| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Review
Volume 15, Number 1, January 2023, pages 10-22
Improving the Emergency Department Management of Sickle Cell Vaso-Occlusive Pain Crisis: The Role and Options of Sublingual and Intranasally Administered Analgesia
Ademola S. Ojoa, d, Olumayowa G. Odipeb, Oluwanifemi Owosenic
aDepartment of Medicine, Howard University Hospital, Washington DC, USA
bDepartment of Pediatrics and Child Health, Queen’s Medical Center, Nottingham University Hospitals NHS Trust, Nottingham, UK
cDepartment of Pharmaceutical Sciences, Howard University College of Pharmacy, Washington DC, USA
dCorresponding Author: Ademola S. Ojo, Department of Internal Medicine, Howard University Hospital, Washington DC, USA
Manuscript submitted November 1, 2022, accepted December 10, 2022, published online January 24, 2023
Short title: Intranasal and Sublingual Analgesics in SCD Patients
doi: https://doi.org/10.14740/jocmr4841
- Abstract
- Introduction
- Pathophysiology of Acute SCD Pain
- Physiology of the Intranasal and Sublingual Route of Drug Administration
- Administration of Intranasal and Sublingual Medications
- Intranasally Administered Analgesic Agents
- Sublingually Administered Analgesic Agents
- Improving the ED-Based Management of Vaso-Occlusive Pain Crisis
- Conclusions
- References
| Abstract | ▴Top |
Vaso-occlusive crisis (VOC), characterized by periods of excruciating pain is the most common clinical manifestation of sickle cell disease (SCD), often resulting in emergency room presentation. These patients often experience long wait times in the emergency department before receiving their first dose of analgesia. This delay results from the complexities of the emergency care system. Using the intranasal or sublingual approach to administering analgesia to SCD patients with VOC offers a fast, safe, noninvasive, atraumatic, and easily accessible route of administration which could reduce the time to first dose of analgesia. With the evolving advances in the development and delivery of analgesic medications, providers should be conversant with the nuances of intranasal and sublingual analgesia in the management of acute vaso-occlusive pain crisis. This review explores the pharmacokinetic profiles, dosages, and administration of intranasal and sublingual analgesics with relevance to the SCD population.
Keywords: Sickle cell disease; Vaso-occlusive crisis; Analgesics; Intranasal; Sublingual; Emergency department; Opioid; NSAID
| Introduction | ▴Top |
Sickle cell disease (SCD) is the most common monogenic disease globally with millions of people affected and an estimated 300,000 children born with this disorder annually [1]. It is a heterogeneous disease primarily affecting individuals of African, Caribbean, Saudi Arabian, and Indian ancestry [2]. Certain individuals in the Mediterranean and Spanish-speaking regions of Central and South America are also affected by this condition [2]. The presence of abnormal beta-globin alleles due to a mutated hemoglobin beta (HBB) gene on chromosome 11 p15.4 with the inheritance of at least one hemoglobin S allele, in combination with another pathogenic HBB variant underlies the myriads of pathological manifestations of this disease [3]. The spectrum of SCD includes the homozygous and most severe form of hemoglobin SS (HbSS) referred to as sickle cell anemia, as well as other less common compound heterozygous forms; HbSC, HbSDPunjab, HbSOArab, HbS/β0-thalassemia, and HbS/β+-thalassemia [4]. HbS undergoes polymerization upon deoxygenation, forming polymers with a resulting alteration in red blood cell (RBC) morphology (sickling) [3].
Besides hemolysis and the resulting chronic anemia, vaso-occlusive crisis (VOC) is the most common clinical manifestation of SCD [5]. These episodes of excruciating pain are the most common reason for hospitalization in SCD [6]. As the mortality rate from SCD continues to decline due to advances in intervention, data from the Healthcare Cost and Utilization Project (HCUP) and Agency for Healthcare Research and Quality (AHRQ) show a trend toward increased hospitalization for painful crisis in the United States [7]. While most episodes of sickle cell pain are managed at home, acute painful crisis often leads to emergency department (ED) presentation [8].
The National Heart, Lung, and Blood Institute (NHLBI) guideline for the management of acute pain crisis in SCD recommends an individualized, rapid administration of analgesia with a goal of administration within 1 h of ED arrival followed by reassessment and repeat dosing every 15 to 30 min until the pain is controlled [9]. However, in many EDs, patients often wait for many hours before having intravenous access placed. The common reason for the delays includes large patient volumes with competing priorities, inadequate staffing, and difficult intravenous access (“hard stick”) requiring ultrasound guidance or special procedures to secure access [10]. Based on this, part of the recommended framework by the NHLBI for optimal pain management in acute vaso-occlusive pain crisis is the use of alternatives to intravenous pain medications [9]. In the setting of acute VOC, intramuscular administration is undesirable as it inflicts more pain. Subcutaneous analgesia is beneficial, but the onset of action is relatively slow, and administration involves needle sticks, while oral medications have a longer onset of action. In the setting of excruciating pain often experienced in VOC, fast administration and rapid onset of analgesia are essential to optimal patient care.
The use of an intranasal or sublingual approach to administering analgesia to SCD patients with VOC offers a fast, noninvasive, atraumatic, and easily accessible route of administration which has been shown to reduce the time-to-administration of analgesia [11]. With the evolving advances in the development and delivery of analgesic medications, providers should be conversant with the nuances of intranasal and sublingual analgesia in the management of acute vaso-occlusive pain crisis. In this review, we discuss the physiology of intranasal and sublingual drug administration and factors to consider when using these routes for the administration of analgesics in SCD. In addition, we explore the available options of intranasal and sublingual analgesics, pharmacokinetics, dosage, and side effects, and outline strategies to improve the ED-based management of vaso-occlusive pain crisis.
| Pathophysiology of Acute SCD Pain | ▴Top |
The pain of VOC is often described as a throbbing and sharp pain of sudden onset, preceded by 1 or 2 days of prodromal symptoms, with a peak on the third day and a gradual resolution at about a week [12]. Although the course of acute VOC pain could be unpredictable, the common underlying theme for this vascular phenomenon is a complex interaction of cellular and molecular cascades culminating in blood flow disruption in the microcirculation, leading to tissue ischemia, hypoxia, and inflammation (Fig. 1) [13].
