| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Short Communication
Volume 15, Number 1, January 2023, pages 51-57
Reduced Time to Procedure for Gastrointestinal Bleeding After Warfarin Reversal With Four-Factor Complex Concentrate as Compared to Plasma
Hannah Spectora, Hannah L. McRaea, Tanzy Loveb, Kalynn Northamc, Khaled Refaaia, Marian A. Rollins-Ravald, Majed A. Refaaia, e
aDepartment of Pathology and Laboratory Medicine, Transfusion Medicine Division, University of Rochester Medical Center, Rochester, NY, USA
bDepartment of Biostatistics and Computational Biology, University of Rochester Medical Center, Rochester, NY, USA
cDepartment of Pharmacy, University of North Carolina Medical Center, Chapel Hill, NC, USA
dDepartment of Pathology, University of New Mexico, Albuquerque, NM, USA
eCorresponding Author: Majed A. Refaai, Department of Pathology and Laboratory Medicine, Transfusion Medicine Division, University of Rochester Medical Center, Rochester, NY 14642, USA
Manuscript submitted December 14, 2022, accepted January 16, 2023, published online January 24, 2023
Short title: Warfarin Reversal in GI Bleeds
doi: https://doi.org/10.14740/jocmr4856
| Abstract | ▴Top |
Background: Bleeding is a serious adverse effect of vitamin K antagonists (VKAs). Anticoagulation reversal is required in some acute cases. This is usually accomplished by plasma transfusion or four-factor prothrombin complex concentrate (4F-PCC). The aim of this study was to gain insight into the clinical course of patients with gastrointestinal (GI) bleeding who require VKA reversal.
Methods: Medical records were collected from two centers from patients who presented to the emergency department (ED) for GI bleeding and received 4F-PCC or plasma for VKA reversal between January 2015 and December 2020. ED, hospital, intensive care unit (ICU) length of stay (LOS) as well as time from admission to GI procedure were determined.
Results: 4F-PCC patients (n = 49) as compared to plasma (n = 63) patients were found to have a greater number of comorbidities (average of 4.2 vs. 2.7 comorbidities/patient) and more ICU admissions (47% vs. 21%). Time to GI procedure was significantly decreased in the 4F-PCC group (median (interquartile range (IQR)) 19.47 (9.23 - 30.25) vs. 27.88 (21.38 - 45.00) h; P = 0.01). When adjusting for comorbidities, differences in time to GI procedures were also significant in favor of 4F-PCC regardless of any comorbidities (P = 0.014), in atrial fibrillation (P = 0.045) and in hypertension (P = 0.02). The 4F-PCC patients had shorter LOS in the ED and ICU.
Conclusions: Our study demonstrated that compared to plasma, 4F-PCC was utilized in more acutely ill patients with higher rates of comorbidities and ICU admission. Nevertheless, the patients who received 4F-PCC had faster access to GI procedure and shorter ED and ICU LOS.
Keywords: Anticoagulation reversal; Gastrointestinal hemorrhage; Prothrombin complex concentrate; Plasma; Warfarin
| Introduction | ▴Top |
Warfarin is an oral anticoagulant that functions as a vitamin K antagonist (VKA), thereby reducing the prevalence of vitamin K-dependent coagulation factors (VKDFs) [1]. Warfarin is used to prevent and treat a variety of prothrombotic states including venous thrombosis and thrombosis associated with atrial fibrillation (A-Fib) [2]. In the presence of additional factors including liver dysfunction, antibiotic therapy or disruption to the gut flora (i.e., decreased vitamin K absorption), malnutrition (i.e., decreased vitamin K intake), and prior history of bleeding, patients may become over-anticoagulated [3]. Among the adverse effects most associated with warfarin therapy is gastrointestinal (GI) bleeding, a potentially life-threatening complication with an estimated incidence of about 12% of warfarin-treated patients [4]. The risk of GI bleeding further increases with length of warfarin treatment and the degree of elevation of international normalized ratio (INR) [5]. Additionally, an increased risk of re-bleeding in patients with previous GI bleeding has been reported [6, 7]. With the recent increase in usage of direct oral anticoagulants (DOACs) such as apixaban and rivaroxaban, it is unclear whether warfarin has a higher or similar risk for GI bleeding in patients with A-Fib, according to three systematic reviews [8-10].
Patients experiencing warfarin-associated GI bleeding are usually treated with an endoscopy to help diagnosis and treat the bleeding [11]. This procedure is recommended to be done after at least partial reversal of anticoagulation during major bleeding, although INR correction may not be essential before endoscopy [12]. In many of these patients, urgent warfarin reversal is required upon presentation to the emergency department (ED) before the bleeding can be identified and terminated. This is usually dependent on the type and severity of bleeding, as well as the urgency level [5, 13-15].
