated that chronically inflamed colonized surfaces (e.g., periodontitis) are especially necessary for the mucosal translocation of opportunistic pathogens including Gemella species [37].
There were two otherwise healthy patients in our review who encountered septic arthritis of a native joint with no potential identifiable risks [14, 18]. This observation agreed with previous literature that reported G. morbillorum infections in a few immunocompetent patients with no underlying conditions [2].
The pathogenesis of invasive infections caused by G. morbillorum remains poorly understood, and the virulence factors are not well-studied [35]; however, the production of exopolysaccharides may be associated with these infections [4]. Furthermore, animal models demonstrated in vivo reduction in the basal levels of interleukin (IL)-12 and interferon (IFN)-gamma together with down-modulation of IL-4 that was induced by G. morbillorum [38].
The source of G. morbillorum infection was documented in more than half of the cases (56%) with odontogenic and GI sources being the most reported infectious focus, consistent with a previous review of G. morbillorum infective endocarditis [35]. The preliminary assessment of a potential source should include a detailed history (notably for recent dental infections and GI interventions) and a thorough physical examination (including evaluation of teeth and oropharynx) [13, 22-24]. Additionally, an echocardiographic evaluation is recommended for underlying cardiac vegetations in the setting of suspected hematogenous seeding [24]. An endoscopic examination with gastroscopy and colonoscopy with appropriate imaging of the abdomen and pelvis may be necessary and warranted if a GI source is suspected [22]. Singer et al described a case of G. morbillorum bacteremia in the setting of PJI whereas a subsequent diagnostic workup revealed an undiagnosed chronic liver disease with ascites and a colonic polyp that were presumed as the likely GI source of bacteremia from gut translocation [24]. Notably, none of the patients in the present study had an associated colorectal cancer; in fact, colorectal cancers had been reported with G. morbillorum bacteremia and infective endocarditis [39, 40], and evaluation for an occult colorectal malignancy was suggested in the previous literature for patients with unexplained G. morbillorum bacteremia [40]. Source control is vital, as demonstrated in one patient with osteomyelitis and infective endocarditis who was found to have a concurrent dental abscess, requiring drainage [24]. Nevertheless, a comprehensive assessment may not always detect an underlying evident focus [19].
Endovascular infections were present in five patients with bacteremia in our study [13, 19, 23-25], four of these were due to infective endocarditis [19, 23-25], and one case involved an infected hemodialysis access site [13]; therefore, a high suspicion index is warranted in patients with G. morbillorum bacteremia in order to exclude associated infective endocarditis [19, 23-25].
Positive blood cultures were documented in half of the patients (50%) with OAI in this review; this finding can be explained by the microbiological observation that G. morbillorum is reportedly difficult to isolate from routine cultures owing to its fastidious nature and its very slow growth rate, resembling that of viridian streptococci [2, 14, 23]. This fastidious growth would likely be an explanation for the under-reporting of G. morbillorum infections in the available literature [35]. Fortunately, modern microbiological identification methods, such as direct matrix-associated laser desorption/ionization-time of flight (MALDI-TOF), have facilitated faster detection of G. morbillorum [40]. Earlier pathogen identification with the availability of susceptibility data by employing MALDI-TOF resulted in the prompt institution of targeted antibiotic therapy compared to traditional bacterial culturing methods [10]. Moreover, molecular diagnostic techniques, such as polymerase chain reaction (PCR) and 16S ribosomal RNA detection with gene sequencing, have allowed rapid and accurate diagnosis of various G. morbillorum infections [41-43].
Antibiotic susceptibility patterns noted in this study were in line with the available literature reporting on other G. morbillorum infections such as infective endocarditis [35]. Similar to other viridian streptococci, G. morbillorum was reportedly susceptible to penicillin, erythromycin, vancomycin, amoxicillin/clavulanate, cefotaxime, cefepime, ciprofloxacin, imipenem, and gentamicin [19, 35]. Non-susceptibility to penicillin and oxacillin [27] and gentamicin [18], as reported in this study, was rare. Vancomycin was an effective alternative in patients with penicillin allergy [13]. The total duration of culture-guided antimicrobial therapy ranged from 6 to 12 weeks with a mean of 7 weeks, in agreement with the current evidence of medical management of OAI that recommended a minimum of 6 weeks of systemic pathogen-directed antibiotic treatment that augments surgical debridement of the affected bone or joints [11]. The extended duration of medical treatment of more than 8 weeks in some patients [15, 22, 23] could be explained by their poor immune status [15] and the presence of implants [22, 23] that warranted a longer duration of antimicrobial therapy [11].
