| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Review
Volume 14, Number 7, July 2022, pages 251-259
Carbapenemase Inhibitors: Updates on Developments in 2021
Maroun Bou Zerdana, i , Sally Al Hassanb, i, Waleed Shakerc, Rayan El Hajjard, Sabine Allame, Morgan Bou Zerdanf, Amal Najig, Nabil Zeineddineh, j
aDepartment of Internal Medicine, SUNY Upstate Medical University, Syracuse, NY, USA
bDepartment of Internal Medicine, American University of Beirut Medical Center, Beirut, Lebanon
cDepartment of Internal Medicine, New Cross Hospital, Wolverhampton, UK
dDepartment of Pathology, Anatomy, and Cell Biology, Thomas Jefferson University, Philadelphia, PA, USA
eFaculty of Medicine, University of Balamand, Beirut, Lebanon
fFaculty of Medicine, American University of Beirut, Beirut, Lebanon
gDivision of Infectious Diseases, Department of Medicine, Emory University School of Medicine, Atlanta, GA, USA
hDepartment of Infectious Disease, SUNY Upstate Medical University, Syracuse, NY, USA
iThese authors contributed equally to this article.
jCorresponding Author: Nabil Zeineddine, Department of Infectious Disease, SUNY Upstate Medical University, Syracuse, NY, USA
Manuscript submitted June 13, 2022, accepted July 21, 2022, published online July 29, 2022
Short title: Antibiotic Resistance and Carbapenemase Inhibitors
doi: https://doi.org/10.14740/jocmr4764
| Abstract | ▴Top |
Carbapenem resistance, an emerging global health problem, compromises the treatment of infections caused by nosocomial pathogens. Preclinical and clinical trials demonstrate that a new generation of carbapenemases inhibitors, together with the recently approved avibactam, relebactam and vaborbactam, would address this resistance. Our review summarizes the latest developments related to carbapenemase inhibitors synthesized to date, as well as their spectrum of activity and their current stage of development. A particular focus will be on β-lactam/β-lactamase inhibitor combinations that could potentially be used to treat infections caused by carbapenemase-producer pathogens. These new combinations mark a critical step forward the fight against antimicrobial resistance.
Keywords: Carbapenem resistance; Carbapenemase inhibitors; Pathogens
| Introduction | ▴Top |
Carbapenem resistance has been declared a worldwide problem as per the 2017 World Health Organization [1]. To address this global pandemic crisis, it is pivotal to understand the mechanism of resistance and better classify the responsible pathogens. The main mechanism of resistance is the hydrolysis of carbapenems via the production of carbapenem-hydrolyzing enzymes [2]. β-lactamase enzymes are classified by the Ambler Classification System into four groups (A, B, C, and D) based on their central catalytic domain and substrate preference [3]. In general, class A, B and D enzymes utilize carbapenemases for resistance while class C enzymes mainly hydrolyze cephalosporins. Moreover, class A, C and D enzymes have serine in their central catalytic domain, while class B enzymes have zinc and are considered metallo-β-lactamases (MBLs) [4] (Table 1).
 Click to view | Table 1. Summary and Classification of Carbapenemases Enzymes |
Class A enzymes can be chromosomally encoded (SME, NmcA, SFC-1, BIC-1, PenA, FPH-1, SHV-38) or plasmid-encoded (KPC, GES, FRI-1) [5]. Chromosomally encoded class A carbapenemases are rare and are found in a limited number of Serratia and Enterobacter isolates [6-8]. However, plasmid-encoded class A carbapenemases are widely spread and have been isolated predominantly in Klebsiella pneumoniae [9, 10]. Specifically, the KPC family from class A has enzymes that can hydrolyze a huge variety of β-lactams substrates, which gives these carbapenemases the power to spread and develop resistance, making them one of the most difficult carbapenemases to control [11].