Restoring VKDF (factors II, VII, IX, and X) is the more effective treatment option in warfarin reversal. Plasma transfusion with or without vitamin K has historically been used. However, several safety issues are of concern when using plasma, including the risk of allergic reaction, viral transmission, transfusion-related acute lung injury (TRALI), and transfusion-associated circulatory overload (TACO) due to the large volume necessary to raise factor levels [16-18].
Four-factor prothrombin complex concentrate (4F-PCC) is a purified concentrate of VKDF that is administered in a smaller volume and shorter time, effectively restores VKDF, and rapidly reverses anticoagulation, which is critical in life-threatening bleeding [19-21]. 4F-PCC products such as Kcentra© (CSL Behring, King of Prussia, PA, USA) are derived from human plasma and contain VKDF (factors II, VII, IX, and X) as well as the antithrombotic proteins C and S. Kcentra© has been approved by the FDA for the urgent reversal of VKA in patients with life-threatening bleeding and/or those undergoing emergent invasive procedures. 4F-PCCs are generally preferred over 3F-PCC (factors II, IX, and X) because they restore INR more efficiently [22]. Apart from a low risk of thromboembolism from PCC administration, there are no other additional risks associated with PCC as compared to plasma transfusion for warfarin reversal [20].
The aim of this multicenter retrospective study was to analyze the time spent in the hospital by warfarin-associated GI bleeding patients who receive 4F-PCC versus plasma for warfarin reversal.
| Materials and Methods | ▴Top |
This retrospective study of medical and laboratory records was conducted between January 1, 2015 through December 31, 2020 at the University of Rochester Medical Center and the University of North Carolina Medical Center. Patients taking warfarin that were ≥ 18 years of age with an INR ≥ 2 who presented to the ED with GI bleeding requiring anticoagulation reversal were eligible for inclusion. Only patients managed by 4F-PCC or plasma with or without vitamin K were included in this analysis. Patients who received 3F-PCC or other reversal agents for warfarin reversal, received > 1 dose of 4F-PCC, and/or trauma patients were excluded. Subjects were stratified into two groups according to the warfarin reversal product they received: plasma or 4F-PCC. The collected data included patient demographics, past medical history, medications, date and time of ED admission, transfusion information, results of GI diagnostic procedures, results of laboratory tests, and clinical outcomes. The study was approved by the Research Study Review Board and the Office of Human Research Ethics. All procedures were conducted in compliance with the ethical standards of the responsible institution on human subjects and the Helsinki Declaration.
The GI procedures of interest included endoscopy, colonoscopy, sigmoidoscopy, enteroscopy, cholecystectomy, gastroscopy, capsule endoscopy, and tagged GI bleed scan. The length of time from ED admission to GI procedure, and ED, hospital, and ICU (if applicable) length of stay (LOS) were also calculated. The primary outcome of interest in this study was the time from ED admission to the first diagnostic GI procedure. The secondary outcomes of interest were ED, hospital and ICU (if applicable) LOS. Exploratory outcomes were common comorbidities that were correlated with a shorter time to the diagnostic GI procedure.
The Pearson’s Chi-squared test with Yates’ continuity correction and Wilcoxon rank sum were used for statistical comparison between plasma and 4F-PCC patients. The difference between patients who received plasma and 4F-PCC was estimated for each of the four clinical measures (ED, ICU, and hospital LOS, as well as time to first diagnostic GI procedure), adjusting separately for each of 30 comorbidities or for having any comorbidities. The most common comorbidities include recurrent GI bleeding, hypertension (HTN), cerebrovascular accident (CVA), deep vein thrombosis (DVT), valve disease, A-Fib, coronary artery disease (CAD), and renal failure. The adjusted comparison was done using a Kruskal-Wallis rank sum test between the four groups: 4F-PCC and plasma with and without the comorbidity. P-values < 0.05 were considered statically significant.