It is notable to find that all patients with reported outcomes had satisfactory results with nearly complete recovery of functional status within varied periods of follow-up running from 6 weeks to several months. This observation may suggest good outcomes when G. morbillorum-OAIs are diagnosed and adequately treated with a combination of surgical control of sepsis source and culture-based antimicrobial therapy.
Our systematic review has some limitations that necessitate cautious interpretations of the results. Firstly, the number of included studies is very small. Secondly, presumably due to the clinical rarity of G. morbillorum infections, the review only included case reports; hence, the quality of evidence was low. Thirdly, the possibility of publication bias may exist. Due to the lack of large-sized studies in the current literature that report on G. morbillorum-associated OAI, our systematic review of the available case reports may be the only methodology possible to provide the clinical and microbiological features of these rare infections.
| Conclusions | ▴Top |
G. morbillorum is an emerging pathogen for OAI in certain patients with specific risk factors. This review reported the demographic, clinical, and microbiological features of OAIs caused by G. morbillorum. Careful evaluation searching for an underlying infectious focus of G. morbillorum is warranted to identify and control the source. A high suspicion index for an associated endovascular infection in the presence of G. morbillorum bacteremia is necessary.
Acknowledgments
None to declare.
Financial Disclosure
This research received no external funding.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
Conceptualization: ES. Methodology: ES, MF, MA, and PP. Formal analysis: ES and EA. Resources: ES and EA. Writing-original draft preparation: ES, MF, MA, and PP. Supervision and editing: JS. All authors were involved in drafting and revising the manuscript and approved the final version.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Kilpper-Balz R, Schleifer KH. Transfer of Streptococcus morbillorum to the Genus Gemella as Gemella morbillorum comb. nov. Int J Syst Bacteriol. 1988;38(4):442-443
- Debast SB, Koot R, Meis JF. Infections caused by Gemella morbillorum. Lancet. 1993;342(8870):560.
pubmed - Ramanathan A, Gordon SM, Shrestha NK. A case series of patients with Gemella endocarditis. Diagn Microbiol Infect Dis. 2020;97(1):115009.
doi pubmed - Vasishtha S, Isenberg HD, Sood SK. Gemella morbillorum as a cause of septic shock. Clin Infect Dis. 1996;22(6):1084-1086.
doi pubmed - Said M, Tirthani E. Gemella morbillorum- and Capnocytophaga sp.-related mycotic thoracic aortic aneurysm and mediastinal abscess: an unusual case report, a treatment challenge, and a review of literature. Cureus. 2021;13(9):e17728.
doi pubmed pmc - Yamakawa H, Hayashi M, Tanaka K, Kuwano K. Empyema due to Gemella morbillorum is diagnosed by 16S ribosomal RNA gene sequencing and a phylogenetic tree analysis: a case report and literature review. Intern Med. 2015;54(17):2231-2234.
doi pubmed - Hsu CY, Su YC, Wang TL, Chong CF, Chen CC. Gemella morbillorum liver abscess. Scand J Infect Dis. 2007;39(6-7):637-638.
doi pubmed - Chotai S, Moon HJ, Kim JH, Kim JH, Chung HS, Park YK, Kwon TH. Brain abscess caused by Gemella morbillorum: case report and review of the literature. Turk Neurosurg. 2012;22(3):374-377.
doi pubmed - Ueberroth BE, Roxas R. Gemella morbillorum isolated from a pelvic abscess in an HIV-positive patient with squamous cell carcinoma of the perianal region. BMJ Case Rep. 2019;12(5):e227352.