Class B carbapenemases are distinguished by having zinc ions on their active site. These ions interact with the β-lactams leading to their hydrolysis. The mechanism of action of these MBLs facilitates their inhibition by metal ion chelators, such as EDTA, but not by the regularly used lactam inhibitors. They are divided into chromosomal (CcrA, CphA, L1) and plasmid-encoded (VIM, IMP, GIM, SIM) variants. Chromosomally encoded class B carbapenemases are mainly found in opportunistic pathogens and are therefore not common in nosocomial bacteria. Since the spread of chromosomal MBLs is directly dependent on the prevalence of the offending pathogen, they are relatively rarer to detect [12, 13]. On the contrary, the plasmid-encoded class B carbapenemases are transferrable, so their prevalence has increased over the years, with some (VIM, IMP) even spreading beyond their countries of origin. They are found predominantly in Pseudomonas aeruginosa, Acinetobacter baumannii and Enterobacterales [11, 14].
Class C enzymes, as mentioned earlier, are known to hydrolyze mainly cephalosporins. Recently five enzymes in this group (ACT-1, DHA-1, CMY-2, CMY-10, and ADC-68) were found to exhibit carbapenem catalytic activity as well, imposing further therapeutic threat against the use of multiple antibiotic classes [15].
Class D enzymes, also called OXAs (oxacillinases) due to their ability to hydrolyze isoxazolyl penicillin oxacillin, are widely found in gram-negative bacteria. Some of these enzymes are found to possess carbapenemase activity, thus given the name carbapenem-hydrolyzing class D β-lactamases (CHDLs). The majority of the clinically significant CHDLs (OXA-23, OXA-24/40, OXA-51, OXA-58, and OXA-143) are found in the A. baumannii strains. Of those, OXA-51 is the most widespread. OXA-48 is also found in K. pneumonia and less often in some members of the Enterobacteriaceae family [16-22].
| Carbapenemase Inhibitors | ▴Top |
Diazabicyclooctanes (DBOs) derived inhibitors
DBOs were thought to be potential β-lactam mimics; however, early synthetic DBOs had no antimicrobial uses [23, 24]. Nevertheless, continuing efforts in their development had made them potentially potent inhibitors of β-lactamases. DBOs consist of a five-membered ring with an amide group that carbamylates and subsequently deactivates the serine residues of class A and class C enzymes. Additionally, it has shown unpredictable activity against class D enzymes and no effect on class B metalloenzymes [24].
Avibactam
Up until 2015, antibiotics with extensive side effect profile, such as aminoglycosides and colistin, have been considered the optimal modality for managing carbapenem-resistant Enterobacteriaceae (CRE). In 2015, the Food and Drug Administration (FDA) approved the use of avibactam-ceftazidime, a combination of a β-lactam/β-lactamase inhibitor (BLI) [25, 26]. It has a broader spectrum of activity when compared to current BLIs (clavulanic acid, tazobactam, and sulbactam) [27]. Moreover, when paired with ceftazidime, a third-generation cephalosporin, it has restored the antimicrobial activity against a wide range of class A and C β-lactamases, along with K. pneumoniae carbapenemase (KPC) carbapenemases, extended spectrum beta-lactamases (ESBLs), and AmpC enzymes. A recent study by Niu et al proved that aztreonam-avibactam combination has an activity against MBL-producing K. pneumoniae, a matter which has been challenging for many antimicrobials [28]. Nevertheless, ceftazidime alone stands effective against OXA-48-like carbapenemases in addition to its good antipseudomonal activity. Together when combined they offer extensive action against β-lactamase producing Enterobacteriaceae as well as against P. aeruginosa with derepressed AmpC [29]. The combination of aztreonam in combination with ceftazidime-avibactam has provided an upgrade on the individual ability of aztreonam and ceftazidime-avibactam individually to fight against serine-beta-lactamase (SBL) and MBL-producing Enterobacterales (P. aeruginosa). In a study designed to evaluate the bactericidal activity of different antibiotic combinations against SBL and MBL-producing P. aeruginosa isolates, there was a significant increase in bactericidal activity in 4/5 of the isolates upon combining aztreonam with ceftazidime-avibactam, as opposed to using aztreonam, aztreonam-avibactam, and ceftazidime-avibactam which showed no bactericidal activity against any of the isolates [30].
Aztreonam-avibactam combination is superior to relebactam, clavulanate, and vaborbactam in the treatment of multidrug-resistant (MDR) S. maltophilia. Though there may be some decreased susceptibility by some strains in part due to overexpression of intrinsic beta-lactamases and efflux pumps [31].
