| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 15, Number 1, January 2023, pages 38-50
Clinical, Hematological, Biochemical and Radiological Characteristics for Patients With Splenic Infarction: Case Series With Literature Review
Mariko Hakoshimaa, Kazuya Kitakazea, Hiroki Adachia, Hisayuki Katsuyamaa, Hidekatsu Yanaia, b
aDepartment of Diabetes, Endocrinology and Metabolism, National Center for Global Health and Medicine Kohnodai Hospital, Chiba, Japan
bCorresponding Author: Hidekatsu Yanai, Department of Diabetes, Endocrinology and Metabolism, National Center for Global Health and Medicine Kohnodai Hospital, 1-7-1 Kohnodai, Ichikawa, Chiba 272-8516, Japan
Manuscript submitted October 26, 2022, accepted December 9, 2022, published online January 24, 2023
Short title: Characteristics for Patients With Splenic Infarction
doi: https://doi.org/10.14740/jocmr4836
| Abstract | ▴Top |
Background: Splenic infarction is a frequently missed diagnosis in acute clinical conditions and is often under-diagnosed due to the lack of high-quality evidence on pathophysiology of splenic infarction. Due to the scarcity of such evidence, no consensus guidelines regarding the diagnostic approach and management of patients with splenic infarction exist. Most of published articles on splenic infarction are case reports and there was no systematic review on splenic infarction.
Methods: We conducted a retrospective analysis of all radiologically confirmed cases of splenic infarction patients with any history of admission at National Center for Global Health and Medicine Kohnodai Hospital, from 2014 to 2020. Further, to understand the pathophysiology that causes splenic infarction, we searched the literatures on splenic infarction.
Results: We found 18 patients with splenic infarction. The average age was 78 years, and about half of patients had abdominal pain; however, the other half did not have abdominal pain. One-third of patients with splenic infarction died. Leukocytosis with neutrophilia, a decrease of lymphocytes, anemia, hypoalbuminemia, and liver dysfunction were observed. Fibrinogen was decreased and D-dimer was remarkably elevated. Lactate dehydrogenase (LDH) and C-reactive protein (CRP) were remarkably increased. Six patients (33.3%) had cancer, four patients (22.2%) had atrial fibrillation, and four patients (22.2%) had infection. We found 466 case reports on splenic infarction published from 1975 to 2021. Recently, the number of case reports on splenic infarction due to infection, especially, coronavirus disease 2019 (COVID-19), has been remarkably increasing. Furthermore, we found that leukocytosis, a decrease of lymphocytes, elongated activated partial thromboplastin time, decrease of fibrinogen, liver dysfunction, elevation of LDH and blood urea nitrogen can be the prognosis predicting factors for patients with splenic infarction.
Conclusion: Our study elucidated clinical, hematological, biochemical and radiological characteristics for patients with splenic infarction. We newly found significant differences in blood cell counts, coagulation markers, transaminases, LDH and blood urea nitrogen between patients who died and those who survived, suggesting that these parameters can be the prognosis predicting factors for splenic infarction. Further, our systematic review on case reports about splenic infarction showed the etiology of splenic infarction and the trend of the causative diseases.
Keywords: Computed tomography; Infection; Malignancy; Prognosis; Splenic infarction; Survival
| Introduction | ▴Top |
Splenic infarction is caused by an impaired blood supply to the spleen due to occlusion of the splenic artery or one of its subbranches. Although splenic infarction typically presents with left upper quadrant abdominal pain, fever, chills and nausea, asymptomatic cases may also occur. Childers et al reported a coronavirus disease 2019 (COVID-19) patient with incidental and asymptomatic splenic infarction [1]. Due to an improvement of accessibility of contrast-enhanced computed tomography (CT) scans, splenic infarction is frequently detected as an incidental finding in acute clinical settings. Such properties of splenic infarction do not allow performing prospective studies and randomized controlled trials (RCTs) to establish its characterization and treatments. Further, very few case series provide a current, comprehensive, and detailed description of splenic infarction. Schattner et al performed a retrospective chart review complemented by imaging evaluation and patient follow-up [2]. Thirty-two adult patients with a confirmed diagnosis of acute splenic infarction discharged over 10 years from a single academic center were studied. Their study suggested that splenic infarction is a frequently missed diagnosis in acute clinical conditions and is often under-diagnosed because splenic infarction patients show a wide range of non-specific symptoms, and acute causative comorbidities for splenic infarction make the diagnosis of splenic infarction difficult [2]. In fact, it was reported that only 10% of patients diagnosed as splenic infarction were found antemortem [3]. Weber et al reported a multi-center observational study using a total of 161 patients: 34 patients with acute renal infarction, 104 patients with acute splenic infarction and 23 patients with both acute renal and splenic infarctions [4]. They concluded that acute splenic infarction is heterogenous entity in regards to their clinical presentation, etiology, associated venous or arterial thrombosis. Yen et al reported a multi-center retrospective study using 130 patients with splenic infarction in emergency departments [5]. A total of 130 cases were included, two-thirds of whom presented with abdominal pain. Atrial fibrillation was the most common associated predisposing condition, followed by hematological diseases. Their study showed that a history of hypertension, atrial fibrillation, a laboratory result of leukocytosis or thrombocytopenia may provide a clue for clinicians to include splenic infarction in the list of differential diagnosis.
The clinical relevance of splenic infarction as an incidental finding on abdominal imaging is uncertain. The incidence, underlying etiology and prognostic relevance of splenic infarction are poorly characterized. Due to the scarcity of high-quality evidence, no consensus guidelines regarding the diagnostic approach and management of patients with splenic infarction exist. To elucidate clinical, hematological, biochemical and radiological characteristics for splenic infarction, we conducted a retrospective analysis of patients with splenic infarction detected by contrast-enhanced CT scans, with any history of admission at National Center for Global Health and Medicine Kohnodai Hospital, from 2014 to 2020.
Furthermore, most of published articles on splenic infarction are case reports and there was no systematic review on splenic infarction. To understand the pathophysiology that causes splenic infarction, we searched the literatures on the etiology of splenic infarction.
| Materials and Methods | ▴Top |
Study design and ethics
Research design is a retrospective observational study. Informed consent was obtained by the opt-out approach. The study protocol was approved by the Ethics Committee of the National Center for Global Health and Medicine (NCGM-S-004344-00), and the study was performed in accordance with the Declaration of Helsinki.
Study population
The patients diagnosed with splenic infarction by contrast-enhanced CT scans, from January 1, 2014 to March 12, 2020 at National Center for Global Health and Medicine Kohnodai Hospital were recruited by using the radiological interpretation reports on an electronic medical record. The contrast-enhanced CT scan demonstrated a wedge-shaped low-density area suggestive of splenic infarction (Fig. 1). We included patients with partial infarcts.
