| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Case Report
Volume 15, Number 3, March 2023, pages 181-186
Induction Therapy With a Combination of Weekly Adalimumab Plus Intensive Granulocyte and Monocyte Adsorptive Apheresis in Patients With Ulcerative Colitis and Failure of Conventional Agents, Biologics and Janus Kinase Inhibitor
Satoshi Tanidaa, b, d, Keiji Ozekic, Takahito Katanoc, Mamoru Tanakac, Takaya Shimurac, Eiji Kubotac, Hiromi Kataokac, Takuya Takahamab, Shun Sasohb, Yoshimasa Kubotab, Tesshin Banb, Tomoaki Andob, Makoto Nakamurab, Takashi Johb
aEducation and Research Center for Community Medicine, Nagoya City University Graduate School of Medical Sciences, Mizuho-cho, Mizuho-ku, Nagoya, Aichi 467-8601, Japan
bDivision of Gastroenterology, Gamagori City Hospital, Hirata, Gamagori, Aichi 443-8501, Japan
cDepartment of Gastroenterology and Metabolism, Nagoya City University Graduate School of Medical Sciences, Mizuho-cho, Mizuho-ku, Nagoya, Aichi 467-8601, Japan
dCorresponding Author: Satoshi Tanida, Education and Research Center for Community Medicine, Nagoya City University Graduate School of Medical Sciences, Mizuho-cho, Mizuho-ku, Nagoya, Aichi 467-8601, Japan
Manuscript submitted February 9, 2023, accepted March 14, 2023, published online March 28, 2023
Short title: Every-Week ADA and Intensive GMA for Refractory UC
doi: https://doi.org/10.14740/jocmr4887
| Abstract | ▴Top |
Every-week (ew) adalimumab (ADA) maintenance following induction therapy with a standard induction regimen has recently been approved for use in Japan. The efficacy and safety of combination therapy with ew-ADA maintenance following standard induction regimen plus intensive granulocyte and monocyte adsorptive apheresis (GMA) (two sessions/week) for the treatment of refractory ulcerative colitis (UC) displaying failure of conventional, biologics and Janus kinase inhibitor have not been evaluated previously. The present retrospective study evaluated the 10-week efficacy of this combination therapy among refractory UC patients. Six patients were given initial ADA combination therapy (ADA at 160 mg in week 0, ADA 80 mg in week 2, and 40 mg in week 4, followed by ew-ADA at 40 mg/week) plus intensive GMA. One patient (16.6%) achieved clinical remission and two patients (33.3%) achieved endoscopic improvement by week 10. After excluding two patients who discontinued treatment, mean full Mayo score (P = 0.14), endoscopic subscore (P = 0.18) and C-reactive protein level (P = 0.27) at 10 weeks were numerically decreased compared with baseline in the remaining four cases, although the differences were not significant. Use of ew-ADA maintenance following standard induction regimen plus intensive GMA appears unlikely to achieve satisfactory induction of clinical remission in UC patients for whom conventional agents, biologics and Janus kinase inhibitors have failed.
Keywords: Ulcerative colitis; Weekly ADA maintenance; Intensive GMA; Short-term efficacy; Clinical remission
| Introduction | ▴Top |
Ulcerative colitis (UC) is characterized by mucosal ulceration, rectal bleeding, diarrhea, and abdominal pain. Studies have revealed the efficacy and safety of adalimumab (ADA), an anti-tumor necrosis factor (TNF)-α antibody, for the treatment of patients with moderate-to-severe UC who have failed to achieve clinical remission or response to conventional therapies comprising corticosteroids, azathioprine (AZA), and/or 5-aminosalicylic acid [1]. However, the 8-week efficacy of ADA monotherapy for inducing clinical remission in randomized patients who failed to respond conventional therapy was only 16.5% after a standard induction regimen (SIR) (ADA at 160 mg in week 0, followed by 80 mg in week 2 and 40 mg in weeks 4 and 6) [1]. Logistic regression analyses based on pharmacokinetic and pharmacodynamic data from that study, the phase 3 ULTRA 2 study, predicted that higher induction doses of ADA might provide greater efficacy in more patients with moderate-to-severe active UC [1]. A higher induction regimen (HIR) of ADA (160 mg in weeks 0, 1, 2, and 3, followed by 40 mg in weeks 4 and 6) did not provide any greater efficacy regarding 8-week clinical remission rates compared with the SIR [2]. The 52-week clinical remission rate under every-week (ew) ADA at 40 mg for maintenance therapy was significantly higher than that under every-other-week (eow) ADA. Based on such findings, weekly ADA maintenance following SIR (ADA at 160 mg in week 0, followed by 80 mg in week 2 and 40 mg in week 4 and subsequent ew-ADA at 40 mg) has now been approved for use in Japan and the European Union [2]. However, the short-term efficacy of ew-ADA maintenance following SIR remains unclear.