Zidebactam (ZID) and WCK 5153
ZID and WCK 5153 are bicyclo-acyl hydrazides (BCHs), derivatives of the DBOs scaffold, and are used for the treatment of severe infections caused by highly drug-resistant gram-negative bacteria [32, 33]. ZID in combination with cefepime (FEP) is presently being evaluated in clinical trials for infections caused by MDR gram-negative pathogens such as P. aeruginosa and A. baumannii [32]. Despite being synthetized from a DBO scaffold, BCHs were developed with the goal of increasing penicillin-binding protein 2 (PBP2) binding in P. aeruginosa and A. baumannii rather than improving the compound’s β-lactamase-inhibitory action [32, 34]. That contrasts with the first DBO, avibactam, which had a low PBP2 affinity in Enterobacteriaceae, followed by OP0595 (RG 6080), which had a higher PBP2 affinity but was only active against Enterobacteriaceae. ZID and WCK 5153 have shown in P. aeruginosa a high affinity for A. baumannii PBP2 with inhibitory concentration 50 (IC50) of 0.01 g/mL, which was 7 - 8 times lower than imipenem and like meropenem, although both are known to be powerful PBP2-binding drugs. For wild-type and MDR Acinetobacter bacteria, the minimum inhibitory concentrations (MICs) of ZID and WCK 5153 were more than 1,024 µg/mL. Furthermore, combinations of FEP with 8 µg/mL ZID or WCK 5153 and sulbactam with 8 µg/mL ZID or WCK 5153 have resulted in four- and eight-fold decreases in MICs, respectively, and improved antimicrobial activity. Many of the different combinations resulted in complete bacterial elimination after 24 h [32].
Durlobactam (sulbactam/durlobactam)
Sulbactam/durlobactam, previously known as (sulbactam-ETX2514), constitutes a β-lactam/BLI combination used to treat severe A. baumannii-calcoaceticus complex (ABC) organisms, including MDR strains [35, 36]. This combination often inhibits PBP3 and therefore has an inherent action against A. baumannii. However, high MICs are often detected among isolates resistant to carbapenems. When compared to other drugs, sulbactam/durlobactam exhibits high in vitro effectiveness against A. baumannii isolates, including those resistant to imipenem/meropenem, amikacin, minocycline, and colistin. Moreover, it has been shown to be effective when used with current limited antimicrobials used to treat A. baumannii. Sulbactam/durlobactam was found to have a good safety profile, tolerability, and pharmacokinetic properties in phase 1 and phase 2 studies, and is currently being tested in a randomized, controlled phase 3 study in patients with A. baumannii infections, such as hospital-acquired bacterial pneumonia, ventilator-associated bacterial pneumonia, and bacteremia [37].
Nacubactam
Nacubactam, a bridged DBO previously known as RG6080 and OP0595, is a class A and C BLI with intrinsic antibiotic and β-lactam “enhancer” action against Enterobacteriaceae [38, 39]. The structure of nacubactam can be distinguished from avibactam by an aminoethoxy group attached to the carbamoyl side chain. This modification is most likely responsible for nacubactam’s relatively high antibacterial activity. Nacubactam inhibits Escherichia coli PBP2 in a similar manner as ETX2514, WCK 5153, and ZID. Furthermore, when coupled with β-lactams, nacubactam, like mecillinam and other DOBs (WCK 5153 and ZID), was found to act synergistically as a β-lactam enhancer. This is largely attributed to the ability of these combinations to target numerous PBPs. Nevertheless, nacubactam alone was proven to be effective against gram-negative bacteria such as E. coli, Klebsiella spp., Enterobacter spp., and Citrobacter spp. When coupled with β-lactams, its effectiveness is extended to most Enterobacteriaceae isolates generating ESBLs, AmpCs, KPCs, MBLs, OXA-48, as well as ESBL- and AmpC-producing Enterobacteriaceae that lack porins and strains of P. aeruginosa with reduced AmpC, PER, or VEB ESBLs [39]. Of interest, there is growing evidence that meropenem/nacubactam reduces bacterial burden in the lungs of neutropenic mice infected with AmpC and KPC-positive P. aeruginosa [40].