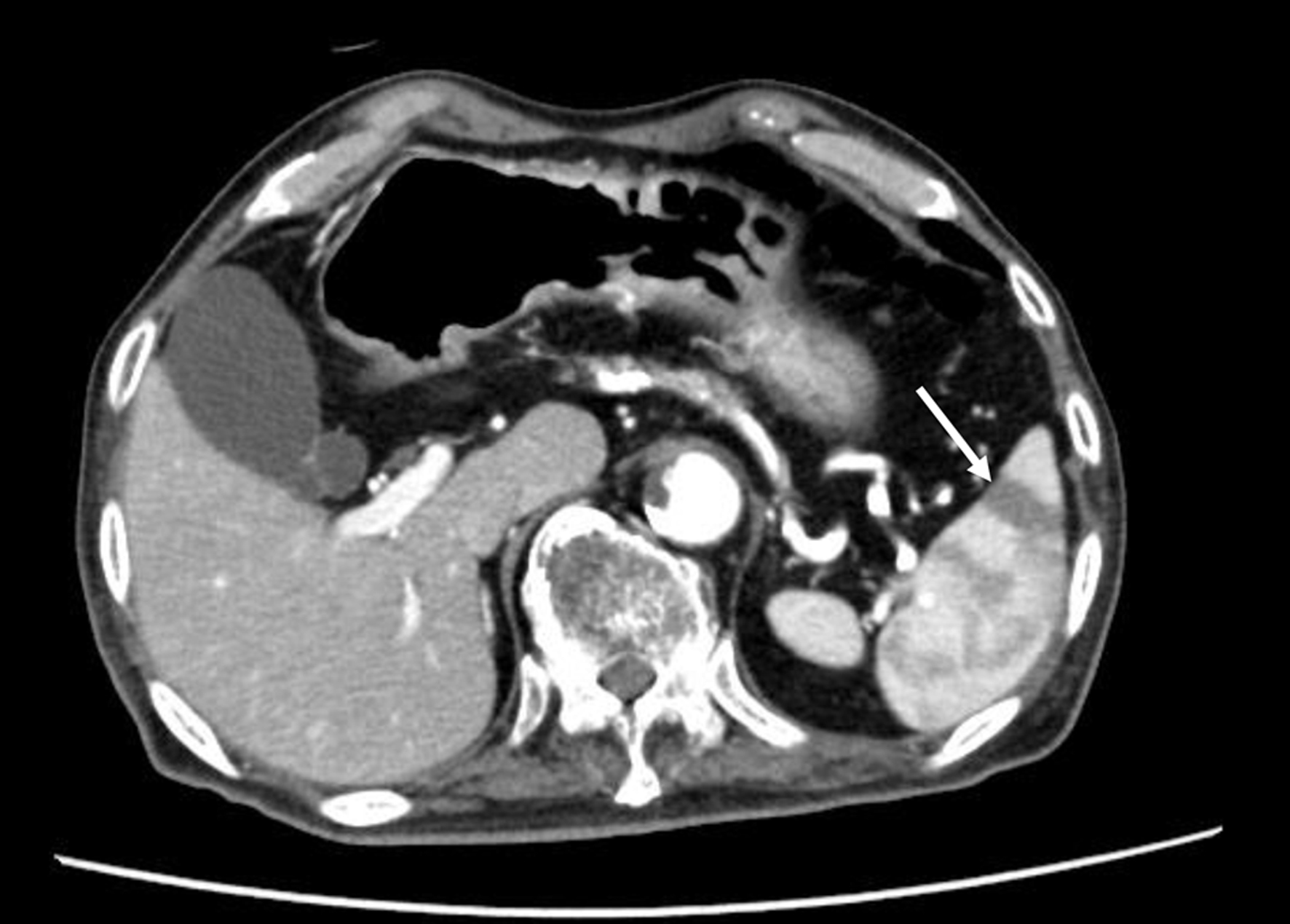 Click for large image | Figure 1. The contrast-enhanced CT scan finding of patients with splenic infarction. The arrow indicates splenic infarction. CT: computed tomography. |
Data obtained from studied participants
The information about medical history and medication were obtained via an electronic medical record. Such information included age, sex, body weight, body height, body mass index (BMI), systolic and diastolic blood pressures, heart rate, body temperature on admission, symptoms, medical treatments, comorbidities and clinical outcome. Although this is a retrospective study and the available data are limited, all available laboratory data on admission were obtained via an electronic medical record. Such data included blood cell counts (white blood cells (WBCs), red blood cells (RBCs), platelets, neutrophils, lymphocytes, monocytes, eosinophils and basophils), biochemistry (albumin, aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transferase (γ-GT), lactate dehydrogenase (LDH), creatine kinase (CK), blood urea nitrogen (BUN), creatinine, C-reactive protein (CRP), and plasma glucose (PG)), and coagulation fibrinolytic system (prothrombin time (PT), activated partial thromboplastin time (APTT), fibrinogen, and D-dimer).
We obtained the radiological (CT) findings (long and short axes of the spleen, maximal cross-sectional area of the spleen and spleen volume). We measured long and short axis and area of spleen at the height of maximal cross-sectional area of spleen. We roughly calculated the volume of spleen by multiplying “height measured at the sagittal section” and “maximal long axis of spleen” and “maximal short axis of spleen”.
Statistical analysis
Statistical analyses were performed by using SPSS version 23 (IBM Co., Ltd, Chicago, IL). All values are expressed as the mean ± standard deviation except for sex. The t-test and χ2 test were used for comparison between the two groups. P value of < 0.05 was considered to be statistically significant, and P value of < 0.1 was considered to have a tendency.
Search for literatures on splenic infarction
To find out the etiology of splenic infarction, we searched the case reports on splenic infarction from 1975 to 2021 by using PubMed. For the literatures search, we used the following keywords combination; “splenic infarction AND case report”, “spleen infarction AND case report”, “infarction of spleen AND case report”, “thrombosis of spleen vessel AND case report”, “thrombosis of spleen vein AND case report”, and “spleen venous thrombosis and AND case report.
| Results | ▴Top |
Clinical characteristics of patients with splenic infarction
We found 18 patients from the radiological interpretation reports, and we confirmed the existence of splenic infarction in contrast-enhanced CT with radiologists. Clinical characteristics of patients with splenic infarction are shown in Table 1. The average age was 78 years. Of patients with described symptoms, about half of patients had abdominal pain, and the other half did not have abdominal pain. The mean body temperature on admission was over 38 °C. Fever was defined as body temperature over 38 °C or 100.4 °F, and fever was observed in 57.1% of patients. The mean heart rate was over 90 beats/min. Tachycardia was observed in 40% of patients. One-third of patients with splenic infarction died after the diagnosis of splenic infarction.