| Results | ▴Top |
Out of 129 patients, 112 (plasma n = 63 and 4F-PCC n = 49) met the inclusion/exclusion criteria and were included in this study. Table 1 provides a summary of selected patient characteristics and laboratory results. The age and gender were similar between both groups (P = 0.699 and 0.885, respectively). The 30-day mortality rate was 3.6% (n = 4), one 4F-PCC patient and three plasma patients. The average number of plasma units administered in the plasma patients was 2.75 ± 1.36 units. Of note, there were no records indicating if this amount was balanced according to the patients’ weight. The average dose of 4F-PCC administered was 1,742 ± 1,500 units. In comparison to the plasma patients, significantly lower hemoglobin (P = 0.028) and hematocrit (P = 0.027) were observed in the 4F-PCC group at time of admission along with a trend toward a higher INR (5.77 ± 5.94 vs. 4.01 ± 2.53; P = 0.08). Moreover, the first INR after reversal within 24 h was significantly higher for plasma patients compared to 4F-PCC (1.95 ± 0.69 vs. 1.27 ± 0.21, respectively, P < 0.0001). A higher percentage of 4F-PCC patients received oral or intravenous vitamin K (89.8%) compared to plasma patients (69.8%). Furthermore, for those who received vitamin K, the average dose was greater for 4F-PCC patients (10 vs. 7.5 mg, P = 0.009). The rate of ICU admission was higher for patients who received 4F-PCC (47% vs. 21%), although their ICU LOS tended to be shorter (median (interquartile range (IQR)) 1.98 (1.54 - 3.25) vs. 2.63 (2.12 - 4.85) days, Fig. 1). 4F-PCC patients also tended to have a shorter ED LOS (median (IQR) 6.08 (4.17 - 11.37) vs. 6.68 (5.08 - 13.72) h, Fig. 2).
 Click to view | Table 1. Laboratory Data and Patient Characteristics at Time of Admission |
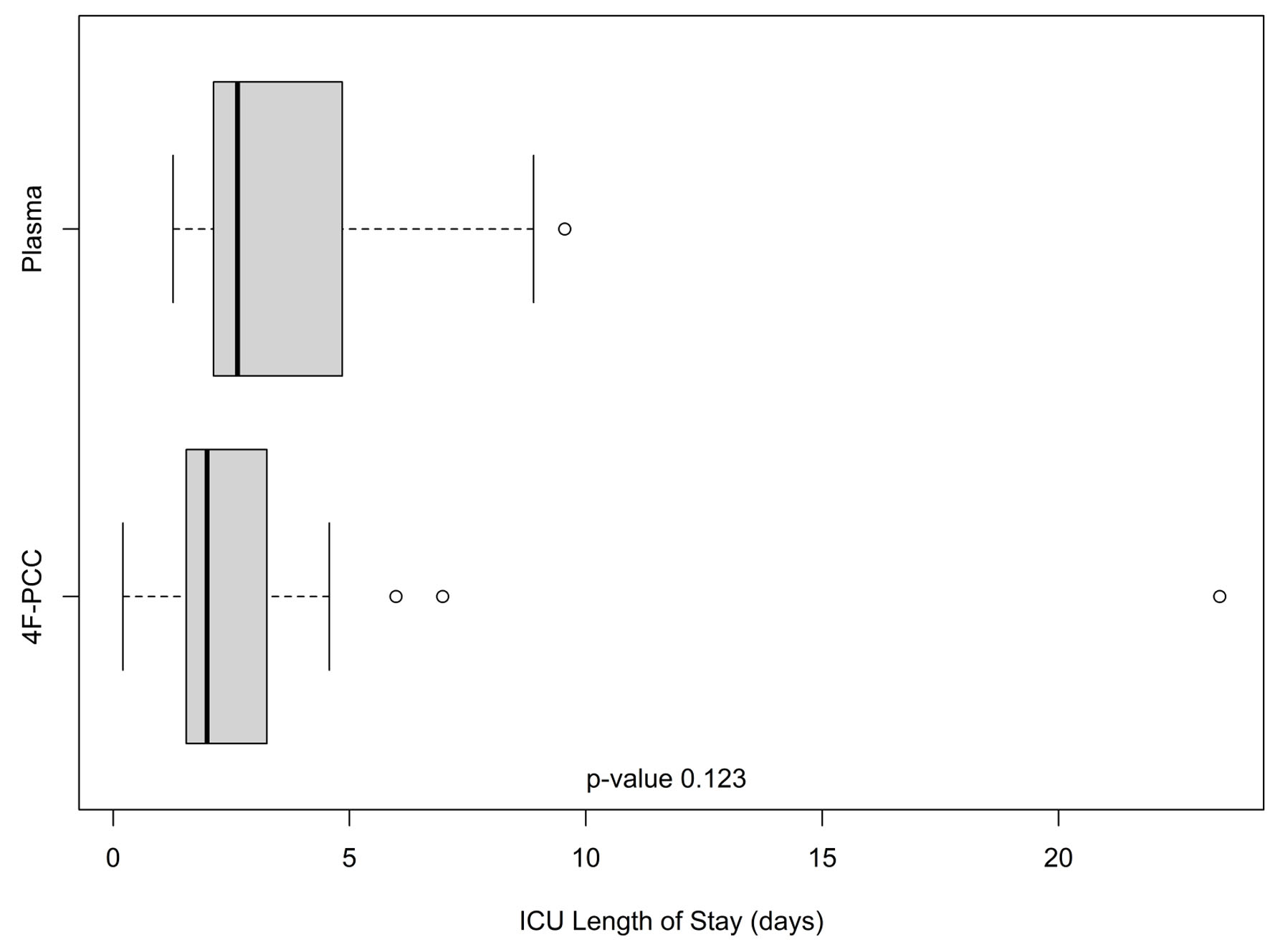 Click for large image | Figure 1. Intensive care unit lengths of stay (in days) in the plasma and 4F-PCC groups. In each boxplot, the bold line is the median, the other lines are the quartiles of the data with outlier values displayed individually as points. 4F-PCC: four-factor prothrombin complex concentrate. |