doi pubmed pmc - Saad E, Tummala A, Agab M, Rodriguez-Nava G. Gemella morbillorum as the culprit organism of post-colonoscopy necrotizing perineal soft tissue infection in a diabetic patient with Crohn's disease. J Med Cases. 2022;13(3):99-103.
doi pubmed pmc - Masters EA, Ricciardi BF, Bentley KLM, Moriarty TF, Schwarz EM, Muthukrishnan G. Skeletal infections: microbial pathogenesis, immunity and clinical management. Nat Rev Microbiol. 2022;20(7):385-400.
doi pubmed pmc - Kremers HM, Nwojo ME, Ransom JE, Wood-Wentz CM, Melton LJ, 3rd, Huddleston PM, 3rd. Trends in the epidemiology of osteomyelitis: a population-based study, 1969 to 2009. J Bone Joint Surg Am. 2015;97(10):837-845.
doi pubmed pmc - Omran Y, Wood CA. Endovascular infection and septic arthritis caused by Gemella morbillorum. Diagn Microbiol Infect Dis. 1993;16(2):131-134.
doi pubmed - Roche M, Smyth E. A case of septic arthritis due to infection with Gemella morbillorum. J Infect. 2005;51(3):e187-189.
doi pubmed - Giger A, Yusuf E, Manuel O, Clerc O, Trampuz A. Polymicrobial vertebral osteomyelitis after oesophageal biopsy: a case report. BMC Infect Dis. 2016;16:141.
doi pubmed pmc - Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906.
doi pubmed - Slim K, Nini E, Forestier D, Kwiatkowski F, Panis Y, Chipponi J. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg. 2003;73(9):712-716.
doi pubmed - van Dijk M, van Royen BJ, Wuisman PI, Hekker TA, van Guldener C. Trochanter osteomyelitis and ipsilateral arthritis due to Gemella morbillorum. Eur J Clin Microbiol Infect Dis. 1999;18(8):600-602.
doi pubmed - Czarnecki A, Ong GH, Pieroni P, Trepman E, Embil JM. Gemella morbillorum septic arthritis of the knee and infective endocarditis. Am J Orthop (Belle Mead NJ). 2007;36(1):E7-9.
pubmed - Young Ann J, Kwon JC, Eun Song J, Hyung Kim M, Hyun Oh D, Park Y, Ah Kim Y, et al. Sternal osteomyelitis with a mediastinal abscess caused by Gemella morbillorum following blunt force trauma. Intern Med. 2013;52(4):511-514.
doi pubmed - von Essen R, Ikavalko M, Forsblom B. Isolation of Gemella morbillorum from joint fluid. Lancet. 1993;342(8864):177-178.
doi pubmed - Pardo-Pol A, Perez-Prieto D, Alier A, Ilzarbe L, Sorli L, Puig L, Martinez-Diaz S, et al. Acute hematogenous periprosthetic hip infection by Gemella morbillorum, Successfully treated with debridement, antibiotics and implant retention: a case report and literature review of osteoarticular gemella morbillorum infections. Trop Med Infect Dis. 2022;7(8):191.
doi pubmed pmc - Medina-Gens L, Bordes-Benitez A, Saez-Nieto JA, Pena-Lopez MJ. Infection of a total hip arthroplasty due to Gemella morbillorum. Enferm Infecc Microbiol Clin. 2007;25(8):553.
doi pubmed - Singer Z, Leis B, Nosib S, Kogilwaimath S. Gemella morbillorum endocarditis and osteomyelitis in a patient with ankylosing spondylitis. J Assoc Med Microbiol Infect Dis Can. 2021;6(1):69-72.
doi pubmed pmc - Eisenberger U, Brunkhorst R, Perharic L, Petersen R, Kliem V. Gemella morbillorum—spondylodiscitis in a patient with a renal graft. Nephrol Dial Transplant. 1998;13(6):1565-1567.
doi pubmed - Sono T, Takemoto M, Shinohara K, Tsuchido Y. An uncommon case of pyogenic spondylodiscitis caused by gemella morbillorum. Case Rep Orthop. 2018;2018:3127613.