 Click to view | Table 1. Clinical Characteristics of Patients With Splenic Infarction (n = 18) |
Laboratory medical characteristics of patients with splenic infarction
Laboratory medical characteristics of patients with splenic infarction are shown in Table 2. Leukocytosis and neutrophilia, and anemia were observed, and lymphocytes was decreased. Fibrinogen was decreased and D-dimer was remarkably elevated. Hypoalbuminemia and liver dysfunction were observed. LDH and CRP were remarkably increased.
 Click to view | Table 2. Laboratory Medical Characteristics of Patients With Splenic Infarction (n = 18) |
CT findings of patients with splenic infarction
CT findings of patients with splenic infarction are shown in Table 3.
 Click to view | Table 3. Computed Tomographic Findings of Spleen in Patients With Splenic Infarction |
Age, sex, comorbidity, anticoagulant use and clinical outcome of patients with splenic infarction
Age, sex, comorbidity, anticoagulant use and clinical outcome of patients with splenic infarction are shown in Table 4. Six patients (33.3%) had malignant diseases, four patients (22.2%) had atrial fibrillation, and four patients (22.2%) had infection. One patient had protein C deficiency. Three patients (16.7%) developed splenic infarction in spite of anticoagulant use. Anticoagulant therapy was started in four patients (22.2%) after the development of splenic infarction. One-third of patients with splenic infarction died.
 Click to view | Table 4. Age, Sex, Comorbidity, Presence of Abdominal Pain, Body Temperature, Heart Rate, Anticoagulant Use, and Clinical Outcome of Patients With Splenic Infarction |
Clinical, laboratory medical and radiological characteristics of splenic infarction patients who died or survived
Clinical characteristics of splenic infarction patients who died or survived are shown in Table 5. There were no significant differences in sex, age, BMI, body temperature, blood pressure and heart rate between two groups.
 Click to view | Table 5. Clinical Characteristics of Splenic Infarction Patients Who Died or Survived |
Laboratory medical characteristics of splenic infarction patients who died or survived are shown in Table 6. In blood cell counts, WBCs were significantly increased in patients who died than patients who survived. Lymphocytes were significantly decreased in patients who died than patients who survived. APTT was significantly longer in patients who died than patients who survived. Fibrinogen was significantly reduced in patients who died. D-dimer was non-significantly but extremely elevated in patients who died. Serum levels of AST, ALT, LDH and BUN were significantly higher in patients who died than patients who survived.
 Click to view | Table 6. Laboratory Medical Characteristics of Splenic Infarction Patients Who Died or Survived |
CT findings of spleen in splenic infarction patients who died or survived are shown in Table 7. Short axis of spleen in patients who died was significantly longer than that in patients who survived. Maximal cross-sectional area of spleen in patients who died tended to be significantly larger than that in patients who survived. Although a significant difference was not obtained, the volume of spleen in patients who died was 1.8 times larger than that in patients who survived.
 Click to view | Table 7. Computed Tomographic Findings of Spleen in Splenic Infarction Patients Who Died or Survived |
The etiology of splenic infarction found by literature search
We showed the etiology of splenic infarction by searching case reports on splenic infarction published from 2019 to 2022 in Table 8 [6-67]. We found 65 case reports on splenic infarction during the searched period. The case reports on splenic infarction due to infection were the most common, and followed by malignancy and RBC abnormality. Among case reports due to infection, case reports on the development of splenic infarction due to COVID-19 were the most common.
 Click to view | Table 8. The Etiology of Splenic Infarction Found by Literature Search |
The changes over time in the number of case reports on the causative diseases of splenic infarction
We found 466 case reports on splenic infarction published from 1975 to 2022. We classified them into five subgroups by the causative diseases for splenic infarction: 1) infection; 2) malignancy including neoplasm and myeloproliferative diseases; 3) abnormal RBC (sickle cell disease, hereditary spherocytosis), abnormal hemoglobin (hemoglobin SE, β-thalassemia) and coagulation abnormality (protein C deficiency, protein S deficiency and antiphospholipid syndrome); 4) vasculitis; and 5) cardiovascular disease (CVD) including atrial fibrillation. The numbers of case reports on splenic infarction due to infection, malignancy, abnormal RBCs, vasculitis, and CVD were 229, 92, 96, 24 and 25, respectively.
The changes over time in the number of published case reports on splenic infarction due to each category are shown in Figure 2. Recently, the number of case reports on splenic infarction due to infection has been remarkably increasing. The number of case reports due to other categories has been relatively constant over the years.