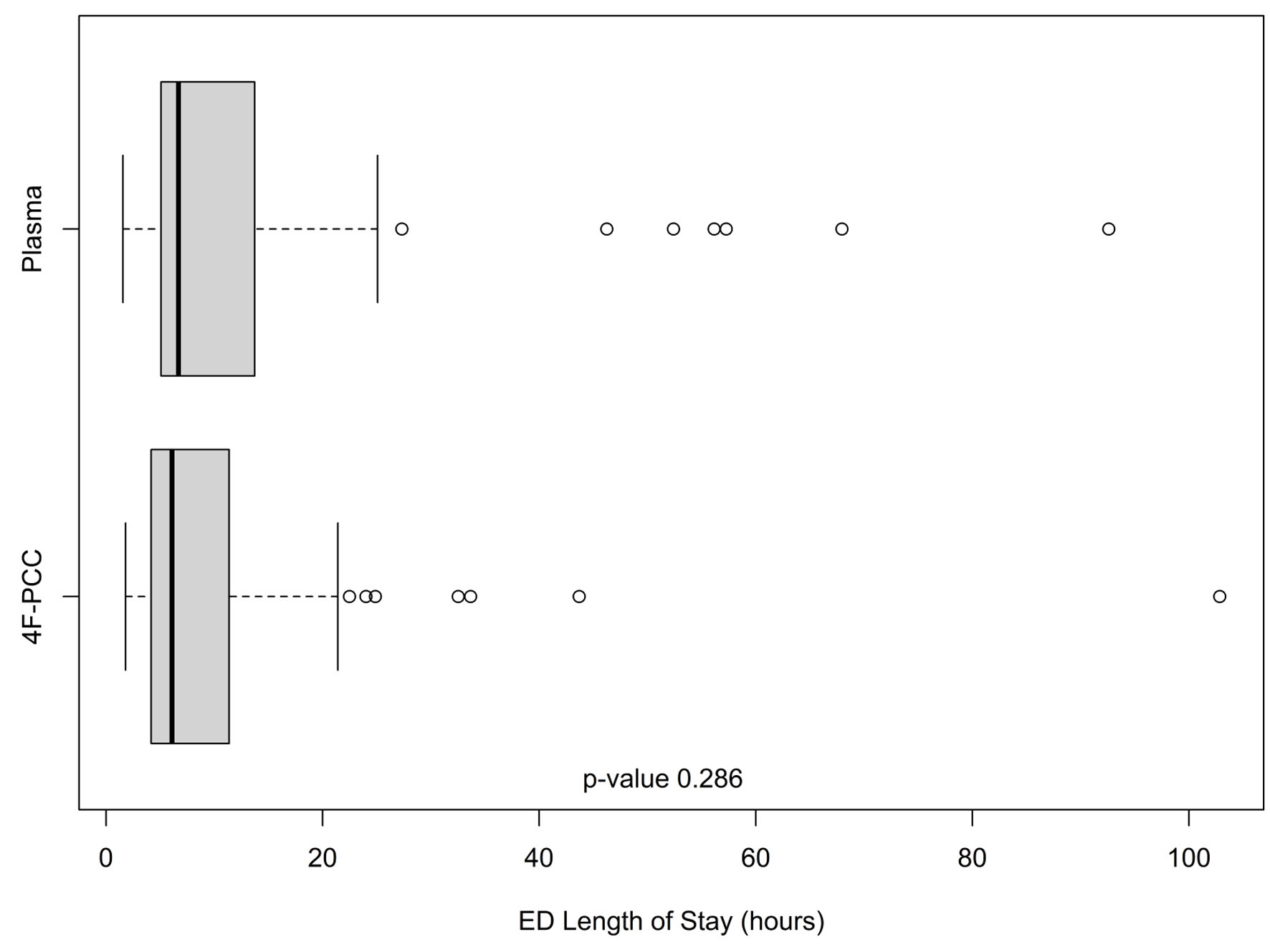 Click for large image | Figure 2. Emergency department lengths of stay (in h) in the plasma and 4F-PCC groups. In each boxplot, the bold line is the median, the other lines are the quartiles of the data with outlier values displayed individually as points. 4F-PCC: four-factor prothrombin complex concentrate. |
4F-PCC patients were noted to have greater numbers of comorbidities compared to the plasma group: average of 4.2 vs. 2.7 comorbidities per patient (P < 0.001). More 4F-PCC patients had a history of recurrent GI bleeding (65% vs. 22%, P = 0.001) and CVA (41% vs. 9%, P = 0.002), while more plasma patients had a history of DVT (2% vs. 13%, P = 0.013) and valve disease (28% vs. 53%, P < 0.001). The prevalence rates of other comorbidities including A-Fib, CAD and renal failure were similar among the two groups. When adjusted for the presence of any comorbidity, plasma patients had shorter hospital stays than 4F-PCC patients (P = 0.019). A greater proportion of patients who received plasma had GI procedures (83% vs. 76%); however, the time (h) to GI procedure was significantly decreased for patients in the 4F-PCC group (median (IQR) 19.47 (9.23 - 30.25) vs. 27.88 (21.38 - 45.00) h, P = 0.01; Fig. 3). History of A-Fib, hypertension (HTN), or CVA were associated with more significant differences in time to GI procedure between the 4F-PCC and plasma groups. Compared to patients who received plasma and patients in either group who did not have a history of A-fib, time to GI procedure was lowest for those treated with 4F-PCC who had a history of A-Fib (P = 0.045). This trend was the same for patients with HTN, with the lowest time to GI procedure being people with HTN who received 4F-PCC (P = 0.021). Conversely, patients who received 4F-PCC who had a history of CVA had the longest time to GI procedure compared to plasma patients with a history of CVA and patients who never had a CVA (P = 0.049).
