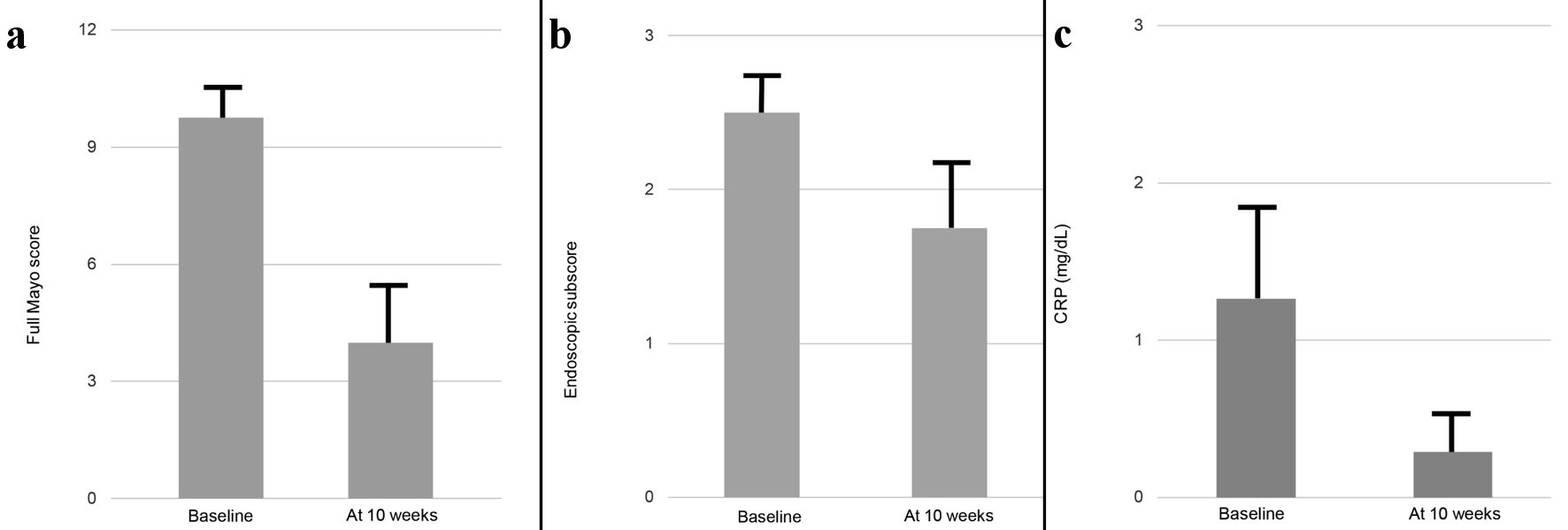
Figure 1. Changes in mean full Mayo score (a), endoscopic subscore (b) and CRP value (c) between baseline and 10 weeks in six cases. Cases 1 and 2, in which the patients received additional drug because of insufficient response to this combination therapy, were excluded from analysis. Data are presented as mean ± standard error. Comparisons were made using the Wilcoxon signed-rank test for paired data. A significance level of 0.05 was used for all statistical tests, and two-tailed tests were applied as appropriate. CRP: C-reactive protein.