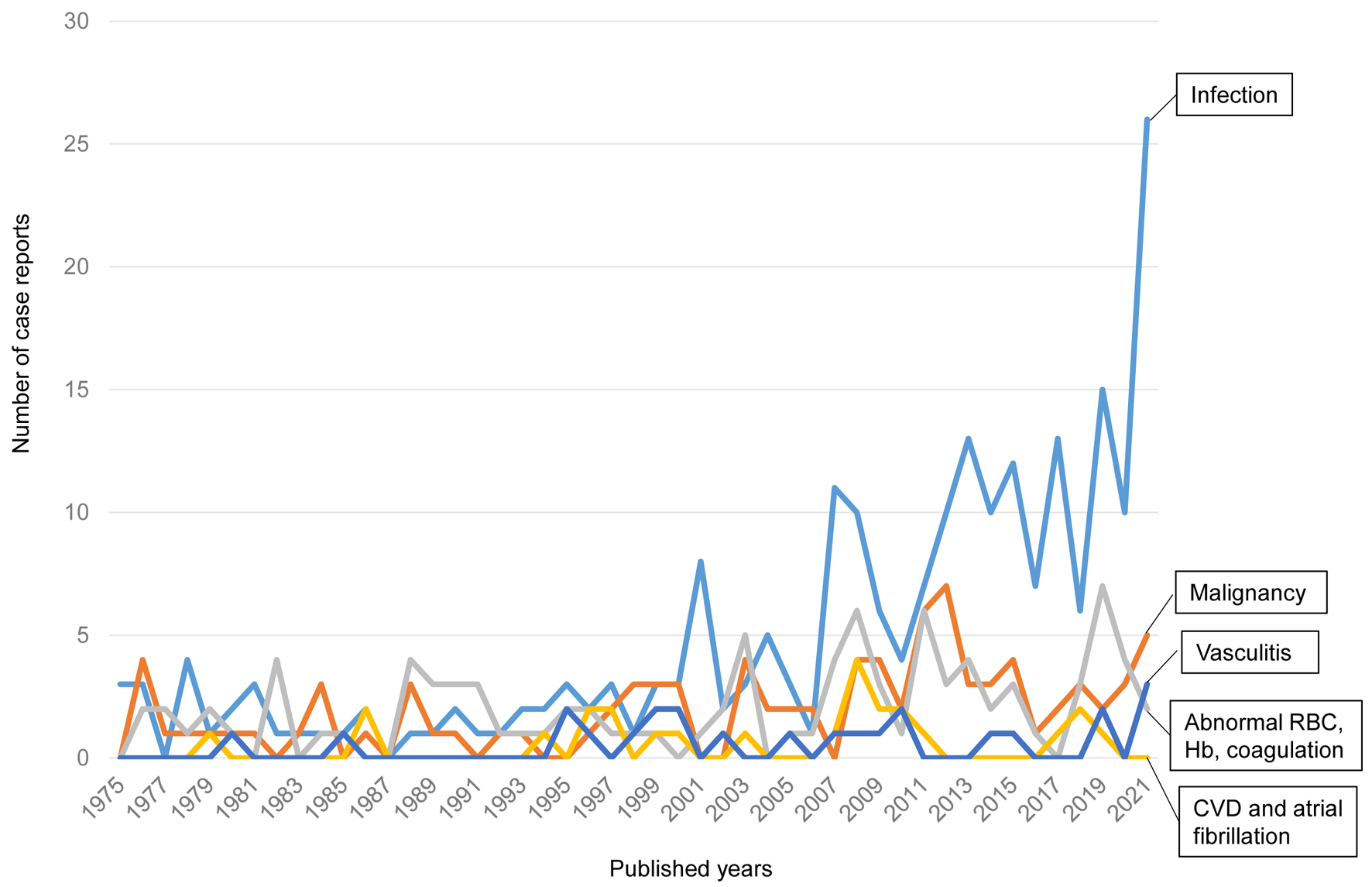 Click for large image | Figure 2. The changes over time in the number of published case reports on splenic infarction. CVD: cardiovascular disease; Hb: hemoglobin; RBCs: red blood cells. |
| Discussion | ▴Top |
Splenic infarction is a frequently missed diagnosis in acute clinical conditions and is often under-diagnosed due to the lack of high-quality evidences on pathophysiology of splenic infarction. As far as we searched, there was no meta-analysis and systematic review on splenic infarction, and there were only two original articles which investigated a relatively large number of cases. Even in the recent years, the reports on splenic infarction have been mainly case reports. Here, we studied clinical characteristics, laboratory medical examination and radiological findings in patients with splenic infarction which was diagnosed by using contrast-enhanced abdominal CT. Furthermore, we systematically reviewed case reports on splenic infarction by using those published from 1975 to 2022.
Weber et al reported a multi-center observational study using a total of 161 patients: 34 patients with acute renal infarction, 104 patients with acute splenic infarction and 23 patients with both acute renal and splenic infarctions [3]. In their study, the mean age was 63.2 years old. The average age of our splenic infarction patients was 78 years. Yen et al reported a multi-center retrospective study using 130 patients with splenic infarction in emergency departments [4]. In their study, 45.4% of patients were over 65 years old. Splenic infarction is likely to develop in the elderly.
Of our patients with described symptoms, about half of patients had abdominal pain, and the other half did not have abdominal pain. In Weber’s study, the main symptom was abdominal pain; however, its frequency was low (26.4%). In Yen’s study, 71.5% of patients presented with abdominal pain [4]. Although the most common symptom of splenic infarction was abdominal pain in three studies including ours, its frequency varied.
In the study by Weber et al and Yen et al, 16.4% and 10.8% of patients showed fever, respectively. The mean body temperature of our patients was over 38 °C. Fever was observed in 53.3% of patients. However, it remains unclear whether fever was due to splenic infarction or the underlying diseases which induced splenic infarction such as infection. Our study included four patients with infection, but did not include COVID-19 patients. A higher proportion of tachycardia was observed in Yen’s study. However, Weber et al did not report heart rate in splenic infarction patients. The mean heart rate was 91 beats/min in our study and tachycardia was observed in 40% of patients, suggesting that tachycardia may be one of clinical signs for splenic infarction. However, tachycardia is a very non-specific sign, and three of five patients with tachycardia showed abdominal pain, and tachycardia might have been due to pain. Although abdominal pain, tachycardia and fever are non-specific symptoms, such symptoms were relatively common in patients with splenic infarction. As shown in Table 4, all patients with splenic infarction showed either abdominal pain or fever or tachycardia. In patients with partial splenic infarction, they can be un-symptomatic or show no abnormal laboratory data. We should determine the range of splenic infarction that can remain silent in the future.
Yen et al reported that a laboratory result of leukocytosis or thrombocytopenia may provide a clue for clinicians to include splenic infarction in the list of differential diagnosis. In our study, leukocytosis was also observed; however, thrombocytopenia was not detected. Our study newly indicated neutrophilia, anemia, a decrease of lymphocytes, a decrease of fibrinogen, an increase of D-dimer, hypoalbuminemia, liver dysfunction, an increase of LDH and CRP as the possible markers for splenic infarction.
Our study was the first to report the measurements of spleen. The previous study using abdominal CT scans of 238 consecutive living donors for liver transplantation showed that the mean volume of the spleen of healthy Japanese people was 123 ± 45 cm3 [68]. Although our method to measure spleen volume was rough, the spleen volume in patients with splenic infarction was about 2.4 times larger than those in healthy people, suggesting that splenic infarction induced enlargement of spleen.
Yen et al reported that atrial fibrillation was the most common associated pre-disposing condition for splenic infarction, followed by hematological disease [4]. Our study also showed that 22% of patients had atrial fibrillation. Our study showed that malignancy such as pancreatic carcinoma, malignant lymphoma, and colon cancer, and infection such as septic shock, infective endocarditis, pyelonephritis and diabetic gangrene, induced the development of splenic infarction.
In Yen’s study, the mortality rate was 6.97%. High mortality rate (30.7%) was found in patients with infective endocarditis [4]. The mortality rate in our study was 33.3%, which was higher than that in Yen’s study. Our study did not include COVID-19 patients. A higher proportion of the elderly people in our study, and different clinical settings between the emergency department and general hospital, can explain the different mortality rates. Our patients who died were complicated with various severe diseases such as abdominal aortic dissection, pancreatic carcinoma, septic shock, heart failure, and hyperglycemic dehydration. Rather than dying from splenic infarction, our patients died from diseases that caused splenic infarction. The mean age of patients who died was 82.3 years old which was 7 years older than that of patients who survived, indicating that aging may be associated with death in splenic infarction patients.