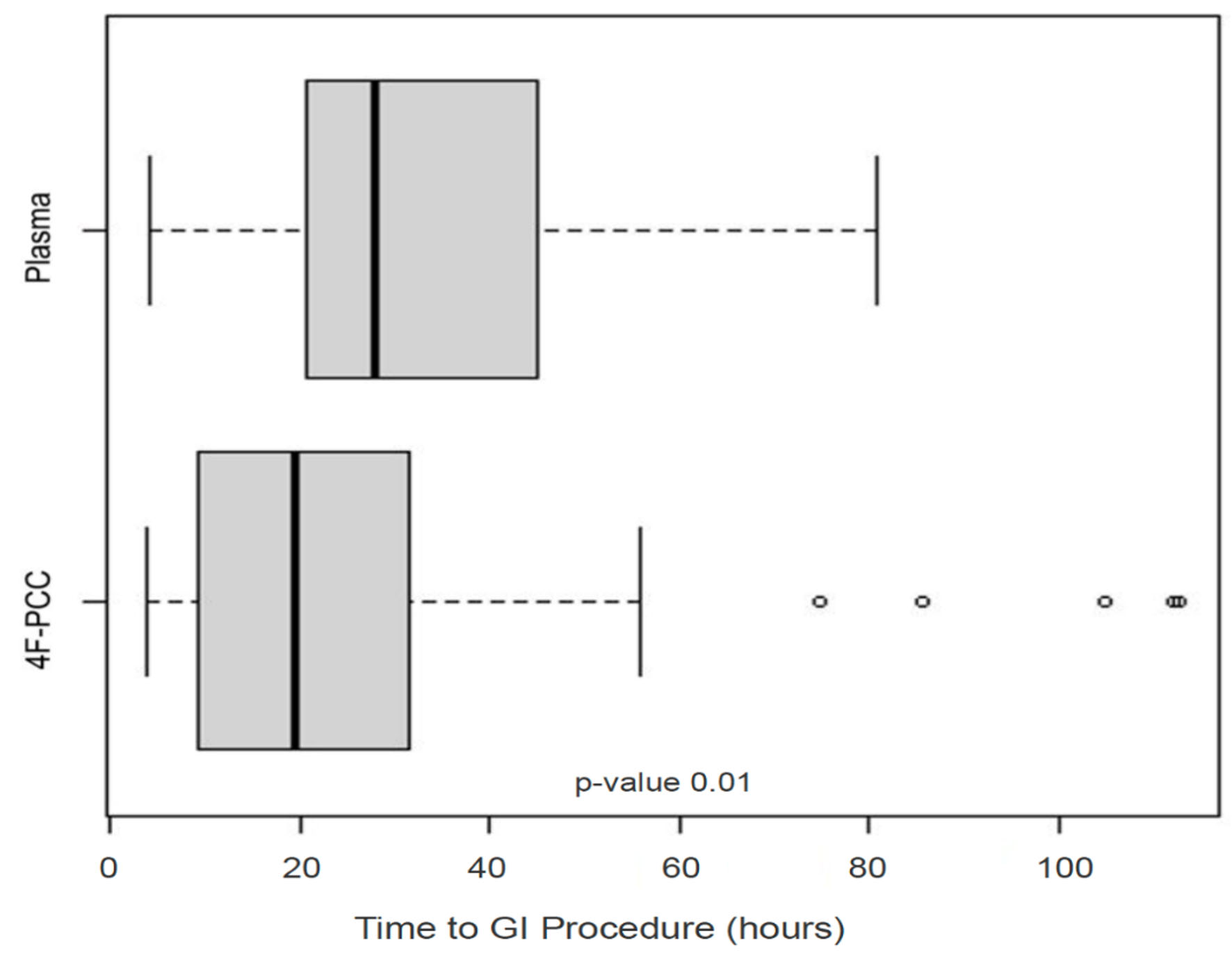 Click for large image | Figure 3. Time from admission to GI procedure (in h) in the plasma and 4F-PCC groups. In each boxplot, the bold line is the median, the other lines are the quartiles of the data with outlier values displayed individually as points. GI: gastrointestinal; 4F-PCC: four-factor prothrombin complex concentrate. |
| Discussion | ▴Top |
It has been well established that 4F-PCC is the preferred product for warfarin reversal over plasma as it has less transfusion-related risks and faster time to correction [13, 14, 19, 20]. More specifically, the use of 4F-PCC for VKA reversal in acutely bleeding patients requiring surgery has been previously shown to be non-inferior to plasma [21]. Our results agree with previous studies in that 4F-PCC allowed for more normalized INR correction and less time to GI procedure, although 4F-PCC patients were admitted in more critical conditions. This study was the first to find a difference in the time to GI procedure comparing plasma and 4F-PCC patients for warfarin reversal in warfarin-associated GI bleeding.
Since GI procedures are often used to identify the source of bleeding in this patient population, shorter time to the GI procedure will help to identify the source of bleeding faster, reduce the time and amount of bleeding, reduce blood product transfusions, and may overall improve clinical outcome. Endoscopy has been recommended for patients with upper GI bleeding on warfarin with a supra-therapeutic INR [12, 23]. In patients with an INR of 1.5 - 2.5, endoscopy may be performed during the administration of reversal agents to reverse the effects of warfarin. However, in patients with an INR > 2.5, reversal agents should generally be administered before endoscopy [11]. Therefore, the faster and more