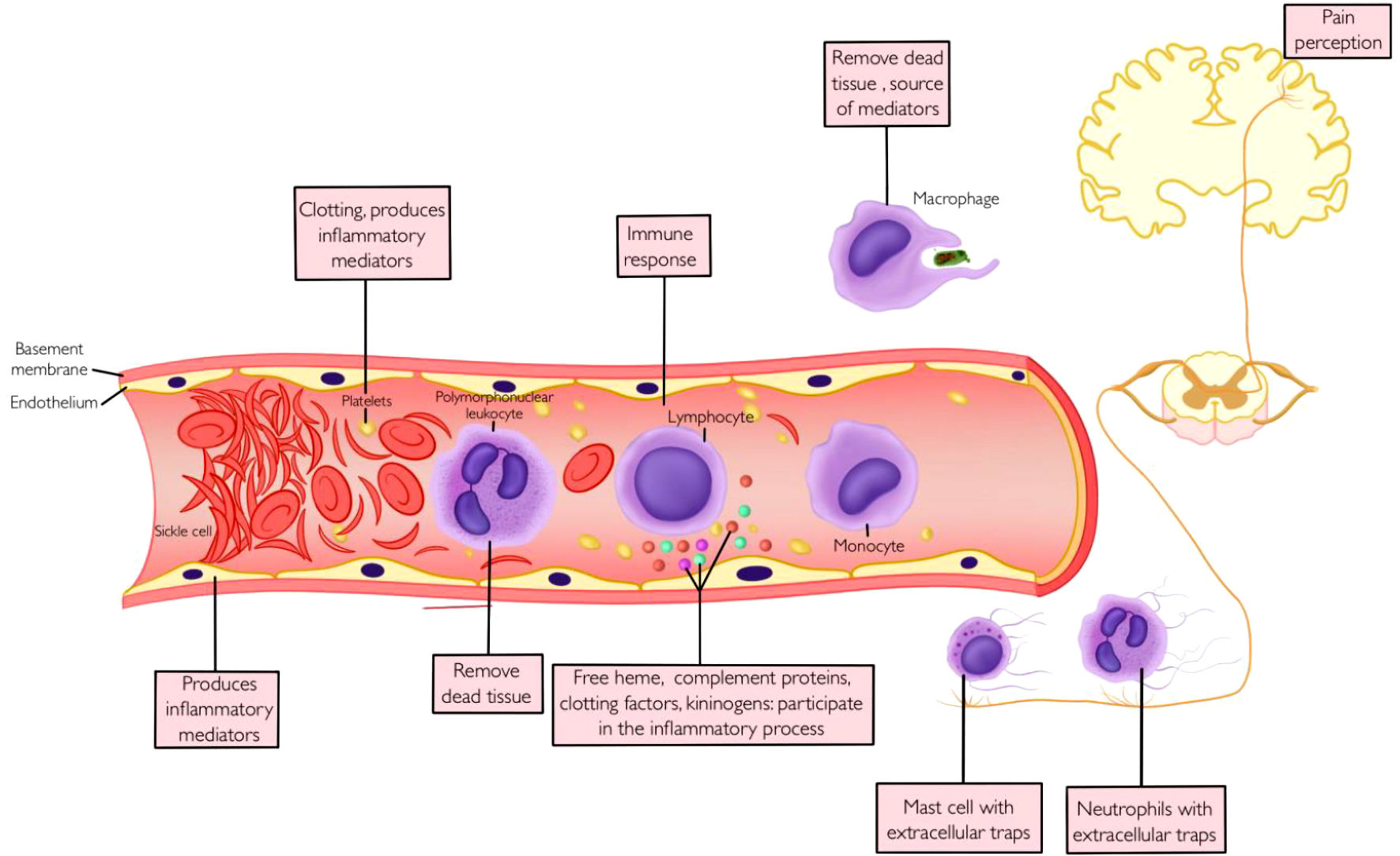 Click for large image | Figure 1. Vaso-occlusion, inflammation and pain. |
Hemoglobin polymerization
Deoxygenated sickle hemoglobin undergoes polymerization, forming linear polymers leading to an alteration in the shape of RBCs (sickling) [13]. These deformed RBCs lack the flexibility to navigate the microcirculation, leading to vascular occlusion and tissue ischemia [14]. The recurrent cycle of sickling-unsickling leads to damage to RBCs resulting in hemolysis, increased adhesion to vascular endothelium, microcirculation occlusion, ischemia-reperfusion, and downstream organ dysfunction [15].
Sickle cells, endothelium, and adhesion molecules
VOC involves a complex interaction of cellular elements and connective tissue matrix resulting in the formation of microthrombi and vascular occlusion [16]. Sickle RBCs are rigid and fragile and are abnormally adherent to endothelial cells, platelets, and adherent leukocytes [15]. When lysed, these cells release proinflammatory hemoglobin particles and generate reactive oxygen species [16]. The resulting proinflammatory and oxidative milieu promotes endothelial activation and increases expression of adhesion molecules [15]. These receptors include CD36 on sickle erythrocytes, α4β1 integrin on leukocytes, CD239 (basal cell adhesion molecule/Lutheran adhesion glycoprotein) on activated endothelial cells, and intercellular adhesion molecule 4 (ICAM-4) on RBCs [17, 18]. In addition, activated endothelial cells increase the expression of P-selectin, E-selectin, ICAM-1, and vascular cell adhesion molecule 1, all of which promote leukocyte recruitment as well as platelet aggregation [18]. Activated endothelial cells, platelets, and leukocytes are major sources of proinflammatory cytokines such as interleukin-1 (IL-1), tumor necrosis factor (TNF), chemokines, and eicosanoids, all of which propagate the cycle of endothelial activation, cellular adhesion, complement, and coagulation system activation and inflammation [19].
Inflammation and pain
Inflammatory modulators released by cellular elements at the site of an ongoing inflammatory process create a noxious environment that sensitizes and excites nociceptors, lowering the threshold for action potential initiation and impulse conduction across these neurons [20]. Damage to nerve endings in the oxidative and noxious environment as well as direct injury from extracellular entrapments by innate immune cells generate a neural impulse which is transmitted to the dorsal root ganglion [15, 21]. The nociceptive impulse is transmitted to the central nervous system (CNS) where it undergoes further modulation. In SCD, the repeated cycle of ischemia/reperfusion creates a chronic inflammatory state, leading to increased sensitization of the neural circuit involved in the pain pathway, creating a state of hyperalgesia and chronic pain [22].
| Physiology of the Intranasal and Sublingual Route of Drug Administration | ▴Top |
Intranasal route
The nasal cavity is divided into two halves by a nasal septum, each made up of three regions: the vestibule, respiratory and olfactory regions [23]. The nasal vestibule connects the nostrils to the rest of the nasal cavity and is lined by stratified squamous keratinized epithelium in its first part with coarse hair for air filtration and respiratory epithelium in its second part [24]. The respiratory region contributes to the largest area of the nasal cavity and is lined by the respiratory epithelium (Fig. 2) [23]. This region functions in humidification, warming, and filtration of air and it is the site responsible for most of the absorption of drug particles during intranasal administration [24]. The mucosa in this area is 0.3 - 0.5 mm thick and with an overall surface area of 120 cm2 (Fig. 2) [25]. The epithelium of the respiratory region is made up of pseudostratified ciliated columnar epithelium and is covered with a protective layer of mucus secreted by the epithelial goblet cells [26]. These trap inhaled particles and are swept into the nasopharynx through the rhythmic beating of the cilia apparatus [23]. The presence of microvilli on the columnar epithelial cells increases the surface area of absorption [27]. The olfactory region is located on the roof of the nasal cavity and contains specialized sensory epithelium whose axons project into the CNS, providing a direct route of medication delivery into the CNS [27]. The presence of an extensive vascular supply, submucosa veins with thin walls, a well-developed submucosa lymphatic network, highly permeable epithelium, and the ability to bypass the first-pass metabolism in the liver makes the nasal epithelium an interesting site for medication delivery [28, 29].