Granulocyte and monocyte adsorptive apheresis (GMA) with Adacolumn® (JIMRO, Takasaki, Japan) is an efficacious and safe therapeutic option for patients with mild-to-moderate UC that proves refractory to pharmacotherapy [3]. Furthermore, intensive GMA, involving two sessions per week, appears superior to weekly GMA in terms of both remission rate and time to remission among patients with refractory UC [4]. However, this therapy is also considered to be limited because the efficacy of intensive GMA for inducing clinical remission in patients with severe UC is unsatisfactory [5].
We report herein a retrospective assessment of the 10-week efficacy of combination therapy with ew-ADA maintenance following SIR plus intensive GMA for UC patients in whom conventional agents, biologics and Janus kinase inhibitors had failed.
| Case Report | ▴Top |
Investigations
Between October 2021 and December 2022, six consecutive patients with moderate and severe UC were recruited to this study from Nagoya City University Hospital and Gamagori City Hospital. Patients receiving combination therapy comprising ew-ADA maintenance following SIR plus intensive GMA for corticosteroid-refractory or corticosteroid-dependent UC or for refractory UC that showed loss of response to AZA, ustekinumab (UST) and tofacitinib (TOF) were enrolled.
All procedures in the present study were performed in compliance with the Declaration of Helsinki. All study protocols were approved by the Ethics Committee at Nagoya City University (approval no. #60-210043) as the central review board for both participating institutions. As this retrospective study was categorized as a low-risk project given the use of approved treatments and obtaining express consent from each person was not feasible, informed consent was considered to have been obtained in the form of an opt-out available on the website of Nagoya City University Hospital during enrollment.
Treatment and assessments
Disease activities and severities were assessed using full Mayo score [6] at baseline and at 10 weeks, and a partial Mayo score when worsening rectal bleeding or an increase in stool frequency was observed or another treatment was added within the 10-week study period. Patients were enrolled to this study according to the following criteria: 1) corticosteroid-refractory or corticosteroid-dependent UC; or 2) loss of response to AZA, UST and TOF; and 3) moderate-to-severe UC (full Mayo score at baseline: 6 - 12), including an endoscopic subscore of 2 - 3 despite concurrent treatment. Patients who failed to complete 10-week combination therapy of ew-ADA maintenance following SIR plus intensive GMA were excluded from clinical assessment as discontinued cases. Only corticosteroid dosages were tapered off, as appropriate. The primary outcome was the clinical remission rate after 10 weeks of ew-ADA maintenance following SIR plus intensive GMA. Clinical remission was defined as a full Mayo score ≤ 2 with no individual subscore > 1. Other assessments at 10 weeks included clinical response (decrease in full Mayo score ≥ 3 from baseline and ≥ 30% plus ≥ 1-point decrease from baseline in rectal bleeding score (RBS) or absolute RBS of 0 or 1) and endoscopic improvement (Mayo endoscopy subscore: 0 - 1) [7]. Among the four patients remaining after excluding two discontinued cases, changes in full Mayo scores, Mayo endoscopic subscore and C-reactive protein (CRP) values at baseline and 10 weeks were also evaluated. Data on any adverse events were recorded, including date of onset, severity, outcome, and relationship of such events to the administered therapies.