complete correction associated with 4F-PCC administration may be more beneficial for patients with a higher INR on admission. Another study found that complete INR correction may not be necessary before surgery for patients who receive 4F-PCC and have massive bleeding. Unlike plasma, 4F-PCC was able to restore depleted VKDF quickly and successfully without always normalizing INR [24]. Additionally, higher doses of vitamin K have also been found to be associated with faster INR change during warfarin reversal [25]. Thus, the increased rate of administration and higher average dose of vitamin K in 4F-PCC patients suggests that there was a need for more emergent INR reversal due to critical clinical status before diagnostic GI procedure.
It is unclear if warfarin carries a higher risk of GI bleeding compared to DOACs. While one systematic review found that the risk between some DOACs and warfarin are similar in A-Fib patients, another found that, in the Asian population with A-Fib, warfarin had a significantly higher risk of GI bleeding [8, 9]. Although other interventions exist for acute bleeding on DOACs, PCC administration has been shown to lead to similar clinical outcomes when used for DOAC reversal as compared to warfarin [26]. Nevertheless, as demonstrated in this study 4F-PCC seems to be a fast, safe, and effective reversal agent for warfarin-associated GI bleeding.
Although the retrospective nature and small number of patients in this study make it difficult to derive concrete conclusions from the medical record, we suspect based on our own clinical practice that the patients who had more severe comorbidities were selected to receive 4F-PCC due to the more emergent need for warfarin reversal. Decreased LOS in the 4F-PCC group in the ED is dependent on multiple factors but includes acuity of patient condition. In addition, the 4F-PCC group having worse clinical status upon admission likely contributed to their higher rate of ICU admission.