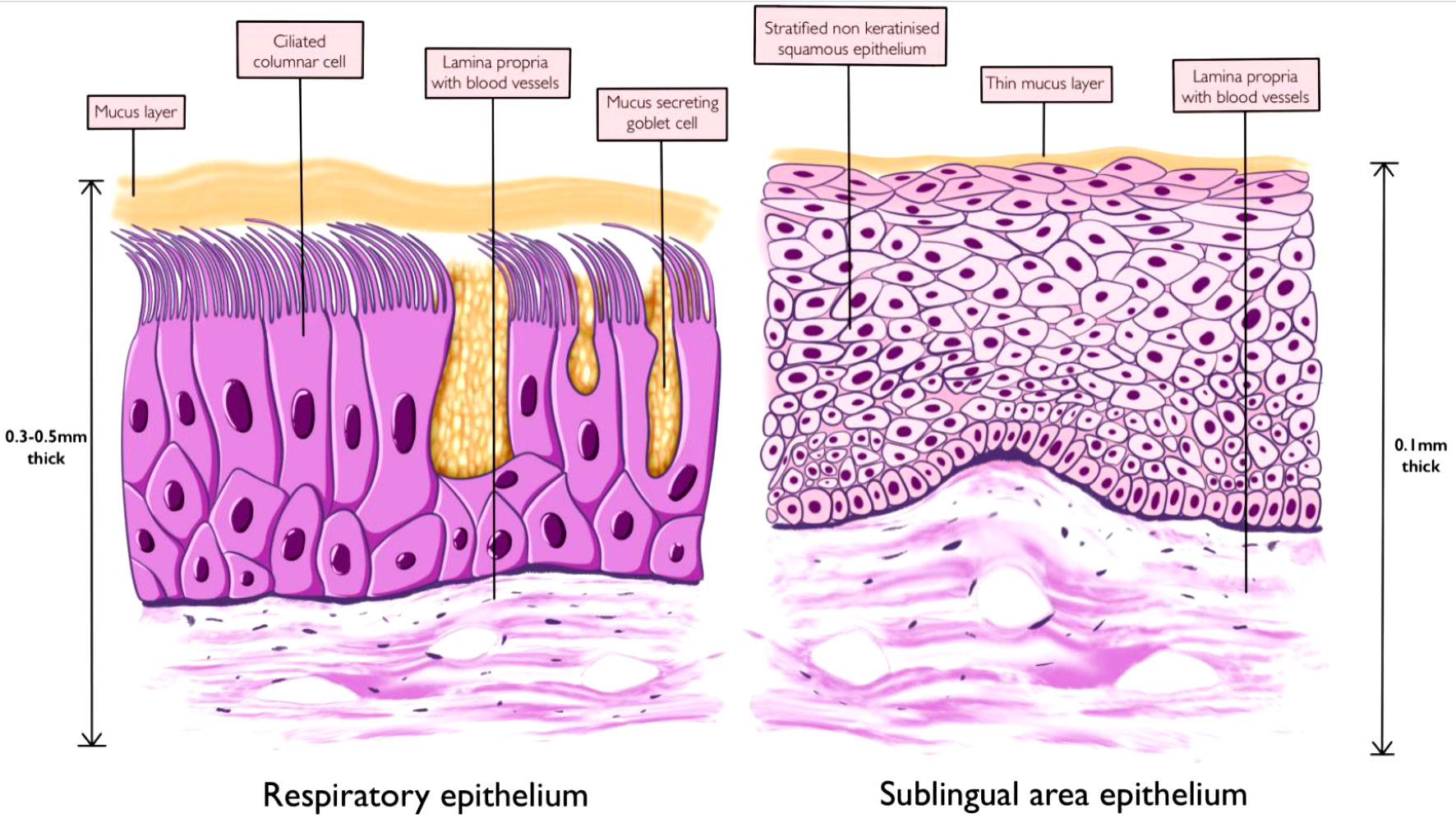 Click for large image | Figure 2. Nasal respiratory epithelium and sublingual area epithelium. |
Sublingual route
The sublingual region on the floor of the oral cavity under the tongue provides an alternative route of medication administration. The direct absorption of medications into the systemic circulation via this route bypasses the first-pass hepatic metabolism effect and is suitable for medications with a high first-pass metabolism or degradation in the gastrointestinal tract. The sublingual region covers a surface area of about 26 cm2, making up about 11-30% of the surface area of the oral cavity [30, 31]. The stratified squamous non-keratinized epithelium of this region is made up of eight to 12 layers of cells and covered by a thin layer of mucus derived from saliva (Fig. 2) [30]. The underlying lamina propria and submucosa are rich in lymphatics and blood vessels derived from branches of the lingual artery and the accompanying veins [32]. Compared to the buccal mucosa and other regions of the oral cavity, the sublingual region has the thinnest mucosa in the oral cavity with a thickness of 0.1 - 0.15 mm [33]. The presence of a very thin mucosa and the sophisticated vascular supply makes it a fascinating route of drug administration. In addition to these parameters, other factors that could influence the rate of drug absorption across the mucosa include the rate of flow of saliva, oral pH, and the duration of medication contact with the oral mucosa [33].
| Administration of Intranasal and Sublingual Medications | ▴Top |
Intranasal
The intranasal route of administration offers an option for medication delivery that is capable of achieving good bioavailability comparable to that seen following intravenous or intramuscular administration [34]. It is particularly suitable for medications that have a high first-pass metabolism in the liver or those that are poorly absorbed in the gastrointestinal tract [28]. For absorption to occur, medications must penetrate the nasal mucus layer and epithelial cells. This is dependent on nasal mucociliary clearance, a natural protective mechanism that transports surface mucus from the nasal cavity towards the nasopharynx through a coordinated beating of the ciliary apparatus [35].
The central principle of optimal administration of intranasal medications is the delivery of a highly concentrated small volume of medication to the nasal cavity while limiting medication runoff into the pharynx [36]. This reduces the volume of medications to be administered and reduce the frequency of administration per dosing. The optimal volume of medication administered per nostril should be less than 1 mL [28]. This is maximized through the use of both nostrils per administration. Options for intranasal medication delivery include topical application, sniffing, application as drops, and the use of atomization devices (the preferred and most efficient) [37]. Mucosa atomization devices (MADs) aerosolize medications, creating a fine mist that is rapidly absorbed into the nasal mucosa (Fig. 3) [38]. Medication inhalation into the lungs or blowing the nostril after intranasal medication administration should be avoided to improve medication absorption [39].
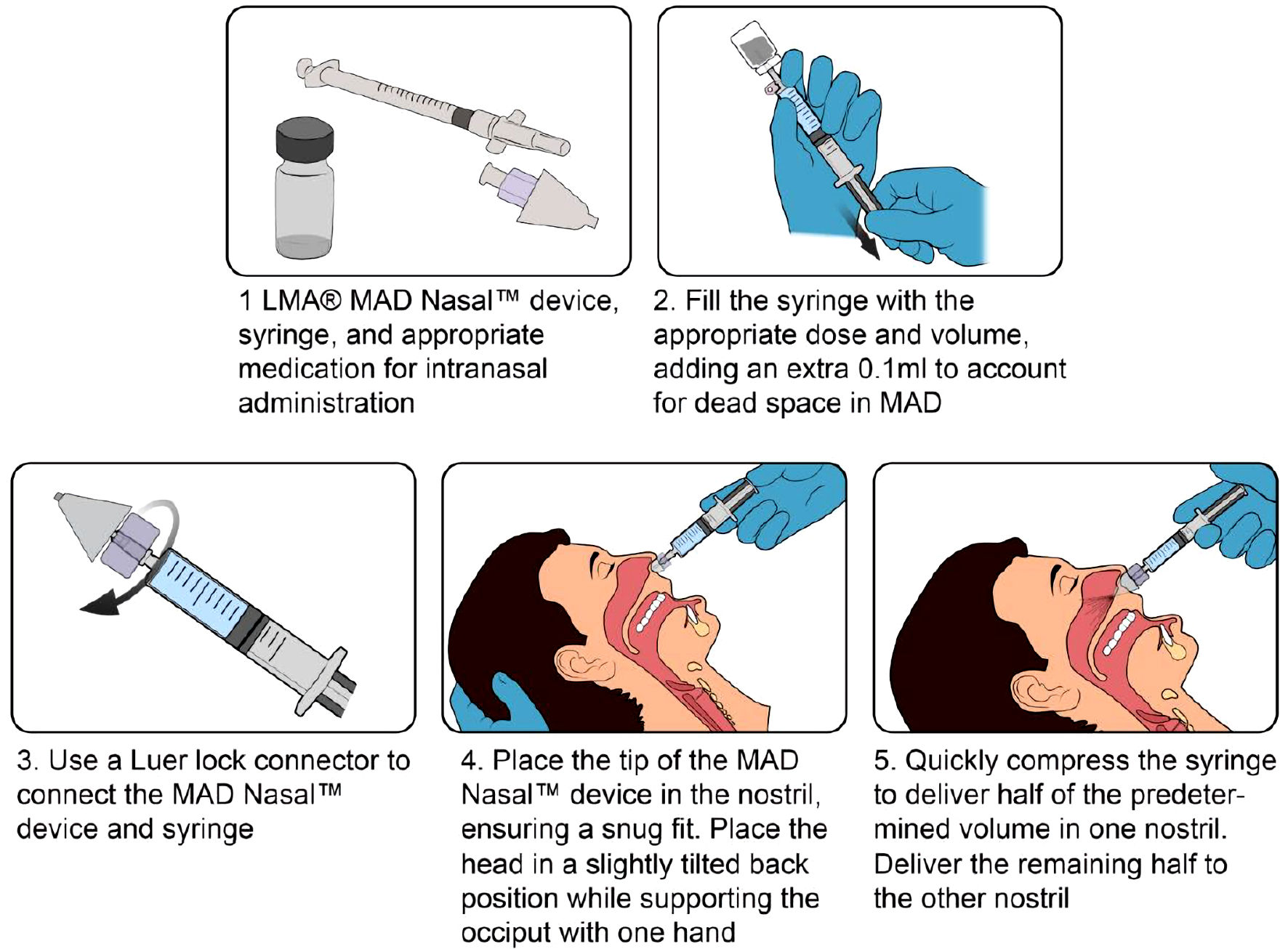 Click for large image | Figure 3. Technique for intranasal medication administration using an LMA® mucosa atomization device. |
Not all patients are candidates for intranasal medication application. Individuals with active rhinitis have increased mucosa mucus secretion, which reduces the rate of medication absorption. Nasal obstruction from tumors, polyps, and chronic sinusitis with enlarged turbinate creates a barrier to medication delivery [40]. Other relative contraindications to intranasal medication delivery include facial injuries, ciliary dysfunction syndromes, nasal bleeds, recent nasal vasoconstrictor use, and mucosa erosion [28]. Generally, medications should be avoided in those with a history of allergy to those medications. The adverse event profile of intranasally administered medications includes mucosa irritation and other systemic effects associated with a specific medication. There are limited data on possible interactions between medications if administered concurrently via the intranasal route. However, this potential limitation should be considered when administering intranasal medications.