Statistical methods
The data are presented as mean ± standard error, and comparisons were made using the Wilcoxon signed-rank test for paired data. Statistical analyses were performed using SPSS version 27 software (IBM, Chicago, IL, USA). A significance level of 0.05 was used for all statistical tests, and two-tailed tests were applied when appropriate.
| Results | ▴Top |
The demographic data of all study participants are shown in Table 1. Mean age was 34.5 years (range, 24 - 48 years), and mean disease duration was 3.75 years (range, 0.15 - 10 years). In terms of location, UC was extensive colitis in three cases, left-sided colitis in one case and distal colitis in two cases. Concurrent medications included 5-aminosalicylate, corticosteroids and AZA. Of the six patients, three were corticosteroid-dependent, two were corticosteroid-refractory, and one experienced loss of response to both UST and TOF but had not received anti-TNF-α antibodies. Mean full Mayo score and CRP level at baseline were 10.1 points and 1.53 mg/dL, respectively. Mean dose of concomitant corticosteroid was 19.0 mg/day in five patients, with one patient using local rectal foam budesonide.
 Click to view | Table 1. Baseline Demographic Variables of the Six Cases |
All six patients were initially provided combination therapy comprising ADA at 160 mg in week 0, followed by 80 mg in week 2 and 40 mg in week 4, with ew-ADA at 40 mg maintenance plus intensive GMA (two sessions per week). Of the six patients receiving combination therapy with ew-ADA maintenance following SIR plus intensive GMA, the proportion of patients who achieved clinical remission by week 10 (primary efficacy end point) was 16.6% (one of six patients). The proportions of patients achieving clinical response and endoscopic improvement (defined as endoscopy subscore ≤ 1) were 50% (3/6) and 33.3% (2/6), respectively. In addition, all three patients who received corticosteroids or budesonide and completed 10-week combination therapy with ew-ADA maintenance following SIR plus intensive GMA were able to discontinue corticosteroids or budesonide at 10 weeks. Median full Mayo score at baseline was 11 (interquartile range, 10 - 11) and median CRP level at baseline was 1.10 mg/dL (interquartile range, 0.03 - 2.83) (Table 2). Two cases (cases 1 and 2) received additional treatment of AZA at 4 and 8 weeks, respectively, because of insufficient response to combination therapy. These two patients were excluded from clinical assessments as discontinued cases. For the remaining four cases, mean full Mayo score and endoscopic subscore changed from baseline values of 9.75 ± 0.94 and 2.5 ± 0.28, respectively, to 4.0 ± 1.73 and 1.75 ± 0.48, respectively, at 10 weeks (Fig. 1a, b). No significant differences were observed between baseline and 10 weeks in terms of changes to mean full Mayo score (P = 0.14) or endoscopic subscore (P = 0.18). Mean CRP levels for the four patients receiving combination therapy with ew-ADA maintenance following SIR plus intensive GMA were 1.26 ± 0.59 mg/dL at baseline and 0.29 ± 0.24 mg/dL at 10 weeks. No significant decreases in CRP levels at 10 weeks were seen compared with baseline (P = 0.27) (Fig. 1c).
 Click to view | Table 2. Clinical Course Within 10 Weeks |