Yen et al reported that Quick Sequential Organ Failure Assessment (qSOFA) score ≥ 1, body temperature > 38 °C, and malignancy history were associated with mortality in patients with splenic infarction [4]. We compared clinical, laboratory medical and radiological findings in splenic infarction patients who died with those in patients who survived. We did not find a significant difference in clinical characteristics between patients who died and patients who survived. We newly found significant differences in leukocytosis, a decrease of lymphocytes, APTT, fibrinogen, AST, ALT, LDH and BUN between patients who died and patients who survived, suggesting that these parameters can be the prognosis predicting factors for patients with splenic infarction. CRP was higher in patients who survived than those who died; however, this difference did not reach a statistical significance. However, any venous thromboembolism increases D-dimer, and decreases fibrinogen. Albumin is an acute phase reactant and would decrease in any sick patient. LDH and CRP are non-specific inflammatory markers which can rise in any inflammatory scenarios. To elucidate and validate the prognosis predicting factors for patients with splenic infarction, we should perform further studies by using a greater number of patients.
Furthermore, our study revealed that short axis and maximal cross-sectional area of spleen in patients who died were greater than those in patients who survived. The volume of spleen in patients who died was 1.8 times larger than that in patients who survived. In a former study involving 18 patients with granulomatosis with polyangiitis, splenomegaly was resolved after treatment [69], suggesting that splenomegaly may reflect the severity of splenic infarction due to the occlusion of splenic vessels. However, it remains unknown whether it is true or not in this study that the large volume of spleen can be a predicting factor for mortality in patients with splenic infarction.
Three patients (16.7%) developed splenic infarction in spite of anticoagulant use. Anticoagulant therapy was started in four patients (22.2%) after the development of splenic infarction. The usefulness of anticoagulant therapy for splenic infarction remains unknown in this study, which should be elucidated in the future.
As our systematic review of case reports on splenic infarction showed, the number of case reports on splenic infarction has been increasing in recent years, due to an improvement of accessibility of enhanced CT scans. Infection (cytomegalovirus and Epstein-Barr virus infection, and COVID-19), malignancy (carcinoma, malignant lymphoma and leukemia) and abnormal RBC (hereditary spherocytosis and sickle cell disease), abnormal hemoglobin (hemoglobin SE disease and β-thalassemia), coagulation abnormality (protein C and S deficiency, antiphospholipid syndrome) and vasculitis (anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis, eosinophilic granulomatosis with polyangiitis), and atrial fibrillation induced the development of splenic infarction. The number of case reports on splenic infarction due to infection has been remarkably increasing in recent years. Especially very recently, the number of case reports on splenic infarction due to COVID-19 has been rapidly increasing. Before the pandemic of COVID-19, infective endocarditis is the most common cause of infection which induced splenic infarction. The number of case reports due to other categories has been relatively constant over the years. Although we have looked for what is the primary cause for splenic infarction in the retrospective study or literature review, it was hard to determine such causes due to complicated pathophysiology.
The past decade has witnessed the increasing understanding of the biology of autophagy and its roles in various kinds of disorders. Both the protective and detrimental effects of autophagy in the pathogenesis and progression of acute myocardial infarction under ischemic or ischemia/reperfusion injuries were discussed [70]. Baseline autophagy or adaptively induced autophagy contributed to the alleviation of ischemic or ischemia/reperfusion damage while overwhelmingly induction of autophagy was detrimental during acute myocardial infarction. However, the role of autophagy in splenic infarction remains unknown, and this interesting theme should be studied in the future.
Limitations of the study should be addressed. The number of patients was small. This is a single-center study. Due to the retrospective nature, this study is inherently limited by the missing data. Especially, the number of patients whose D-dimer was measured was very small. We showed clinical, laboratory medical and radiological characteristics of splenic infarction patients who died or survived. In patients who died some laboratory data are worse, but heart rate and blood pressure did not differ. It remains unclear what was the reason for the patients who died. In short, is the death a direct result from splenic infarction or from other cause? When patients are sick, laboratory data can be worse. If death is from non-splenic infarction causes, such causes might have influenced on worse laboratory data in splenic infarction patients who died. I think this is one limitation of the study. However, it is also difficult to conduct a prospective comparative study among splenic infarction patients due to its scarcity and sporadic nature.
Conclusion
The summary of present study is shown in Figure 3. Our study elucidated clinical, hematological, biochemical and radiological characteristics for patients with splenic infarction. We newly found significant differences in leukocytosis, a decrease of lymphocytes, APTT, fibrinogen, AST, ALT, LDH and BUN between patients who died and patients who survived, suggesting that these parameters can be the prognosis predicting factors for patients with splenic infarction. Further, our systemic review on case reports about splenic infarction showed the etiology of splenic infarction and the trend of the causative diseases.