Sublingual
Whether tablets, films, or sprays, sublingual medications are placed directly under the tongue and are easily self-administered. Patients should be kept in an upright position while placing sublingual medications to reduce the risk of aspiration [32]. The advantages of this route include easy accessibility, rapid absorption, and onset of action for selected preparations, and the effect of medications could be easily terminated by spitting out the medication [32]. However, it is not the optimal choice for administering large doses and sustained-release medications due to possible interference with eating or drinking. In addition, an unpleasant taste or mucosa irritation may create barriers to compliance with use.
| Intranasally Administered Analgesic Agents | ▴Top |
Although acetaminophen, non-steroidal anti-inflammatory drugs (NSAIDs), and opioids are the most frequently used classes of analgesics in clinical practice, there is a limited number of medications that are currently approved for intranasal use.
Intranasal fentanyl
Fentanyl is a short-acting synthetic opioid that has found use in both prehospital and hospital settings due to its ability to provide fast relief of acute pain and the relative ease of medication titration [28]. It is potent (50 to 100 times more potent than morphine), highly lipophilic, and rapidly absorbed through the nasal mucosa [41]. In addition, in contrast to other routes of administration in which medications must cross the blood-brain barrier (BBB) before penetrating the CNS, intranasal fentanyl can penetrate the CNS without crossing the BBB through direct access via the olfactory nerve and trigeminal nerves [42]. The onset of action is within 2 to 10 min, reaching a peak plasma concentration within 12 to 21 min [41, 43-45]. The duration of effect is at least 60 min, although this is dose-dependent, with one study reporting a return to pre-analgesic pain score after 120 min following 75 µg administration and 240 min following administration of 200 µg [41, 43]. This profile makes intranasal fentanyl an excellent option for the initial management of acute severe pain in various settings and has demonstrated effectiveness in the management of post-procedure pain, pain associated with wound care, breakthrough pain in cancer patients, and non-operative acute pain in the ED [43].
Few studies have evaluated the benefit of intranasal fentanyl in the ED management of VOC pain. Myrick et al reported a significant shortening of time to initiation of analgesia for children with acute sickle cell pain (43 mins for intranasal fentanyl vs. 75 min for intravenous opioids) after intranasal fentanyl was added to the ED pain order set [46]. Paquin et al reported a 41-min reduction in analgesic administration time when intranasal fentanyl was used in the ED [47]. Although there is limited evidence on the comparative efficacy of intranasal fentanyl over intravenous morphine in acute sickle cell VOC pain, studies comparing intranasal fentanyl to intravenous morphine in other types of acute pain show similar effectiveness in providing rapid analgesia [48, 49].
Dosage
Intranasal fentanyl has a good bioavailability (55-89%), as such, the dosage often used is similar to that used for intravenous administration [43, 45]. For adults, an initial dose of 0.5 - 2 µg/kg is recommended (maximum dose of 100 µg/dose) while the initial recommended dosage in children is 1 - 1.5 µg/kg (maximum dose of 100 µg/dose) [28, 50, 51].
Intranasal hydromorphone
Often used in its intravenous form, hydromorphone is one of the most commonly used opioids in the ED. It is eight times more potent than morphine and provides a rapid onset of analgesia for individuals with moderate to severe pain. Its lipophilicity and low-molecular weight make hydromorphone suitable for intranasal absorption [52]. Following intranasal administration, the onset of action is rapid (about 5 min), achieving a peak plasma concentration within 20 to 30 min and producing an analgesic effect lasting 90 to 180 min [53-55]. It has a bioavailability of 50-60% [53-56]. Two studies evaluated the use of intranasal hydromorphone in the management of acute pain in the ED [55, 56]. Tsze et al evaluated intranasal hydromorphone in 35 children who had moderate to severe pain [56]. They were made up of three groups of 15, 11, and nine children who received a total dose of 0.03, 0.045, and 0.06 mg/kg, respectively. All participants experience a > 40% reduction in the pain score within 5 - 15 min of medication administration and the duration of analgesia lasted more than 1 h in 85.7% of the patients. Wermeling et al studied the effect of 2, 4, 6, and 8 mg single-dose intranasal hydromorphone on 113 patients with acute trauma-related pain divided into four groups: 19 (2 mg), 33 (4 mg), 28 (6 mg), and 19 (8 mg) [55]. All patients in the 4, 6, and 8 mg groups experienced a 39-44% reduction in pain score within 30 min while the 2 mg group experienced a 24% reduction in pain score.
Dosage
An initial dose of 4 - 8 mg should be considered in adults with moderate to severe pain [53-55]. Lower doses may be used in elderly patients and those at a high risk of respiratory depression [28]. Given the limited available evidence on its use, intranasal hydromorphone should be used with caution in children. If indicated, a dose of 0.03 - 0.06 mg/kg may be an appropriate initial dose [56].
Intranasal ketorolac
Similar to other NSAIDs, ketorolac is a cyclo-oxygenase (COX) enzyme inhibitor, inhibiting both COX-1 and COX-2 [57]. It has a higher affinity for COX-1 compared to other NSAIDs and possesses a potent analgesic effect [57]. When compared to morphine, a 30 mg single dose of intramuscular ketorolac was shown to provide a similar analgesic effect to 6 - 12 mg of intramuscular morphine [58]. The Food and Drug Administration (FDA) approved intranasal ketorolac tromethamine for short-term (up to 5 days) management of moderate to severe pain that requires an opioid level of analgesia in May 2010. Since then, many studies have assessed the effectiveness in the management of acute pain in the ED, intraoperative analgesia, postoperative pain management, migraine, and severe renal colic [59-62]. Gaul et al assess the use of intranasal ketorolac in 28 adults presenting to the ED with acute pain and reported a median decrease of 5 points in pain score in a median time of 5 min after administration with minimal adverse events [59]. Turner et al reported good pain control among patients with moderate to severe endodontic pain who received intranasal ketorolac for endodontic procedures [60]. Similarly, Rao et al compared intranasal ketorolac to sumatriptan and placebo for the treatment of acute migraine and reported superiority of ketorolac over placebo, and a non-inferiority to sumatriptan for aborting acute migraine episodes [61]. Nasal burning and an altered sense of taste are the adverse events reported in that study.
Following intranasal administration, the onset of action is rapid (5 - 20 min), achieving a peak plasma concentration within 30 - 52 min and producing an analgesic effect lasting about 180 min [57, 59, 63-65]. Intranasal ketorolac has a good bioavailability (67-75%), although inferior to that achievable via the intramuscular route (up to 100%) [66].
Dosage
Intranasal ketorolac is available in the USA as nasal spray bottles, with each 1.7 g bottle made up of eight sprays (one spray is equivalent to 15.75 mg in a 100 µL solution) [67]. For adults < 65 years, the dose is 31.5 mg (one 15.75-mg spray in each nostril) every 6 - 8 h (maximum dose of 63 mg/day) [67]. For adults who are ≥ 65 years of age, have renal impairment, or are less than 50 kg, the dose is 15.75 mg (one 15.75-mg spray in one nostril) every 6 - 8 h. Intranasal ketorolac is currently not approved for use in children.
Table 1 summarized the profile of analgesics administered via the intranasal route [28, 41, 43-45, 50, 51, 53-57, 59, 63-67].