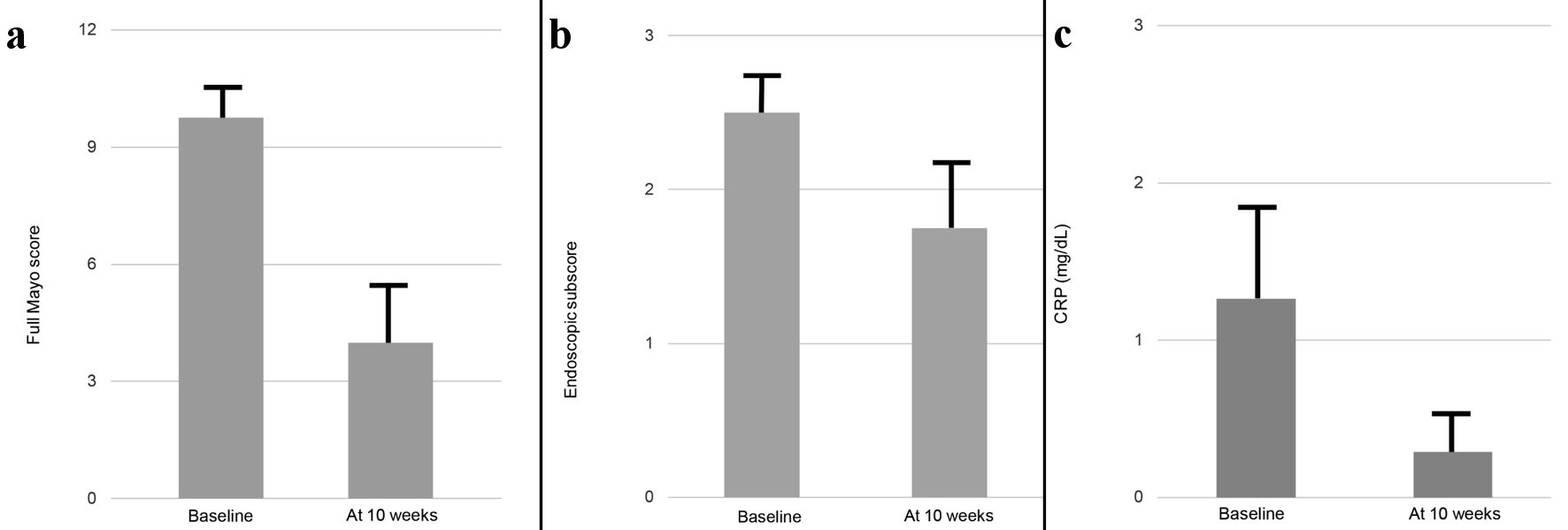 Click for large image | Figure 1. Changes in mean full Mayo score (a), endoscopic subscore (b) and CRP value (c) between baseline and 10 weeks in six cases. Cases 1 and 2, in which the patients received additional drug because of insufficient response to this combination therapy, were excluded from analysis. Data are presented as mean ± standard error. Comparisons were made using the Wilcoxon signed-rank test for paired data. A significance level of 0.05 was used for all statistical tests, and two-tailed tests were applied as appropriate. CRP: C-reactive protein. |
Safety
No adverse events were observed. Exaggeration of symptoms with no improvement of UC was seen in two cases. No occurrence of lymphoma, non-melanoma skin cancer, leukemia, or tuberculosis were observed in the present study. Combination therapies with ew-ADA maintenance following SIR plus intensive GMA were safe and well-tolerated.
| Discussion | ▴Top |
We have reported herein the inadequate efficacy of ew-ADA maintenance following SIR plus intensive GMA in six consecutive cases with refractory UC.
In clinical settings, ADA offers an efficacious therapy for induction and maintenance of clinical remission in patients with moderately-to-severely active UC [7, 8]. The overall 8-week clinical remission rate during SIR with ADA in the ULTRA 2 study was 16.5% and that of patients allocated to receive placebo was 9.3% (P = 0.019). However, this result for SIR ADA was considered limited and logistic regression analysis of pharmacokinetic and pharmacodynamic outcomes suggested that greater efficacy may be obtained with a higher induction dose of ADA. In addition, in European countries, patients who experience a decrease in their response to eow-ADA 40 mg as the recommended maintenance dose appear to benefit from an increase in dose to ew-ADA 40 mg or eow-ADA 80 mg [9]. Based on such findings, the phase 3, double-blinded, randomized, multicenter SERENE UC study was conducted to compare the safety and efficacy of higher and standard ADA dosing regimens for induction, and maintenance therapy comparing eow-ADA at 40 mg with ew-ADA at 40 mg in adults with moderate-to-severe active UC [2]. The HIR (with 160 mg at weeks 0, 1, 2, and 3) did not lead to superior clinical or endoscopic efficacy at week 8 compared with SIR, based on the result that the proportion of patients who achieved clinical remission by week 8 (primary efficacy endpoint) was only 13.8% with the HIR as compared to 11.6% in the SIR group (P = 0.297). Further, percentages of patients who achieved endoscopic improvement by week 8 were 30.5% in the HIR and 26.9% in the SIR group. On the other hand, the percentage of patients showing clinical remission by week 52 (maintenance study primary endpoint) was 41.1% in patients randomized to ew-ADA and 30.1% in the integrated study population allocated to eow-ADA (P = 0.045). As a result, ew-ADA appeared significantly superior to eow-ADA. Accordingly, ew-ADA maintenance following SIR was approved in Japan in 2021. However, in clinical settings, the short-term efficacy of ew-ADA maintenance following SIR for refractory UC appears uncertain and is considered limited, suggesting that additional treatment is necessary for active UC patients who are biologically naive and show a loss of response to UST and TOF.