ETX1317
ETX1217 is a DBO serine β-lactam inhibitor. Despite lacking a β-lactam core, EXTA1217 was found to acylate much serine β-lactamases, thus deactivating them [41]. Therefore, it is classified as an antagonist of class A, C, and D serine-lactamases [42]. A combination of its oral prodrug (ETX0282) and cefpodoxime proxetil (an oral prodrug of a third-generation cephalosporin) was also found to improve the efficacy of several β-lactams against a variety of MDR Enterobacteria, including CREs [41].
In vitro, ETX1317 preserves cefpodoxime’s antibacterial activity against organisms immune to fluoroquinolones, cephalosporins, and carbapenems, including Enterobacterales [42]. Moreover, it was shown to be effective against drug-resistant isolates in preclinical infection models [41]. Therefore, ETX1317 has the potential to benefit both patients and the health system by decreasing the risk of nosocomial infections and minimizing the healthcare expenses associated with hospitalizations [42].
WCK
WCK 4234 is a new DBO with a nitrile side chain at the C-2 position; it has strong inhibitory action against carbapenemases of classes A and D, as well as class C enzymes [43]. In a study by Iregui et al, WCK 4234 increased carbapenem activity against isolates generating KPC, AmpC, and OXA-lactamases [44].
The combination of meropenem and WCK 4234 was effective in mouse models infected with carbapenem-hydrolyzing OXA-possessing A. baumannii [43]. Moreover, meropenem and WCK 4234 were shown to be highly effective against Enterobacteriaceae, including KPC-producing K. pneumoniae isolates [43, 44]. WCK 4234 also enhanced carbapenem activity against MDR A. baumannii expressing OXA-23, OXA-24/40, and OXA-58 carbapenemases and hyper-producing the chromosomal OXA-51 carbapenemases [44, 45]. Moreover, the combination of meropenem and WCK 4234 was effective in vivo against A. baumannii isolates generating OXA-23 or OXA-26 [44]. In vivo studies also showed an MIC for meropenem with WCK 4234 of 8 µg/mL against OXA-23 and OXA-26, indicating an eight-fold reduction when compared to meropenem alone (MIC: 64 µg/mL). The WCK 4234/meropenem combination was also found to be effective in the treatment of MDR A. baumannii-induced mouse peritonitis and neutropenic lung infection [45].
GT-055(GT-1/GT-055)
Many E. coli, K. pneumoniae, and Acinetobacter spp. MDR strains were shown to be susceptible to GT-1, a new siderophore cephalosporin [46, 47]. Some strains, however, have shown extremely high GT-1 MICs. Except for YMC2011/11/B144, non-susceptibility to GT-1 was frequently associated with the presence of AmpC-lactamase DHA-1, for which GT-1 MICs ranged from 4 to 64 g/mL. The high potency of the synergistic combination of GT-055 and GT-1 in the presence of -lactamases in CTX-M- (CTX-M-14, CTX-M-15, CTX-M-27, CTX-M-55, CTX-M-65), SHV- (SHV-12, SHV-83), DHA-1-, and SIM-1-producing strains is another feature of this novel antibiotic [47]. Additionally, GT-055 has inherent action against several Enterobacteriaceae isolates, which likely contributes to its effectiveness against E. coli and K. pneumoniae isolates when combined with GT-1 [47].
Boronic acid derived inhibitors
Taniborbactam (FEP/taniborbactam)
VNRX-5133 (taniborbactam), a bicyclic boronate, is a novel BLI under clinical testing. VNRX-5133 inhibits SBLs and some MBLs, such as NDM-1 and VIM-1/2 [48, 49]. However, the activity of VNRX-5133 against IMP-1 and tested B2/B3 MBLs was reduced or non-existent. Crystallographic findings show that bicyclic boronates have the capacity to block SBLs and MBLs by adding a tetrahedral (sp3) boron species [50]. Moreover, a study by Wang et al has shown that taniborbactam enhanced FEP activity in the same way that avibactam promoted ceftazidime efficiency against 66 KPC-2 producers, 30 carbapenem-non-susceptible Enterobacteriaceae, and 28 meropenem-susceptible P. aeruginosa. Of interest, FEP/taniborbactam was more effective than ceftazidime/avibactam against 56 ESBL-producing, 61 AmpC-producing, 32 ESBL and AmpC co-producing Enterobacteriaceae, 87 NDM-producing, and 21 MBL-producing Enterobacteriaceae predicted by phenotypic mCIM and eCIM, 82 Enterobacteriaceae that were sensitive to all tested β-lactams as well as 22 carbapenem-non-susceptible P. aeruginosa [49]. Taniborbactam concentrations needed to restore the activity of FEP were 4 mg/L for Enterobacterales, 32 mg/L for P. aeruginosa, 4 mg/L for E. coli, and 16 mg/L for K. pneumoniae [51].