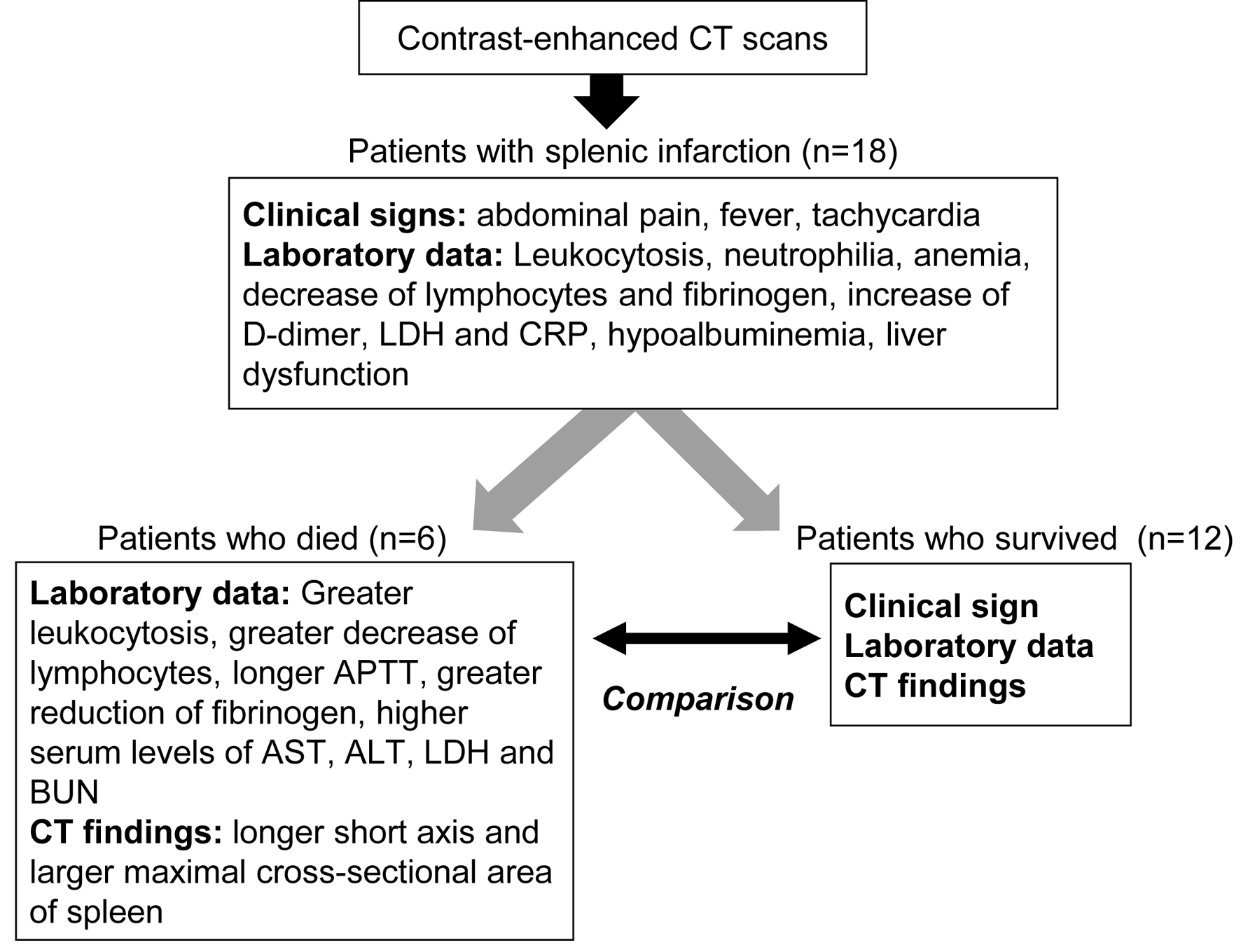 Click for large image | Figure 3. The summary of present study. APTT: activated partial thromboplastin time; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BUN: blood urea nitrogen; CRP: C-reactive protein; CT: computed tomography; LDH: lactate dehydrogenase. |
Acknowledgments
We thank the staffs of the Division of Research Support, National Center for Global Health and Medicine Kohnodai Hospital.
Financial Disclosure
Authors have no financial disclosures to report.
Conflict of Interest
The authors declare that they have no conflict of interest concerning this article.
Informed Consent
Not applicable.
Author Contributions
HY contributed to conceptualization, supervision, writing - original draft, review and editing. MH, KK, and HA contributed to formal analysis and data curation. HK contributed to conceptualization and data curation. All authors have read and agreed to the published version of the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
APTT: activated partial thromboplastin time; ALT: alanine aminotransferase; AST: aspartate aminotransferase; BMI: body mass index; BUN: blood urea nitrogen; CRP: C-reactive protein; CK: creatine kinase; CT: computed tomography; CVD: cardiovascular disease; γ-GT: γ-glutamyl transferase; LDH: lactate dehydrogenase; PG: plasma glucose; PT: prothrombin time; qSOFA: Quick Sequential Organ Failure Assessment; RBCs: red blood cells; RCTs: randomized controlled trials; WBCs: white blood cells
| References | ▴Top |
- Childers J, Do TVC, Smith F, Vangara A, Ganti SS, Akella R. Incidental and asymptomatic splenic infarction and infrarenal thrombus in a COVID-19 patient. Cureus. 2022;14(7):e26555.
doi - Schattner A, Adi M, Kitroser E, Klepfish A. Acute splenic infarction at an academic general hospital over 10 years: presentation, etiology, and outcome. Medicine (Baltimore). 2015;94(36):e1363.
doi pubmed - O'Keefe JH, Jr., Holmes DR, Jr., Schaff HV, Sheedy PF, 2nd, Edwards WD. Thromboembolic splenic infarction. Mayo Clin Proc. 1986;61(12):967-972.
doi pubmed - Weber E, Grangeon F, Reynaud Q, Hot A, Seve P, Jardel S, Tazarourte K, et al. Acute renal and splenic infarctions: a review. QJM. 2020;113(3):186-193.
doi pubmed - Yen CC, Wang CK, Chen SY, Gao SY, Lo HY, Ng CJ, Chaou CH. Risk assessment and prognostic analysis of patients with splenic infarction in emergency department: a multicenter retrospective study. Sci Rep. 2021;11(1):21423.
doi pubmed - Blackwood GA, Danta M, Gett R. Acute cytomegalovirus infection associated with splenic infarction: a case report and review of the literature. Cureus. 2022;14(3):e23404.
doi pubmed - Pakkiyaretnam M, Horne C, Sarr L, Parsad M, Chong J. Cytomegalovirus-associated splenic infarction in a young female patient. Cureus. 2020;12(10):e10801.
doi pubmed - Schattner A, Dubin I, Glick Y. Cytomegalovirus-associated splenic infarction. Am J Med. 2020;133(3):e104-e105.
doi pubmed - Ma Z, Wang Z, Zhang X, Yu H. Splenic infarction after Epstein-Barr virus infection in a patient with hereditary spherocytosis: a case report and literature review. BMC Surg. 2022;22(1):136.
doi pubmed - Nishioka H, Hayashi K, Shimizu H. Case report: splenic infarction in infectious mononucleosis due to Epstein-Barr virus infection. Am J Trop Med Hyg. 2021;106(2):623-625.
doi pubmed - Hasibi M, Zargaran M, Asadollahi-Amin A. Infectious mononucleosis complicated with bilateral peritonsillar abscess and splenic infarction. Case Rep Infect Dis. 2021;2021:6623834.
doi pubmed - Patruno JV, Milross L, Javaid MM. Not quite a mono spot diagnosis. Splenic infarction complicating infectious mononucleosis. Am J Med. 2021;134(5):e306-e307.
doi pubmed - Thida AM, Ilonzo I, Gohari P. Multiple splenic infarcts: unusual presentation of hereditary spherocytosis associated with acute Epstein-Barr virus infection. BMJ Case Rep. 2020;13(7):e235131.
doi pubmed - Pervez H, Tameez Ud Din A, Khan A. A Mysterious Case of an Infarcted Spleen due to Kissing Disease: A Rare Entity. Cureus. 2020;12(1):e6700.