GMA is presently available in Europe and Japan for the treatment of active UC that may have become refractory to standard pharmacotherapies, including TNF-α blockers and UST. GMA depletes elevated and activated myeloid lineage leucocytes and is associated with a marked downregulation of inflammatory cytokines including interleukin (IL)-1β, IL-6, IL-8, and TNF-α and expression of leukocyte-related adhesion molecules [10-12]. Our previous study investigating the efficacy of combination therapy with SIR ADA plus intensive GMA for 10-week induction in 10 patients with refractory UC showed that rates for clinical remission and endoscopic improvement of 55.6% and 66.7%, respectively. This led to speculation that adding intensive GMA to ADA might be effective as a combination therapy for inducing clinical remission and endoscopic improvements [13]. However, the present study found that the rates of clinical remission and endoscopic improvement at 10 weeks in six patients receiving combination therapy with ew-ADA maintenance following SIR plus intensive GMA were only 16.6% and 33.3%, respectively. This suggests that dose intensity combination therapy with ew-ADA maintenance following SIR plus intensive GMA is unsatisfactory for inducing clinical remission and endoscopic improvement and that no additive effects ware recognized for intensive GMA with ew-ADA maintenance following SIR. Patients who were enrolled in the present study were considered to have severe, refractory UC that was resistant to pharmacotherapy. Indeed, four of the six patients displayed severe UC (full Mayo score: 11), and one showed moderately refractory UC with loss of response to multiple drugs, including UST and TOF. Patients presenting with acute severe UC also experience no response to biologics [14]. Further, the percentage of patients who have previously received treatment with biologics and experienced loss of response to initial biologics, then achieve clinical remission when subsequently receiving secondary biologics has been shown to be lower than that of patients who have not experienced exposure to biologics and achieve clinical remission on receiving biologics for the first time [15]. In addition, the key inflammatory cytokine associated with flare and exaggeration of UC might not be TNF-α, but others. The present study has three major limitations: 1) The sample size (n = 6) was too small; 2) Patients with highly heterogeneous UC treatments, displaying corticosteroid-refractoriness or corticosteroid-dependence, and loss of response to AZA, UST or TOF were included; and 3) No control group with or without either ADA or GMA was used. Further prospective studies are needed to clarify the efficacy of combination therapy comprising ew-ADA maintenance following SIR plus intensive GMA.
The safety profile of HIR for ADA showed a small number of serious infections, allergic reactions, worsening or new-onset psoriasis, and tuberculosis comparable with that of the SIR, indicating no additional signals [2]. In the present study, no adverse events occurred within 10 weeks. Combination therapies with ew-ADA maintenance following SIR plus intensive GMA appear safe and well-tolerated.
Learning points
Following SIR plus intensive GMA, ew-ADA maintenance is well-tolerated, but unlikely to satisfactorily induce clinical remission in refractory UC patients.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
Satoshi Tanida has received research grants from AbbVie.
Informed Consent
Informed consent was considered to have been obtained in the form of opt-out on the website during enrollment.
Author Contributions
ST designed and performed the study. ST and KO drafted the manuscript, MT, ST, EK, TT, YK, TB, TA, MN, TS, and SS performed critical editing of the manuscript. ST, KO and TK assisted with and supported sample collection and subsequent analysis with statistics. ST prepared and wrote the manuscript. HK and TJ carefully supervised this study.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
UC: ulcerative colitis; ADA: adalimumab; TNF: tumor necrosis factor; AZA: azathioprine; SIR: standard induction regimen; HIR: higher induction regimen; ew: every-week; eow: every-other-week; GMA: granulocyte and monocyte adsorptive apheresis; UST: ustekinumab; TOF: tofacitinib; CRP: C-reactive protein
| References | ▴Top |
- Sandborn WJ, van Assche G, Reinisch W, Colombel JF, D'Haens G, Wolf DC, Kron M, et al. Adalimumab induces and maintains clinical remission in patients with moderate-to-severe ulcerative colitis. Gastroenterology. 2012;142(2):257-265.e251-253.