Despite having a longer hospital LOS when adjusted for comorbidities, lower hemoglobin, and more elevated INR, the 4F-PCC group had a more complete INR correction, less time to GI procedure, and shorter ED and ICU LOS. We attribute this to the improved efficacy associated with 4F-PCC reversal. However, larger studies are needed to confirm these findings. The small trend toward less time in the ICU after reversal with 4F-PCC suggests that the patient improved more quickly and reduces the overall healthcare costs, although further studies are also needed to confirm this trend.
Acknowledgments
Deborah Sturpe helped to facilitate and execute the project at the University of North Caroline site and reviewed the final manuscript.
Financial Disclosure
This study was funded by CSL Behring.
Conflict of Interest
Majed A. Refaai is on the advisory committee and is a consultant for Cerus, Diagnostica Stago, and CSL Behring. All other authors declare no competing interests.
Informed Consent
Not applicable.
Author Contributions
Hannah Spector and Majed Refaai collected, reviewed and analyzed data, drafted the manuscript, and approved the final version. Tanzy Love reviewed the data and performed statistical analyses. All authors collected data, reviewed the analyses, and read and approved the final version.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
VKA: vitamin K antagonist; VKDF: vitamin K-dependent coagulation factor; A-Fib: atrial fibrillation; GI: gastrointestinal; INR: international normalized ratio; DOAC: direct oral anticoagulant; ED: emergency department; TRALI: transfusion-related acute lung injury; TACO: transfusion-associated circulatory overload; 4F-PCC: four-factor prothrombin complex concentrate; LOS: length of stay; HTN: hypertension; CVA: cerebrovascular accident; DVT: deep vein thrombosis; CAD: coronary artery disease; aPTT: activated partial thromboplastin time; PT: prothrombin time; INR: international normalized ratio; AST: aspartate aminotransferase; ALT: alanine transaminase
| References | ▴Top |
- Hirsh J, Dalen J, Anderson DR, Poller L, Bussey H, Ansell J, Deykin D. Oral anticoagulants: mechanism of action, clinical effectiveness, and optimal therapeutic range. Chest. 2001;119(1 Suppl):8S-21S.
doi pubmed - Kuruvilla M, Gurk-Turner C. A review of warfarin dosing and monitoring. Proc (Bayl Univ Med Cent). 2001;14(3):305-306.
doi pubmed - Chen WC, Chen YH, Hsu PI, Tsay FW, Chan HH, Cheng JS, Lai KH. Gastrointestinal hemorrhage in warfarin anticoagulated patients: incidence, risk factor, management, and outcome. Biomed Res Int. 2014;2014:463767.
doi pubmed - Rubin TA, Murdoch M, Nelson DB. Acute GI bleeding in the setting of supratherapeutic international normalized ratio in patients taking warfarin: endoscopic diagnosis, clinical management, and outcomes. Gastrointest Endosc. 2003;58(3):369-373.
doi pubmed - Petty GW, Brown RD, Jr., Whisnant JP, Sicks JD, O'Fallon WM, Wiebers DO. Frequency of major complications of aspirin, warfarin, and intravenous heparin for secondary stroke prevention. A population-based study. Ann Intern Med. 1999;130(1):14-22.
doi pubmed - Little D, Chai-Adisaksopha C, Hillis C, Witt DM, Monreal M, Crowther MA, Siegal DM. Resumption of anticoagulant therapy after anticoagulant-related gastrointestinal bleeding: A systematic review and meta-analysis. Thromb Res. 2019;175:102-109.
doi pubmed - Sostres C, Marcen B, Laredo V, Alfaro E, Ruiz L, Camo P, Carrera-Lasfuentes P, et al. Risk of rebleeding, vascular events and death after gastrointestinal bleeding in anticoagulant and/or antiplatelet users. Aliment Pharmacol Ther. 2019;50(8):919-929.
doi pubmed - Anghel L, Sascau R, Trifan A, Zota IM, Statescu C. Non-Vitamin K Antagonist oral anticoagulants and the gastrointestinal bleeding risk in real-world studies. J Clin Med. 2020;9(5):1398.
doi pubmed - Yang KT, Sun WC, Tsai TJ, Tsay FW, Chen WC, Cheng JS. The risk of gastrointestinal bleeding between non-vitamin K antagonist oral anticoagulants and vitamin K antagonists in the asian atrial fibrillation patients: a meta-analysis. Int J Environ Res Public Health. 2020;18(1):137.
doi pubmed - Briere JB, Bowrin K, Coleman C, Fauchier L, Levy P, Folkerts K, Toumi M, et al. Real-world clinical evidence on rivaroxaban, dabigatran, and apixaban compared with vitamin K antagonists in patients with nonvalvular atrial fibrillation: a systematic literature review. Expert Rev Pharmacoecon Outcomes Res. 2019;19(1):27-36.
doi pubmed - Hull R, Garcia D. Management of Warfarin-Associated Bleeding or Supratherapeutic INR. Post TW, ed. UpToDate. Accessed on January 8, 2023.