A 4 mg/L taniborbactam was required to achieve > 90% Enterobacterales isolate susceptibility to FEP. However, taniborbactam failed to reach similar susceptibility against Pseudomonas since seven isolates showed resistance even at a concentration of 32 mg/L [51].
Resistant Enterobacterales and P. aeruginosa species were found to have increased expression of VIM and AmpC genes [52].
Human studies done to assess pharmacokinetics of taniborbactam showed no added adverse effects when compared to placebo when treated with doses of up 1,500 mg for a single dose, and a total of 2,250 mg/day for 10 days. Increased doses of taniborbactam were not associated with increased adverse effects. No adverse effects on heart cardio-dynamics were noted (including QTcF changes, heart rate and T-wave morphology) [53].
VNRX-5236 (ceftibuten (CTB)/VNRX-7145)
VNRX-5236 is a very powerful inhibitor of all four Ambler types of β-lactamase enzymes that is also extremely efficient in a wide spectrum of gram-negative bacteria using a cyclic boronate template. The N-(2-aminoethyl)cyclohexylamine side chain of 20 (VNRX-5133) was shown to be important for broad-spectrum β-lactamase inhibition as well as improved gram-negative outer membrane permeability and periplasmic accumulation [54].
The combination of CTB/VNRX-7154 showed similar potency level to ceftazidime-avibactam (IV) and meropenem-avibactam against Enterobacterales-producing SBLs, in in vitro studies. This combination offers an alternative path to treatment since it is readily orally available [55].
CTB/VNRX-5236 has demonstrated potent antibacterial activity against beta-lactamases-producing Enterobacterales including KPC, CTX-M-15, P99AmpC, CMY-2, OXA-1, and OXA-48 [56].
In vivo, efficacy of CTB/VNRX-5236 was assessed in mice injected with K. pneumoniae strain resistant to CTB. Results showed that CTB/VNRX-5326 delivered orally or subcutaneously was able to rescue CTB with median effective dose (ED50) values of 12.9 and 13.5 mg/kg, respectively [56].
QPX7728 (meropenem/QPX7728)
QPX7728 is a BLI that was developed as part of the boronic acid pharmacophore program. It was the first medication from this class to be approved by the FDA and the European Medicines Agency (EMA). When compared to the newly licensed drugs such as avibactam, vaborbactam and relebactam, QPX7728 has a wider beta-lactamase inhibitory spectrum. Drugs from this class are strong inhibitors of Enterobacteriaceae class A carbapenemases, such as KPC, class C beta-lactamases and, some class D enzymes [57, 58]. Of note, this class was the only class among the newly licensed drugs to show significant activity against Baumannii class D carbapenems such as OXA-23, OXA 24/40 and OXA 58 as well as the B1 subclass such as NDM, VIM, and IMP [58, 59]. Other drugs such as durlobactam have shown significant activity against the Acinetobacter OXA enzymes but were not capable of inhibiting class B MBLs. Furthermore, taniborbactam inhibits certain MBLs; however, it has no effect on Acinetobacter’s OXA carbapenemases. Therefore, QPX7728 is a promising candidate for future research because of its ultrabroad-spectrum beta-lactamase inhibitory profile. In summary, antibiotics with QPX7728 are effective against bacteria producing class A ESBLs (CTX-M, SHV, TEM, VEB, PER) and carbapenemases (KPC, SME, NMC-A, BKC-1). QPX7728 also inhibits both plasmid (CMY, FOX, MIR, DHA) and chromosomally encoded (P99, PDC, ADC) class C beta-lactamases and class D enzymes, including carbapenemases such as OXA-48 from Enterobacteriaceae and OXA enzymes from A. baumannii (OXA-23/24/72/58). Many class B MBLs (NDM, VIM, CcrA, IMP, and GIM, but not SPM or L1) are likewise inhibited by QPX7728 [58].