doi - Panes J, Colombel JF, D'Haens GR, Schreiber S, Panaccione R, Peyrin-Biroulet L, Loftus EV, Jr., et al. Higher vs standard adalimumab induction and maintenance dosing regimens for treatment of ulcerative colitis: SERENE UC trial results. Gastroenterology. 2022;162(7):1891-1910.
doi - Ljung T, Thomsen OO, Vatn M, Karlen P, Karlsen LN, Tysk C, Nilsson SU, et al. Granulocyte, monocyte/macrophage apheresis for inflammatory bowel disease: the first 100 patients treated in Scandinavia. Scand J Gastroenterol. 2007;42(2):221-227.
doi - Sakuraba A, Motoya S, Watanabe K, Nishishita M, Kanke K, Matsui T, Suzuki Y, et al. An open-label prospective randomized multicenter study shows very rapid remission of ulcerative colitis by intensive granulocyte and monocyte adsorptive apheresis as compared with routine weekly treatment. Am J Gastroenterol. 2009;104(12):2990-2995.
doi - Hanai H, Watanabe F, Yamada M, Sato Y, Takeuchi K, Iida T, Tozawa K, et al. Adsorptive granulocyte and monocyte apheresis versus prednisolone in patients with corticosteroid-dependent moderately severe ulcerative colitis. Digestion. 2004;70(1):36-44.
doi - Schroeder KW, Tremaine WJ, Ilstrup DM. Coated oral 5-aminosalicylic acid therapy for mildly to moderately active ulcerative colitis. A randomized study. N Engl J Med. 1987;317(26):1625-1629.
doi - Reinisch W, Sandborn WJ, Panaccione R, Huang B, Pollack PF, Lazar A, Thakkar RB. 52-week efficacy of adalimumab in patients with moderately to severely active ulcerative colitis who failed corticosteroids and/or immunosuppressants. Inflamm Bowel Dis. 2013;19(8):1700-1709.
doi - Reinisch W, Sandborn WJ, Hommes DW, D'Haens G, Hanauer S, Schreiber S, Panaccione R, et al. Adalimumab for induction of clinical remission in moderately to severely active ulcerative colitis: results of a randomised controlled trial. Gut. 2011;60(6):780-787.
doi - Humira (INN-adalimumab). Annex I: summary of product characteristics. AbbVie Biotechnology GmbH. Available at: https://www.ema.europa.eu/en/documents/product-information/humira-epar-product-information_en.pdf. January 8, 2021.
- Kashiwagi N, Hirata I, Kasukawa R. A role for granulocyte and monocyte apheresis in the treatment of rheumatoid arthritis. Ther Apher. 1998;2(2):134-141.
doi - Saniabadi AR, Hanai H, Takeuchi K, Umemura K, Nakashima M, Adachi T, Shima C, et al. Adacolumn, an adsorptive carrier based granulocyte and monocyte apheresis device for the treatment of inflammatory and refractory diseases associated with leukocytes. Ther Apher Dial. 2003;7(1):48-59.
doi - Nishise S, Abe Y, Nomura E, Sato T, Sasaki Y, Iwano D, Yoshizawa K, et al. Relationship between tumor necrosis factor-alpha release and granulocyte and monocyte adsorption to cellulose acetate beads. Ther Apher Dial. 2014;18(3):252-257.
doi - Tanida S, Mizoshita T, Nishie H, Ozeki K, Katano T, Kubota E, Kataoka H, et al. Combination therapy with adalimumab plus intensive granulocyte and monocyte adsorptive apheresis in patients with refractory ulcerative colitis. J Clin Med Res. 2015;7(11):884-889.
doi pubmed pmc - Croft A, Walsh A, Doecke J, Cooley R, Howlett M, Radford-Smith G. Outcomes of salvage therapy for steroid-refractory acute severe ulcerative colitis: ciclosporin vs. infliximab. Aliment Pharmacol Ther. 2013;38(3):294-302.
doi - Sands BE, Peyrin-Biroulet L, Loftus EV, Jr., Danese S, Colombel JF, Toruner M, Jonaitis L, et al. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N Engl J Med. 2019;381(13):1215-1226.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