- Zullo A, Hassan C, Radaelli F. Gastrointestinal endoscopy in patients on anticoagulant therapy and antiplatelet agents. Ann Gastroenterol. 2017;30(1):7-14.
- Chai-Adisaksopha C, Hillis C, Siegal DM, Movilla R, Heddle N, Iorio A, Crowther M. Prothrombin complex concentrates versus fresh frozen plasma for warfarin reversal. A systematic review and meta-analysis. Thromb Haemost. 2016;116(5):879-890.
doi pubmed - Milling TJ, Pollack CV. A review of guidelines on anticoagulation reversal across different clinical scenarios - Is there a general consensus? Am J Emerg Med. 2020;38(9):1890-1903.
doi pubmed - Levy JH, Tanaka KA, Dietrich W. Perioperative hemostatic management of patients treated with vitamin K antagonists. Anesthesiology. 2008;109(5):918-926.
doi pubmed - Kor DJ, Stubbs JR, Gajic O. Perioperative coagulation management—fresh frozen plasma. Best Pract Res Clin Anaesthesiol. 2010;24(1):51-64.
doi pubmed - Franchini M, Lippi G. Prothrombin complex concentrates: an update. Blood Transfus. 2010;8(3):149-154.
- Fitzgerald J, Lenihan M, Callum J, McCluskey SA, Srinivas C, van Rensburg A, Karkouti K. Use of prothrombin complex concentrate for management of coagulopathy after cardiac surgery: a propensity score matched comparison to plasma. Br J Anaesth. 2018;120(5):928-934.
doi pubmed - Hickey M, Gatien M, Taljaard M, Aujnarain A, Giulivi A, Perry JJ. Outcomes of urgent warfarin reversal with frozen plasma versus prothrombin complex concentrate in the emergency department. Circulation. 2013;128(4):360-364.
doi pubmed - Smith MN, Deloney L, Carter C, Weant KA, Eriksson EA. Safety, efficacy, and cost of four-factor prothrombin complex concentrate (4F-PCC) in patients with factor Xa inhibitor-related bleeding: a retrospective study. J Thromb Thrombolysis. 2019;48(2):250-255.
doi pubmed - Goldstein JN, Refaai MA, Milling TJ, Jr., Lewis B, Goldberg-Alberts R, Hug BA, Sarode R. Four-factor prothrombin complex concentrate versus plasma for rapid vitamin K antagonist reversal in patients needing urgent surgical or invasive interventions: a phase 3b, open-label, non-inferiority, randomised trial. Lancet. 2015;385(9982):2077-2087.
doi pubmed - DeAngelo J, Jarrell D, Cosgrove R, Camamo J, Edwards C, Patanwala AE. Comparison of 3-factor versus 4-factor prothrombin complex concentrate with regard to warfarin reversal, blood product use, and costs. Am J Ther. 2018;25(3):e326-e332.
doi pubmed - Choudari CP, Rajgopal C, Palmer KR. Acute gastrointestinal haemorrhage in anticoagulated patients: diagnoses and response to endoscopic treatment. Gut. 1994;35(4):464-466.
doi pubmed - Hood C, Goldstein JN, Milling TJ, Refaai MA, Bajcic P, Goldstein B, Sarode R. INR and vitamin K-dependent factor levels after vitamin K antagonist reversal with 4F-PCC or plasma. Blood Adv. 2022.
doi pubmed - Polito NB, Kanouse E, Jones CMC, McCann M, Refaai MA, Acquisto NM. Effect of vitamin K administration on rate of warfarin reversal. Transfusion. 2019;59(4):1202-1208.
doi pubmed - Muller M, Eastline J, Nagler M, Exadaktylos AK, Sauter TC. Application of prothrombin complex concentrate for reversal of direct oral anticoagulants in clinical practice: indications, patient characteristics and clinical outcomes compared to reversal of vitamin K antagonists. Scand J Trauma Resusc Emerg Med. 2019;27(1):48.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