doi - Nofal R, Zeinali L, Sawaf H. Splenic infarction induced by epstein-barr virus infection in a patient with sickle cell trait. J Paediatr Child Health. 2019;55(2):249-251.
doi pubmed - Li J, Zhou L, Gong X, Wang Y, Yao D, Li H. Abiotrophia defectiva as a rare cause of mitral valve infective endocarditis with mesenteric arterial branch pseudoaneurysm, splenic infarction, and renal infarction: a case report. Front Med (Lausanne). 2022;9:780828.
doi pubmed - Hughes HL, Jacob BK. Infective endocarditis in an intravenous drug user: multiple fatal complications. BMJ Case Rep. 2021;14(5):e239376.
doi pubmed - Varga A, Tilea I, Tatar CM, Iancu DG, Jiga MA, Dumbrava RA, Pop M, et al. Native aortic valve endocarditis complicated by splenic infarction and giant mitral-aortic intervalvular fibrosa pseudoaneurysm-a case report and brief review of the literature. Diagnostics (Basel). 2021;11(2):251.
doi pubmed - Mylonas CC, Gomatou G, Poulakou G, Moraitou E, Syrigos K. Human disease caused by <em>Streptococcus alactolyticus</em>: a case report of native valve infective endocarditis and review of the literature. Monaldi Arch Chest Dis. 2020;90(4):638.
doi pubmed - Cheng CW, Feng CM, Chua CS. Invasive pyogenic infection and infective endocarditis due to Streptococcus anginosus: A case report. Medicine (Baltimore). 2019;98(48):e18156.
doi pubmed - Farid S, Esquer Garrigos Z, Sohail MR. Infective endocarditis due to Granulicatella elegans presenting with musculoskeletal symptoms. BMJ Case Rep. 2019;12(8):e229294.
doi pubmed - Park BW, Shin YS, Cho EB, Park EJ, Kim KH, Kim KJ. Two cases of infective endocarditis in patients with atopic dermatitis. Ann Dermatol. 2019;31(1):70-74.
doi pubmed - Javaid U, Young P, Gill G, Bhargava P. Acute complete splenic infarction secondary to COVID-19 infection. Radiol Case Rep. 2022;17(5):1402-1406.
doi pubmed - Rigual R, Ruiz-Ares G, Rodriguez-Pardo J, Fernandez-Prieto A, Navia P, Novo JR, Alonso de Lecinana M, et al. Concurrent cerebral, splenic, and renal infarction in a patient with COVID-19 infection. Neurologist. 2022;27(3):143-146.
doi pubmed - Yildiz E, Satilmis D, Cevik E. Splenic infarction and pulmonary embolism as a rare manifestation of COVID-19. Turk J Emerg Med. 2021;21(4):214-216.
doi pubmed - Tranca SD, Antal O, Farcas AD. Case report: acute splenic artery thrombosis in a COVID 19, postpartum patient. Front Med (Lausanne). 2021;8:698627.
doi pubmed - Moradi H, Mouzannar S, Miratashi Yazdi SA. Post COVID-19 splenic infarction with limb ischemia: A case report. Ann Med Surg (Lond). 2021;71:102935.
doi pubmed - Rehman A, Thoppil AJ, Wallach SL. Portal vein thrombosis and splenic infarction in a COVID-19 patient. Cureus. 2021;13(8):e16843.
doi - Sztajnbok J, Brasil L, Romero LA, Ribeiro AF, Vidal JE, Figueiredo-Mello C, Malaque C. Splenic infarction with aortic thrombosis in COVID-19. Am J Med Sci. 2021;362(4):418-423.
doi pubmed - Norton EJ, Sheikh N. Splenic Infarct due to a patent foramen ovale and paradoxical emboli post-COVID-19 infection: a case study. Cureus. 2021;13(5):14887.
doi - Dagistanli S, Sonmez S. Spleen infarct secondary to thrombus in COVID-19-related splenic vein: A case report. Arab J Gastroenterol. 2021;22(2):180-183.
doi pubmed - Castro GRA, Collaco IA, Dal Bosco CLB, Correa GG, Dal Bosco GB, Correa GL. Splenic infarction as a complication of covid-19 in a patient without respiratory symptoms: A case report and literature review. IDCases. 2021;24:e01062.
doi pubmed - Alvarenga Fernandes D, Batista Araujo Filho JA, Ribeiro de Jesus A. SARS-CoV-2 and splenic infarction: a rarely described thromboembolic presentation. Rev Esp Enferm Dig. 2022;114(1):52-53.
doi - Dennison JJ, Carlson S, Faehling S, Phelan H, Tariq M, Mubarik A. Splenic infarction and spontaneous rectus sheath hematomas in COVID-19 patient. Radiol Case Rep. 2021;16(5):999-1004.
doi pubmed - Ramanathan M, Chueng T, Fernandez E, Gonzales-Zamora J. Concomitant renal and splenic infarction as a complication of COVID-19: a case report and literature review. Infez Med. 2020;28(4):611-615.
- Hafiz W, Alotaibi F, Alneefia R, Alghuraibi E, Basha Ahmed A, Warsi A. Splenic infarction induced by dengue hemorrhagic fever: a rare presentation. Cureus. 2021;13(8):e17072.
doi - Hahn DW, Atkinson CE, Le M. Multiple anatomic sites of infarction in a pediatric patient with M. pneumoniae infection, a case report. BMC Pediatr. 2021;21(1):372.
doi pubmed - Pachet A, Dumestre-Perard C, Moine M, Marlu R, Rubio A, Bost-Bru C. Splenic infarction associated with transient anti-prothrombin antibodies is a rare manifestation of acute Mycoplasma pneumoniae infection. Arch Pediatr. 2019;26(8):483-486.
doi pubmed - Kim SH, Jung HS, Park S. Serial follow-up of malaria-induced splenic infarction: A case report. Ann Hepatobiliary Pancreat Surg. 2020;24(2):239-242.
doi pubmed - Tripathi N, Saha A, Kaur M. Multiple splenic infarcts complicating plasmodium vivax malaria. Pediatr Emerg Care. 2019;35(10):e181-e183.
doi pubmed - Goyal MK, Porwal YC, Gogna A, Gulati S. Splenic infarct with scrub typhus: a rare presentation. Trop Doct. 2020;50(3):234-236.
doi pubmed - Kapoor S, Upreti R, Mahajan M. Splenic infarct secondary to scrub typhus: A rare association. J Vector Borne Dis. 2019;56(4):383-384.