 Click to view | Table 1. Profile of Analgesics Administered via the Intranasal Route |
| Sublingually Administered Analgesic Agents | ▴Top |
Drug absorption through the sublingual route depends on the properties of the drug and the pH of saliva (normal pH: 6.5), although this is highly variable depending on the type of food and drinks one consumes [68]. Transmucosal absorption occurs via transcellular (the main mechanism) and intercellular mechanisms which in turn depend on the lipophilicity (transcellular absorption) and hydrophilicity of the drug (intercellular absorption) [69]. These factors as well as the potency of a medication determine its usefulness as a sublingual analgesic agent. Potent and highly lipophilic agents such as sufentanil, fentanyl, and buprenorphine are more suitable for sublingual use compared to the less potent and strongly hydrophilic agents such as morphine, oxycodone, and hydromorphone [70].
Sublingual sufentanil
Sufentanil is a potent opioid approved for use in its injectable and sublingual form for the treatment of acute severe pain [71]. The injectable form is used mainly as an anesthetic agent either as a primary anesthetic agent or as a component of balanced general or epidural anesthesia. This is due to the high lipophilicity and fast absorption leading to rapid attainment of maximum plasma concentration with an attending development of respiratory depression and apnea in non-intubated patients [70]. As a result, the injectable form is limited to anesthetic use in the operating room. Administration of 30 µg of sufentanil intravenously in a study resulted in a mean peak plasma concentration of > 1,000 pg/mL, a concentration likely to induce apnea while the same dose administered sublingually yielded a peak plasma concentration of 63 µg/mL [72]. In addition, the time to reach the peak plasma concentration was higher following sublingual administration in the study. This slower absorption and lower bioavailability (47-57%) make sublingual sufentanil a desirable analgesic agent with a lower risk of apnea compared to the intravenous form [73]. The onset of analgesia after a single dose of 30 µg sublingual sufentanil is 15 - 30 min, reaching a peak effect at about 60 min and lasting about 180 min [72, 73]. Most clinical trials on sublingual sufentanil have focused on its use for postoperative analgesia following abdominal, thoracic, gynecological procedures and as analgesia for acute trauma in the ED [74-77]. Given its effectiveness in providing a rapid analgesic effect in these settings, SCD patients with acute VOC pain may benefit from its use in the ED.
Dosage
Two forms of sublingual sufentanil nanotablets are currently available for use: a 30-µg tablet to be administered by healthcare personnel and a 15-µg tablet to be used as part of patient-controlled analgesia [73]. The minimum dosing interval for the 30-µg tablet is 1 h while the 15-µg tablet should be administered at a minimum interval of 20 min for no more than 72 h [73]. Sublingual sufentanil is not recommended for use in children.
Sublingual fentanyl
Whether as tablets or sprays, the use of sublingual fentanyl has been well described in the management of breakthrough pain in cancer patients; individuals who are likely opioid tolerant [78-80]. The pharmacokinetic profile of sublingual fentanyl is similar to that of intravenous fentanyl: a rapid rise in plasma concentration and a relatively shorter elimination half-life [81]. The implication of this is that frequent dosing is required for sustained analgesic effect, which in turn creates a variable and increasing plasma concentration [70]. This is usually well tolerated in opioid-tolerant individuals such as cancer patients but could be problematic in opioid naive individuals as it increases their risk of developing respiratory depression and apnea. Similar to cancer patients, SCD patients are likely opioid exposed and tolerant, making them ideal candidates to benefit from the use of sublingual fentanyl in the ED.
The onset of action depends on the dosage form (tablet or spray) with an onset of action of 5 min and time to peak plasma concentration of 40 - 57 min following administration of spray while sublingual tablets have an onset of action at 15 min, reaching a peak concentration in 40 - 75 min [82-84]. The bioavailability of sublingual fentanyl is good (70-76%) with an analgesic effect that lasts at least 60 min [82-84]. The adverse effect profile is similar to other opioids, including nausea, vomiting, and respiratory depression [81].
Dosage
Whether the tablet or spray, the initial recommended dose is 100 µg [85]. An additional 100 µg could be given within 30 - 60 min if the pain is uncontrolled with the first dose [85]. Similar to other sublingual opioids, sublingual fentanyl is not recommended for use in children.
Sublingual buprenorphine
Buprenorphine is a non-selective opioid receptor modulator that acts as a partial µ-receptor agonist and κ-receptor antagonist. It has 25 to 40 times the potency of morphine and has been used as opioid substitution therapy for opioid addiction, as well as an analgesic for cancer-related pain, non-cancer-related chronic pain, postoperative pain, and acute trauma pain [86, 87]. Sublingual buprenorphine is highly lipophilic and well absorbed in the sublingual mucosa with a bioavailability of about 50% (12-94%), although there is a wide inter-patient variability but less intra-individual variability in absorption [88, 89]. In addition, sublingual buprenorphine has a near linear dose-serum concentration relationship from 1 to 32 mg, reaching a peak plasma concentration in 0.5 - 6 h with a mean elimination half-life of 83 h [88, 89]. The onset of action is relatively slow (30 - 60 min), reaching a peak effect at 1 - 4 h, with a duration of effect lasting for 6 - 12 h (for a dose < 4 mg) and 24 - 72 h (for a dose > 16 mg) [90]. While the combination of high potency, good sublingual absorption, and long duration of effect make sublingual buprenorphine an interesting analgesic agent, there are potential limitations to its use in the management of acute VOC pain crises: 1) The agonist effect of buprenorphine has a ceiling effect, with a point at which escalating doses produces no further analgesic effect; 2) Due to its partial agonism on µ-receptors, buprenorphine has a potential to precipitate withdrawal symptoms in individuals who are dependent on pure µ-receptor agonist (10% of SCD patients have physical opioid dependence, and the majority are routinely on opioids for chronic pain control); 3) The wide interpatient variability in bioavailability complicates dosing in an acute setting [68].
Dosage
Although a starting dose of 2 mg of sublingual buprenorphine has been safely used for analgesia in clinical trials, the current use of buprenorphine is largely limited to the treatment of opioid addiction due to its suitability for that purpose, and the availability of alternative opioid analgesic agents that do not have the aforementioned limitations [86, 91].
Sublingual oxycodone
Oxycodone is the gold standard oral opioid medication due to its excellent oral bioavailability (60-80%) and less adverse event profile (less nausea, pruritus, and hallucinations) compared to morphine [92]. However, it is highly hydrophilic and has poor bioavailability following sublingual administration (< 20%), although this depends on salivary pH [93]. Up to 70% bioavailability could be achieved with saliva alkalinization [70]. To achieve a therapeutic concentration of oxycodone via the small-spaced sublingual route would therefore require the administration of a large dose. This profile limits the use of sublingual oxycodone in the emergency management of acute VOC pain.
Profile of analgesics administered via the sublingual route is summarized in Table 2 [72-77, 81-86, 88-90, 91-93].
 Click to view | Table 2. Profile of Analgesics Administered via the Sublingual Route |
| Improving the ED-Based Management of Vaso-Occlusive Pain Crisis | ▴Top |
While ongoing research efforts continue to evaluate interventions aimed at reducing the frequency and severity of SCD crisis, a renewed focus should be directed at ensuring SCD patients receive optimal care in the ED. Receiving prompt analgesia is a vital component of optimal VOC care in the ED. It is worth noting that patients with VOC typically present to the ED on the background of failure to achieve symptomatic relief from their home pain regimen (usually oral opioids and NSAIDs). The intranasal and sublingual routes provide a rapid means to achieve prompt delivery of analgesia on ED arrival. Besides intranasal and sublingual routes, attempts at achieving rapid, easy-to-administer analgesic agents have led to the use of the inhalational route of drug administration, leveraging on the extensive alveoli surface area for absorption of medications. Inhaled methoxyflurane, an anesthetic agent has been used in small sub-anesthetic doses for acute pain management [94]. Although the FDA withdrew methoxyflurane from the market in 2005 due to concern for nephrotoxicity, low-dose methoxyflurane administered via a hand-held device (Penthrox®) is still being used in parts of Europe, Australia, and New Zealand [95]. There are no clinical trials on the use of methoxyflurane for VOC pain. Similarly, nitrous oxide with or without an equimolar mixture of oxygen has been evaluated for the management of VOC pain in clinical trials [96]. However, its use is limited due to concern for an increased risk of nitrous oxide-induced neuropathy and hyperhomocysteinemia [97]. In addition, there is insufficient evidence supporting the clinical benefit of nitrous oxide in the management of VOC. Additional studies with a focus on the SCD population are required to clarify the optimal approach to analgesia for SCD patients presenting to the ED.