β-lactam derived inhibitors, penicillin sulfones
Enmetazobactam (AAI101) (FEP/enmetazobactam)
AAI101, a new drug from the penicillanic acid sulfone family, is an ESBL inhibitor in phase I clinical studies [60]. Its structure is like that of tazobactam with one significant variation; AAI101 has a strategically positioned methyl group, which provides the inhibitor with a net neutral charge allowing it to penetrate bacterial cells more effectively [61]. AAI101 has an inhibitory action against class A lactamases, including ESBLs, carbapenemases, and clavulanic acid-resistant lactamases [61]. Moreover, its addition to FEP has shown to regain its efficacy against an immune population of E. coli and K. pneumoniae [62]. This provides a possible carbapenem-free treatment regimen for infections caused by Enterobacteriaceae ESBLs [61]. In summary, the combination of FEP/emetazobactam provides a novel therapy option for challenging gram-negative bacteria during an age of high bacterial resistance and limited therapeutic alternatives [62].
LN-1-255
Buynak’s penicillin-based sulfone 1 (LN-1-255) has shown significantly more effectiveness than tazobactam and avibactam against relevant CHDLs in A. baumannii both plasmid-encoded OXA-23, OXA-24/40, OXA-58, OXA-143, and OXA-235, and chromosomally encoded OXA-51 as well as OXA-48 produced by K. pneumoniae. LN-1-255 increases imipenem’s in vitro activity by 32- to 128-fold and has high therapeutic effectiveness in vivo [63]. Its efficacy stems from its ability to create an indolizine adduct that is resistant to hydrolysis which is generated by nucleophilic attack of the pyridine nitrogen atom on the conjugated initial imine adduct after the dioxothiazolidine ring is opened [63].
Table 2 summarizes the therapies aimed at treating infections caused by carbapenem-resistant pathogens.
 Click to view | Table 2. Carbapenem-Resistant Pathogens and Their Therapies |
Figure 1 shows the chemical structures of the various carbapenemase inhibitors (biomodel.uah.es).
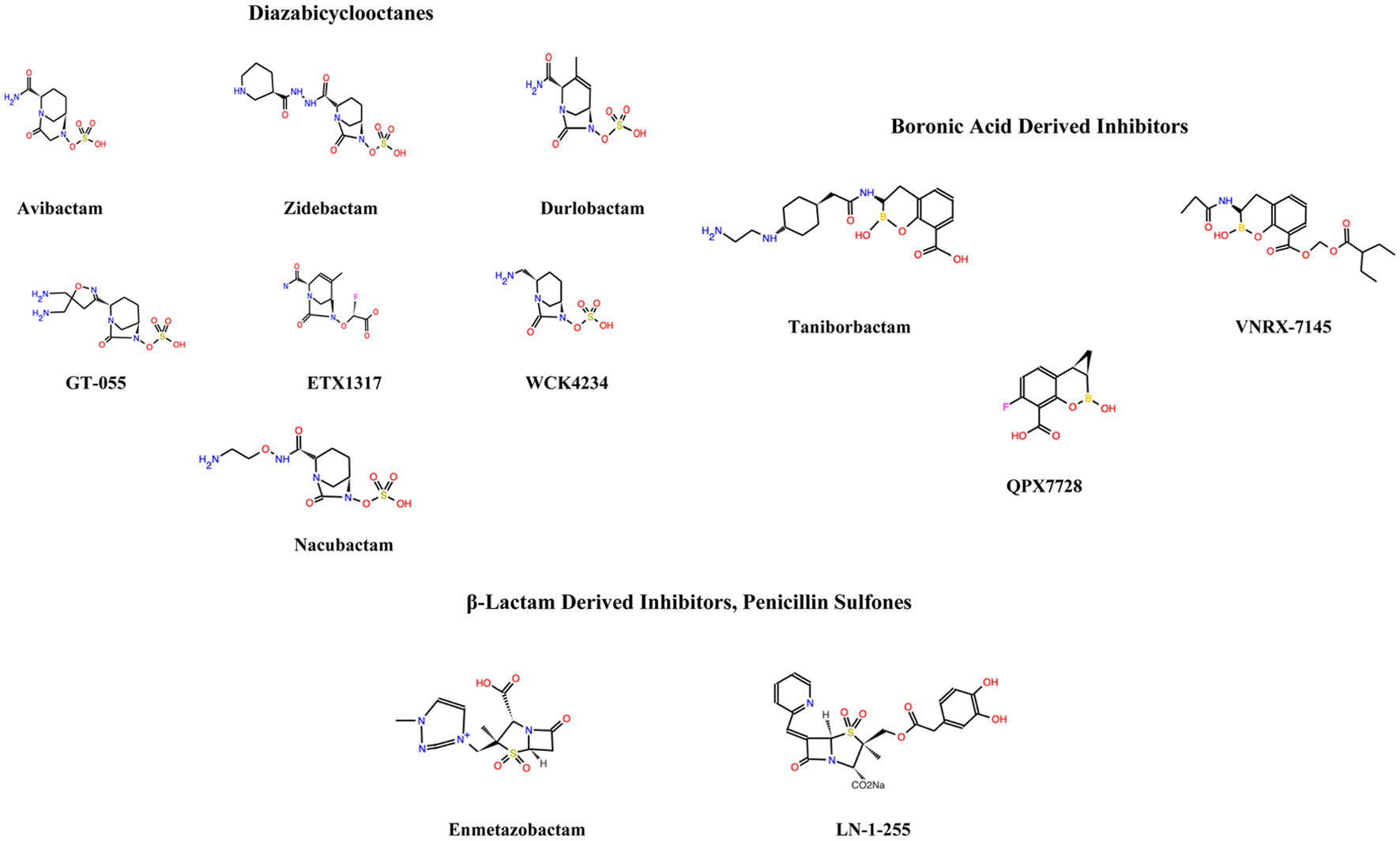 Click for large image | Figure 1. The chemical structures of the various carbapenemase inhibitors (biomodel.uah.es). |
| Conclusion | ▴Top |