doi pubmed - Alkuwaiti FA, Elghoneimy Y, Alabdrabalrasol EA, Alshreadah ST. Unusual Presentation of Aspergillus Pericarditis: A Case Report. Saudi J Med Med Sci. 2019;7(3):175-178.
doi pubmed - Gupta A, Patel P, Manvar K, Kellner T, Guevara E. Splenic infarction in babesiosis: A rare presentation. Clin Case Rep. 2019;7(8):1591-1595.
doi pubmed - Huang P, Li Y. Polycythemia vera presenting with pulmonary embolism and splenic infarction: a case report. J Int Med Res. 2022;50(1):3000605211072801.
doi pubmed - Mehta AM, Seshadri S, Verma S, Madhyastha SP. Essential thrombocythemia with portal vein thrombosis and splenic infarction successfully treated with platelet apheresis. BMJ Case Rep. 2021;14(9):e245267.
doi pubmed - Kuipers RS, Berghuis MAT, Ogilvie AC, van Wissen SA, Riezebos RK. Non-bacterial thrombotic endocarditis manifested by ventricular fibrillation in a patient with low grade ovarian carcinoma: case report and literature review. Eur Heart J Case Rep. 2021;5(4):ytab120.
doi pubmed - Trinci M, Giangregorio C, Calabrese G, Ottaviani P, Riu P, Galluzzo M, Miele V. A rare case of non-traumatic intrasplenic pseudoaneurysms in a patient with acute T-cell lymphoblastic leukemia. J Ultrasound. 2021;24(1):85-90.
doi pubmed - Yokawa K, Inoue T, Yamanaka K, Okada K. Descending aortic replacement for intimal angiosarcoma. Eur J Cardiothorac Surg. 2019;56(6):1204-1205.
doi pubmed - Nakamoto R, Okuyama C, Utsumi T, Yamamoto Y. Splenic marginal zone B-cell lymphoma with splenic infarction in a patient with cold agglutinin disease. Clin Nucl Med. 2019;44(5):e372-e374.
doi pubmed - Chan VSH, Mak YH, Kwong YL, Lam SHY. Non-traumatic splenic rupture secondary to haemorrhagic infarct in diffuse large B-cell lymphoma. BMJ Case Rep. 2019;12(1):e229052.
doi pubmed - Zhang JS, Li L. Laparoscopic ligation of splenic vessels for the treatment of hereditary spherocytosis in children. Pediatr Surg Int. 2020;36(3):365-371.
doi pubmed - Odeh AM, Boumarah KA, Alsumaien WA, Al-Abbad MT, Al-Ali AH, Alammar ZA, Alsuqair H, et al. A huge subcapsular splenic cyst like hematoma in sickle cell anemia. Cureus. 2022;14(2):e22582.
doi - Burley NB, Miller KD. Acute liver failure in sickle cell disease: a perfect storm. Cureus. 2021;13(6):e15680.
doi - Toyoda M, Kitamura T, Nakashima K, Matsunaga Y, Nie M, Miyaji K. Spontaneous splenic rupture, mesenteric ischemia and spinal infarction after aortic repair for acute type A dissection in a patient with sickle cell trait. Gen Thorac Cardiovasc Surg. 2021;69(3):560-563.
doi pubmed - Krepis P, Maritsi DN, Tsolia MN, Vakaki M, Kossiva L. Massive splenic infarction and autosplencetomy: first presentation of homozygous sickle cell disease in a toddler. J Pediatr Hematol Oncol. 2020;42(5):371-372.
doi pubmed - Kumar R, Kapoor R, Singh J, Das S, Sharma A, Yanamandra U, Nair V. Splenic infarct on exposure to extreme high altitude in individuals with sickle trait: a single-center experience. High Alt Med Biol. 2019;20(3):215-220.
doi pubmed - Pajak A, Li JC, Liu A, Nazare S, Smith B. Hemoglobin SE disease presenting as a high-altitude massive splenic infarction complicated by hemorrhagic conversion and splenectomy. Cureus. 2020;12(9):e10321.
doi - Yanamandra U, Bahl R, Mishra K, Kumar S. Letter to the editor: portal vein thrombosis with splenic infarct in beta-thalassemia minor at high altitude. High Alt Med Biol. 2020;21(3):310.
doi pubmed - Hamza M, Sabir Khan H, Arshad A, Ahmed M, Hanif M. Mesenteric ischemia due to thrombosis involving the aorta, celiac artery, and superior mesenteric artery in a young female with protein C deficiency. Cureus. 2019;11(11):e6151.
doi - Pinto V, Ministro A, Carreira NR, Cardoso A, Goncalves CS, Henriques M, Rato J, et al. A catastrophic seronegative anti-phospholipid syndrome: case and literature review. Thromb J. 2021;19(1):103.
doi pubmed - Rajah FT, Alhabobi AA, Aloudah NM, Osman AI, Elhassan EA. Splenic infarcts and pulmonary renal syndrome in a young patient with double-positive anti-GBM and ANCA-associated vasculitis. Saudi J Kidney Dis Transpl. 2021;32(1):240-244.
doi pubmed - Chung SW. Microscopic polyangiitis presenting with splenic infarction: a case report. Medicina (Kaunas). 2021;57(2):157.
doi pubmed - Bottomley MJ, Gibson M, Alchi B. PR3 vasculitis presenting with symptomatic splenic and renal infarction: a case report and literature review. BMC Nephrol. 2019;20(1):84.
doi pubmed - Inaba R, Fuse Y, Kurimoto F, Suzuki S, Watanabe K. A rare case of eosinophilic granulomatosis with polyangiitis presenting as ischemic stroke and splenic infarction. J Stroke Cerebrovasc Dis. 2021;30(3):105539.
doi pubmed - Tanaka Y, Matsumoto M, Yahata T, Mineki T, Oiwa K. Two cases of multiple thromboembolism with asymptomatic atrial fibrillation. Cureus. 2022;14(1):e21645.
doi - Shen LL, Qi W, Duarte J, Nagar A, Merchant NB. Acute renal and splenic infarctions as the initial manifestations of atrial fibrillation. Lancet. 2019;393(10183):1856.
doi pubmed - Kaneko J, Sugawara Y, Matsui Y, Makuuchi M. Spleen size of live donors for liver transplantation. Surg Radiol Anat. 2008;30(6):515-518.
doi pubmed - Pinching AJ, Lockwood CM, Pussell BA, Rees AJ, Sweny P, Evans DJ, Bowley N, et al. Wegener's granulomatosis: observations on 18 patients with severe renal disease. Q J Med. 1983;52(208):435-460.
- Wu D, Zhang K, Hu P. The role of autophagy in acute myocardial infarction. Front Pharmacol. 2019;10:551.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