Apart from the challenges of achieving prompt analgesia delivery, many other aspects of the emergency care system present a formidable barrier to optimal sickle cell care. SCD patients often experience discrimination, bias, and prejudice in the ED and have been shown to experience longer wait times before the initiation of interventions in the ED compared to the general population [98]. In addition, although the prevalence of opioid addiction in SCD is about 5% (similar to the general population), SCD patients are sometimes labeled as “pain seekers”, or “opioid addicts”, which negatively impacts the patient-provider relationship [99]. Interventions to improve the ED management of sickle cell crisis should therefore focus on educating providers on discrimination and bias, adopting strategies to reduce the time to first dose of analgesia, and adopting individualized care for SCD patients beyond the first dose of analgesia.
| Conclusions | ▴Top |
SCD patients often experience long wait times in the ED before receiving their first dose of analgesia. This delay results from the complexities of the emergency care system and is often complicated by negative provider attitudes toward SCD patients. The use of an intranasal or sublingual approach to administering analgesia to SCD patients with VOC may offer a fast, safe, noninvasive, atraumatic, and easily accessible route of administration which has the potential to reduce the time-to-first dose of analgesia. Interventions to improve the ED management of sickle cell crisis should therefore focus on adopting strategies to reduce the time to first dose of analgesia, including the use of sublingual and intranasal routes of analgesia administration as well as educating providers towards reducing discrimination and bias.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Author Contributions
AO conceived and designed the study. AO and OO collected and interpreted all relevant data. AO, OO, and OGO prepared the manuscript. All authors read and approved the final manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Sedrak A, Kondamudi NP. Sickle Cell Disease. In: StatPearls. Treasure Island (FL); 2022.
- Aygun B, Odame I. A global perspective on sickle cell disease. Pediatr Blood Cancer. 2012;59(2):386-390.
doi pubmed - National Center for Biotechnology Information (US). Genes and Disease [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 1998. Anemia, sickle cell. Available from: https://www.ncbi.nlm.nih.gov/books/NBK22238/.
- Ware RE, de Montalembert M, Tshilolo L, Abboud MR. Sickle cell disease. Lancet. 2017;390(10091):311-323.
doi pubmed - Hargrave DR, Wade A, Evans JP, Hewes DK, Kirkham FJ. Nocturnal oxygen saturation and painful sickle cell crises in children. Blood. 2003;101(3):846-848.
doi pubmed - Zakaria OM, Buhalim RA, Al Jabr FA, AlSaeed MN, Al-Hajji IA, Al Saleh YA, Buhalim MA, et al. Reasons for hospitalization of sickle cell disease patients in the eastern province of Saudi Arabia: a single-center study. Cureus. 2021;13(11):e19299.
doi - Fingar KR, Owens PL, Reid LD, Mistry KB, Barrett ML. Characteristics of Inpatient Hospital Stays Involving Sickle Cell Disease, 2000-2016. In: Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD); 2006.
- Osunkwo I, O'Connor HF, Saah E. Optimizing the management of chronic pain in sickle cell disease. Hematology Am Soc Hematol Educ Program. 2020;2020(1):562-569.
doi pubmed - Yawn BP, Buchanan GR, Afenyi-Annan AN, Ballas SK, Hassell KL, James AH, Jordan L, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312(10):1033-1048.
doi pubmed - Shokoohi H, Loesche MA, Duggan NM, Liteplo AS, Huang C, Al Saud AA, McEvoy D, et al. Difficult intravenous access as an independent predictor of delayed care and prolonged length of stay in the emergency department. J Am Coll Emerg Physicians Open. 2020;1(6):1660-1668.
doi pubmed - Corrigan M, Wilson SS, Hampton J. Safety and efficacy of intranasally administered medications in the emergency department and prehospital settings. Am J Health Syst Pharm. 2015;72(18):1544-1554.
doi pubmed - Ballas SK, Gupta K, Adams-Graves P. Sickle cell pain: a critical reappraisal. Blood. 2012;120(18):3647-3656.
doi pubmed - Kato GJ, Piel FB, Reid CD, Gaston MH, Ohene-Frempong K, Krishnamurti L, Smith WR, et al. Sickle cell disease. Nat Rev Dis Primers. 2018;4:18010.
doi pubmed - Darbari DS, Sheehan VA, Ballas SK. The vaso-occlusive pain crisis in sickle cell disease: Definition, pathophysiology, and management. Eur J Haematol. 2020;105(3):237-246.
doi pubmed - Aich A, Jones MK, Gupta K. Pain and sickle cell disease. Curr Opin Hematol. 2019;26(3):131-138.
doi pubmed - Nader E, Romana M, Connes P. The red blood cell-inflammation vicious circle in sickle cell disease. Front Immunol. 2020;11:454.
doi pubmed - Hebbel RP. Adhesive interactions of sickle erythrocytes with endothelium. J Clin Invest. 1997;100(11 Suppl):S83-S86.
- Wick TM, Eckman JR. Molecular basis of sickle cell-endothelial cell interactions. Curr Opin Hematol. 1996;3(2):118-124.
doi pubmed - Teijaro JR, Walsh KB, Cahalan S, Fremgen DM, Roberts E, Scott F, Martinborough E, et al. Endothelial cells are central orchestrators of cytokine amplification during influenza virus infection. Cell. 2011;146(6):980-991.
doi pubmed - Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. 2007;45(2):27-37.
doi pubmed - Gupta K, Harvima IT. Mast cell-neural interactions contribute to pain and itch. Immunol Rev. 2018;282(1):168-187.
doi pubmed - Uhelski ML, Gupta K, Simone DA. Sensitization of C-fiber nociceptors in mice with sickle cell disease is decreased by local inhibition of anandamide hydrolysis. Pain. 2017;158(9):1711-1722.
doi pubmed - Sobiesk JL, Munakomi S: Anatomy, Head and Neck, Nasal Cavity. In: StatPearls. Treasure Island (FL), 2022.
- Patel RG. Nasal Anatomy and Function. Facial Plast Surg. 2017;33(1):3-8.
doi pubmed - Beule AG. Physiology and pathophysiology of respiratory mucosa of the nose and the paranasal sinuses. GMS Curr Top Otorhinolaryngol Head Neck Surg. 2010;9:Doc07.
- Davidovich NE, Kloog Y, Wolf M, Elad D. Mechanophysical stimulations of mucin secretion in cultures of nasal epithelial cells. Biophys J. 2011;100(12):2855-2864.
doi pubmed - Chen CR, Kachramanoglou C, Li D, Andrews P, Choi D. Anatomy and cellular constituents of the human olfactory mucosa: a review. J Neurol Surg B Skull Base. 2014;75(5):293-300.
doi pubmed - Bailey AM, Baum RA, Horn K, Lewis T, Morizio K, Schultz A, Weant K, et al. Review of Intranasally Administered Medications for Use in the Emergency Department. J Emerg Med. 2017;53(1):38-48.
doi pubmed - Furubayashi T, Inoue D, Kimura S, Tanaka A, Sakane T. Evaluation of the Pharmacokinetics of Intranasal Drug Delivery for Targeting Cervical Lymph Nodes in Rats. Pharmaceutics. 2021;13(9):1363.
doi pubmed - Kraan H, Vrieling H, Czerkinsky C, Jiskoot W, Kersten G, Amorij JP. Buccal and sublingual vaccine delivery. J Control Release. 2014;190:580-592.
doi pubmed - Czerkinsky C, Holmgren J. Mucosal delivery routes for optimal immunization: targeting immunity to the right tissues. Curr Top Microbiol Immunol. 2012;354:1-18.
doi pubmed - Hua S. Advances in Nanoparticulate Drug Delivery Approaches for Sublingual and Buccal Administration. Front Pharmacol. 2019;10:1328.