doi pubmed pmc - Garcia-Bordes L, Aguilera-Repiso JA, Serfaty-Soler JC, Collado-Fabregas F, Martinez-Montauti J, de Llobet-Zubiaga JM, Gomez-Bonsfills X. An unusual case of spondylodiscitis. Spine (Phila Pa 1976). 2010;35(5):E167-171.
doi pubmed - Namazi G, Gupta S, Einarsson JI. Deep Pelvic Side Wall Anatomy: A Case of Laparoscopic Management of Vaginal Vault Fistula to the Presacral Area. J Minim Invasive Gynecol. 2022;29(5):583.
doi pubmed - Savides TJ, Margolis D, Richman KM, Singh V. Gemella morbillorum mediastinitis and osteomyelitis following transesophageal endoscopic ultrasound-guided fine-needle aspiration of a posterior mediastinal lymph node. Endoscopy. 2007;39(Suppl 1):E123-124.
doi pubmed - Desmottes MC, Brehier Q, Bertolini E, Monteiro I, Terreaux W. Septic arthritis of the knee due to Gemella morbillorum. Int J Rheum Dis. 2018;21(5):1146-1147.
doi pubmed - Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schunemann HJ, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924-926.
doi pubmed pmc - Ruppen C, Notter J, Strahm C, Sonderegger B, Sendi P. Osteoarticular and skin and soft-tissue infections caused by Streptococcus agalactiae in elderly patients are frequently associated with bacteremia. Diagn Microbiol Infect Dis. 2018;90(1):55-57.
doi pubmed - Cuerel C, Abrassart S, Billieres J, Andrey D, Suva D, Dubois-Ferriere V, Uckay I. Clinical and epidemiological differences between implant-associated and implant-free orthopaedic infections. Eur J Orthop Surg Traumatol. 2017;27(2):229-231.
doi pubmed - Murillo O, Grau I, Lora-Tamayo J, Gomez-Junyent J, Ribera A, Tubau F, Ariza J, et al. The changing epidemiology of bacteraemic osteoarticular infections in the early 21st century. Clin Microbiol Infect. 2015;21(3):254.e251-258.
doi pubmed - Youssef D, Youssef I, Marroush TS, Sharma M. Gemella endocarditis: A case report and a review of the literature. Avicenna J Med. 2019;9(4):164-168.
doi pubmed pmc - Elsissy JG, Liu JN, Wilton PJ, Nwachuku I, Gowd AK, Amin NH. Bacterial septic arthritis of the adult native knee joint: a review. JBJS Rev. 2020;8(1):e0059.
doi pubmed - Flynn KJ, Baxter NT, Schloss PD. Metabolic and community synergy of oral bacteria in colorectal cancer. mSphere. 2016;1(3):e00102-16.
doi pubmed pmc - Ribeiro Sobrinho AP, de Melo Maltos SM, Farias LM, de Carvalho MA, Nicoli JR, de Uzeda M, Vieira LQ. Cytokine production in response to endodontic infection in germ-free mice. Oral Microbiol Immunol. 2002;17(6):344-353.
doi pubmed - FitzGerald SF, Moloney AC, Maurer BJ, Hall WW. Gemella endocarditis: consider the colon. J Heart Valve Dis. 2006;15(6):833-835.
pubmed - Reyes R, 3rd, Abay A, Siegel M. Gemella morbillorum bacteremia associated with adenocarcinoma of the cecum. Am J Med. 2001;111(2):164-165.
doi pubmed - La Scola B, Raoult D. Molecular identification of Gemella species from three patients with endocarditis. J Clin Microbiol. 1998;36(4):866-871.
doi pubmed pmc - Benedetti P, Rassu M, Branscombe M, Sefton A, Pellizzer G. Gemella morbillorum: an underestimated aetiology of central nervous system infection? J Med Microbiol. 2009;58(Pt 12):1652-1656.
doi pubmed - Woo PC, Lau SK, Fung AM, Chiu SK, Yung RW, Yuen KY. Gemella bacteraemia characterised by 16S ribosomal RNA gene sequencing. J Clin Pathol. 2003;56(9):690-693.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