doi pubmed - Prestin S, Rothschild SI, Betz CS, Kraft M. Measurement of epithelial thickness within the oral cavity using optical coherence tomography. Head Neck. 2012;34(12):1777-1781.
doi pubmed - Agarwal SK, Kriel RL, Brundage RC, Ivaturi VD, Cloyd JC. A pilot study assessing the bioavailability and pharmacokinetics of diazepam after intranasal and intravenous administration in healthy volunteers. Epilepsy Res. 2013;105(3):362-367.
doi pubmed - Merkus FW, Verhoef JC, Schipper NG, Marttin E. Nasal mucociliary clearance as a factor in nasal drug delivery. Adv Drug Deliv Rev. 1998;29(1-2):13-38.
doi pubmed - Wolfe TR, Braude DA. Intranasal medication delivery for children: a brief review and update. Pediatrics. 2010;126(3):532-537.
doi pubmed - Djupesland PG. Nasal drug delivery devices: characteristics and performance in a clinical perspective-a review. Drug Deliv Transl Res. 2013;3(1):42-62.
doi pubmed - Using the LMA® MAD Nasal™ Intranasal Mucosal Atomization Device. 2013. https://www.activeforever.com/pdf/mad-nasal-usage-guide.pdf.
- Davis GA, Rudy A, Archer SM, Wermeling DP. Bioavailability of intranasal butorphanol administered from a single-dose sprayer. Am J Health Syst Pharm. 2005;62(1):48-53.
doi pubmed - Kim J, De Jesus O. Medication Routes of Administration. In: StatPearls. Treasure Island (FL), 2022.
- Lotsch J, Walter C, Parnham MJ, Oertel BG, Geisslinger G. Pharmacokinetics of non-intravenous formulations of fentanyl. Clin Pharmacokinet. 2013;52(1):23-36.
doi pubmed - Dhuria SV, Hanson LR, Frey WH, 2nd. Intranasal delivery to the central nervous system: mechanisms and experimental considerations. J Pharm Sci. 2010;99(4):1654-1673.
doi pubmed - Foster D, Upton R, Christrup L, Popper L. Pharmacokinetics and pharmacodynamics of intranasal versus intravenous fentanyl in patients with pain after oral surgery. Ann Pharmacother. 2008;42(10):1380-1387.
doi pubmed - Fisher A, Watling M, Smith A, Knight A. Pharmacokinetic comparisons of three nasal fentanyl formulations; pectin, chitosan and chitosan-poloxamer 188. Int J Clin Pharmacol Ther. 2010;48(2):138-145.
doi pubmed - Paech MJ, Lim CB, Banks SL, Rucklidge MW, Doherty DA. A new formulation of nasal fentanyl spray for postoperative analgesia: a pilot study. Anaesthesia. 2003;58(8):740-744.
doi pubmed - Myrick R, Blakemore S, Waite E, Pernell B, Madan-Swain A, Hilliard L, Lebensburger J. Outpatient pain clinic and intranasal fentanyl to improve sickle cell disease outcomes. Pediatr Blood Cancer. 2020;67(10):e28648.
doi pubmed - Paquin H, Trottier ED, Pastore Y, Robitaille N, Dore Bergeron MJ, Bailey B. Evaluation of a clinical protocol using intranasal fentanyl for treatment of vaso-occlusive crisis in sickle cell patients in the emergency department. Paediatr Child Health. 2020;25(5):293-299.
doi pubmed - Borland M, Jacobs I, King B, O'Brien D. A randomized controlled trial comparing intranasal fentanyl to intravenous morphine for managing acute pain in children in the emergency department. Ann Emerg Med. 2007;49(3):335-340.
doi pubmed - Ali S, Klassen TP. Intranasal fentanyl and intravenous morphine did not differ for pain relief in children with closed long-bone fractures. Evid Based Med. 2007;12(6):176.
doi pubmed - Cole J, Shepherd M, Young P. Intranasal fentanyl in 1-3-year-olds: a prospective study of the effectiveness of intranasal fentanyl as acute analgesia. Emerg Med Australas. 2009;21(5):395-400.
doi pubmed - Borland ML, Bergesio R, Pascoe EM, Turner S, Woodger S. Intranasal fentanyl is an equivalent analgesic to oral morphine in paediatric burns patients for dressing changes: a randomised double blind crossover study. Burns. 2005;31(7):831-837.
doi pubmed - National Center for Biotechnology Information. PubChem Compound Summary for CID 5284570. Hydromorphone. https://pubchem.ncbi.nlm.nih.gov/compound/Hydromorphone. Accessed Aug. 4, 2022.
- Coda BA, Rudy AC, Archer SM, Wermeling DP. Pharmacokinetics and bioavailability of single-dose intranasal hydromorphone hydrochloride in healthy volunteers. Anesth Analg. 2003;97(1):117-123.
doi pubmed - Rudy AC, Coda BA, Archer SM, Wermeling DP. A multiple-dose phase I study of intranasal hydromorphone hydrochloride in healthy volunteers. Anesth Analg. 2004;99(5):1379-1386.
doi pubmed - Wermeling DP, Clinch T, Rudy AC, Dreitlein D, Suner S, Lacouture PG. A multicenter, open-label, exploratory dose-ranging trial of intranasal hydromorphone for managing acute pain from traumatic injury. J Pain. 2010;11(1):24-31.
doi pubmed - Tsze DS, Pan SS, DePeter KC, Wagh AM, Gordon SL, Dayan PS. Intranasal hydromorphone for treatment of acute pain in children: A pilot study. Am J Emerg Med. 2019;37(6):1128-1132.
doi pubmed - He A, Hersh EV. A review of intranasal ketorolac tromethamine for the short-term management of moderate to moderately severe pain that requires analgesia at the opioid level. Curr Med Res Opin. 2012;28(12):1873-1880.
doi pubmed - Brown CR, Mazzulla JP, Mok MS, Nussdorf RT, Rubin PD, Schwesinger WH. Comparison of repeat doses of intramuscular ketorolac tromethamine and morphine sulfate for analgesia after major surgery. Pharmacotherapy. 1990;10(6 Pt 2)):45S-50S.
- Gaul E, Barbour T, Nowacki AS, Mace SE. Intranasal Ketorolac for Acute Pain in Adult Emergency Department Patients. West J Nurs Res. 2022;44(11):1047-1056.
doi pubmed - Turner CL, Eggleston GW, Lunos S, Johnson N, Wiedmann TS, Bowles WR. Sniffing out endodontic pain: use of an intranasal analgesic in a randomized clinical trial. J Endod. 2011;37(4):439-444.
doi pubmed - Rao AS, Gelaye B, Kurth T, Dash PD, Nitchie H, Peterlin BL. A Randomized Trial of Ketorolac vs. Sumatripan vs. Placebo Nasal Spray (KSPN) for Acute Migraine. Headache. 2016;56(2):331-340.
doi pubmed - Nazemian N, Torabi M, Mirzaee M. Atomized intranasal vs intravenous fentanyl in severe renal colic pain management: A randomized single-blinded clinical trial. Am J Emerg Med. 2020;38(8):1635-1640.
doi pubmed - Singla N, Singla S, Minkowitz HS, Moodie J, Brown C. Intranasal ketorolac for acute postoperative pain. Curr Med Res Opin. 2010;26(8):1915-1923.
doi pubmed - Drover DR, Hammer GB, Anderson BJ. The pharmacokinetics of ketorolac after single postoperative intranasal administration in adolescent patients. Anesth Analg. 2012;114(6):1270-1276.
doi pubmed - Brown C, Moodie J, Bisley E, Bynum L. Intranasal ketorolac for postoperative pain: a phase 3, double-blind, randomized study. Pain Med. 2009;10(6):1106-1114.
doi pubmed - McAleer SD, Majid O, Venables E, Polack T, Sheikh MS. Pharmacokinetics and safety of ketorolac following single intranasal and intramuscular administration in healthy volunteers. J Clin Pharmacol. 2007;47(1):13-18.
doi pubmed - Human prescription drug label: SPRIX-ketorolac tromethamine spray, metered. NIH National Library of Medicine DailyMed. Updated October 29, 2012. Available at: https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=09a80ee7-274a-4f96-8b42-480436896050.
- Reisfield GM, Wilson GR. Rational use of sublingual opioids in palliative medicine. J Palliat Med. 2007;10(2):465-475.
doi pubmed - Corbo DC, Liu JC, Chien YW. Drug absorption through mucosal membranes: effect of mucosal route and penetrant hydrophilicity. Pharm Res. 1989;6(10):848-852.
doi pubmed - Miner JR. Sublingual analgesia: a promising proposal for the treatment of pain. Expert Opin Drug Deliv. 2020;17(2):123-126.
doi pubmed - Miner JR, Melson TI, Leiman D, Minkowitz HS, Chiang YK, DiDonato KP, Palmer PP. Pooled Phase III safety analysis of sufentanil sublingual tablets for short-term treatment of moderate-to-severe acute pain. Pain Manag. 2019;9(3):259-271.
doi pubmed - Fisher DM, Chang P, Wada DR, Dahan A, Palmer PP. Pharmacokinetic Properties of a Sufentanil Sublingual Tablet Intended to Treat Acute Pain. Anesthesiology. 2018;128(5):943-952.
doi pubmed - Porela-Tiihonen S, Kokki H, Kokki M. An up-to-date overview of sublingual sufentanil for the treatment of moderate to severe pain. Expert Opin Pharmacother. 2020;21(12):1407-1418.
doi pubmed - Minkowitz HS, Leiman D, Melson T, Singla N, DiDonato KP, Palmer PP. Sufentanil Sublingual Tablet 30 mcg for the Management of Pain Following Abdominal Surgery: A Randomized, Placebo-Controlled, Phase-3 Study. Pain Pract. 2017;17(7):848-858.
doi pubmed - Van Tittelboom V, Poelaert R, Malbrain M, La Meir M, Staessens K, Poelaert J. Sublingual Sufentanil Tablet System Versus Continuous Morphine Infusion for Postoperative Analgesia in Cardiac Surgery Patients. J Cardiothorac Vasc Anesth. 2021;35(4):1125-1133.
doi pubmed - Leykin Y, Laudani A, Busetto N, Chersini G, Lorini LF, Bugada D. Sublingual sufentanil tablet system for postoperative analgesia after gynecological surgery. Minerva Med. 2019;110(3):209-215.
doi pubmed - Miner JR, Rafique Z, Minkowitz HS, DiDonato KP, Palmer PP. Sufentanil sublingual tablet 30mcg for moderate-to-severe acute pain in the ED. Am J Emerg Med. 2018;36(6):954-961.
doi pubmed - Hashemi M, Zali A, Golmakani E, Delshad MH, Shadnoush M, Akbari ME. Efficacy, safety, and tolerability of sublingual fentanyl orally disintegrating tablet in the treatment of breakthrough cancer pain: a randomized, double-blind, placebo-controlled study. Daru. 2021;29(1):51-59.
doi pubmed - Guitart J, Vargas MI, De Sanctis V, Folch J, Salazar R, Fuentes J, Coma J, et al. Efficacy and safety of sublingual fentanyl tablets in breakthrough cancer pain management according to cancer stage and background opioid medication. Drugs R D. 2018;18(2):119-128.
doi pubmed - Alberts DS, Smith CC, Parikh N, Rauck RL. Fentanyl sublingual spray for breakthrough cancer pain in patients receiving transdermal fentanyl. Pain Manag. 2016;6(5):427-434.
doi pubmed - Rauck RL, Oh DA, Singla N, Koch C, Parikh N, Nalamachu S, Yu J, et al. Pharmacokinetics of Fentanyl Sublingual Spray in Opioid-Naive Participants: Results of a Phase 1, Multiple Ascending Dose Study. Clin Drug Investig. 2018;38(8):715-726.
doi pubmed - Lennernas B, Hedner T, Holmberg M, Bredenberg S, Nystrom C, Lennernas H. Pharmacokinetics and tolerability of different doses of fentanyl following sublingual administration of a rapidly dissolving tablet to cancer patients: a new approach to treatment of incident pain. Br J Clin Pharmacol. 2005;59(2):249-253.
doi pubmed - Chwieduk CM, McKeage K. Fentanyl sublingual: in breakthrough pain in opioid-tolerant adults with cancer. Drugs. 2010;70(17):2281-2288.
doi pubmed - Rauck R, Reynolds L, Geach J, Bull J, Stearns L, Scherlis M, Parikh N, et al. Efficacy and safety of fentanyl sublingual spray for the treatment of breakthrough cancer pain: a randomized, double-blind, placebo-controlled study. Curr Med Res Opin. 2012;28(5):859-870.
doi pubmed - Human prescription drug label: SUBSYS-fentanyl spray. NIH National Library of Medicine DailyMed. Updated May 4, 2021. Available at: https://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=18a413e9-11e0-4a8f-86c0-d33b37b7b771.
- Jalili M, Fathi M, Moradi-Lakeh M, Zehtabchi S. Sublingual buprenorphine in acute pain management: a double-blind randomized clinical trial. Ann Emerg Med. 2012;59(4):276-280.
doi pubmed - White LD, Hodge A, Vlok R, Hurtado G, Eastern K, Melhuish TM. Efficacy and adverse effects of buprenorphine in acute pain management: systematic review and meta-analysis of randomised controlled trials. Br J Anaesth. 2018;120(4):668-678.
doi pubmed - Kuhlman JJ, Jr., Lalani S, Magluilo J, Jr., Levine B, Darwin WD. Human pharmacokinetics of intravenous, sublingual, and buccal buprenorphine. J Anal Toxicol. 1996;20(6):369-378.
doi pubmed - Bullingham RE, McQuay HJ, Porter EJ, Allen MC, Moore RA. Sublingual buprenorphine used postoperatively: ten hour plasma drug concentration analysis. Br J Clin Pharmacol. 1982;13(5):665-673.
doi pubmed - Khanna IK, Pillarisetti S. Buprenorphine - an attractive opioid with underutilized potential in treatment of chronic pain. J Pain Res. 2015;8:859-870.
doi pubmed - Mozafari J, Masoumi K, Forouzan A, Motamed H, Saki MA, Dezham M. Sublingual buprenorphine efficacy in renal colic pain relief: a randomized placebo-controlled clinical trial. Pain Ther. 2017;6(2):227-234.
doi pubmed - Huddart R, Clarke M, Altman RB, Klein TE. PharmGKB summary: oxycodone pathway, pharmacokinetics. Pharmacogenet Genomics. 2018;28(10):230-237.
doi pubmed - Weinberg DS, Inturrisi CE, Reidenberg B, Moulin DE, Nip TJ, Wallenstein S, Houde RW, et al. Sublingual absorption of selected opioid analgesics. Clin Pharmacol Ther. 1988;44(3):335-342.
doi pubmed - Fabbri A, Ruggiano G, Garcia Collado S, Ricard-Hibon A, Restelli U, Sbrana G, Marinangeli F, et al. Role of inhaled methoxyflurane in the management of acute trauma pain. J Pain Res. 2020;13:1547-1555.
doi pubmed - Porter KM, Dayan AD, Dickerson S, Middleton PM. The role of inhaled methoxyflurane in acute pain management. Open Access Emerg Med. 2018;10:149-164.
doi pubmed - Gladwin MT, Kato GJ, Weiner D, Onyekwere OC, Dampier C, Hsu L, Hagar RW, et al. Nitric oxide for inhalation in the acute treatment of sickle cell pain crisis: a randomized controlled trial. JAMA. 2011;305(9):893-902.
doi pubmed - Neveu J, Perelman S, Suisse G, Monpoux F. Severe hyperhomocysteinemia and peripheral neuropathy as side effects of nitrous oxide in two patients with sickle cell disease. Arch Pediatr. 2019;26(7):419-421.
doi pubmed - Haywood C, Jr., Tanabe P, Naik R, Beach MC, Lanzkron S. The impact of race and disease on sickle cell patient wait times in the emergency department. Am J Emerg Med. 2013;31(4):651-656.
doi pubmed - Glassberg JA. Improving emergency department-based care of sickle cell pain. Hematology Am Soc Hematol Educ Program. 2017;2017(1):412-417.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