Carbapenems, the most effective β-lactam antibiotics, display a broad spectrum of antibacterial activity. A carbapenem together with a β-lactam ring provides a great stability against hydrolysis by β-lactamases. These agents are mostly used as treatment against severe infections. Mediated by carbapenemases, carbapenem resistance drastically limits treatment options for gram-negative bacteria resistant to most β-lactams and/or all carbapenems. These pathogens often infer resistance to other antibiotics such as aminoglycosides and quinolones, which is problematic. Colistin and fosfomycin are usually the two antibiotics utilized in such scenarios, but their use has been limited due to their toxicity profile. Tigecycline has been utilized as a rescue therapy, but resistance is rapidly increasing as well [64, 65].
Finally, adequate antimicrobial stewardship programs and carbapenems-sparing strategies must be implemented in clinical settings to preserve the effectiveness of these antibiotics [66]. Appropriate infection control and prevention measures along with the rationale use of carbapenems could offset carbapenem resistance and provide us with time to develop new inhibitory molecules.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Author Contributions
MBZ drafted the manuscript. NZ conceived the idea for the paper. All authors contributed to the discussion section. All authors have read and approved the final manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Nordmann P, Poirel L. Epidemiology and diagnostics of carbapenem resistance in gram-negative bacteria. Clin Infect Dis. 2019;69(Suppl 7):S521-S528.
doi pubmed - Nordmann P, Poirel L. Strategies for identification of carbapenemase-producing Enterobacteriaceae. J Antimicrob Chemother. 2013;68(3):487-489.
doi pubmed - Ambler RP. The structure of beta-lactamases. Philos Trans R Soc Lond B Biol Sci. 1980;289(1036):321-331.
doi pubmed - Bush K, Jacoby GA. Updated functional classification of beta-lactamases. Antimicrob Agents Chemother. 2010;54(3):969-976.
doi pubmed - Naas T, Dortet L, Iorga BI. Structural and functional aspects of class a carbapenemases. Curr Drug Targets. 2016;17(9):1006-1028.
doi pubmed - Naas T, Vandel L, Sougakoff W, Livermore DM, Nordmann P. Cloning and sequence analysis of the gene for a carbapenem-hydrolyzing class A beta-lactamase, Sme-1, from Serratia marcescens S6. Antimicrob Agents Chemother. 1994;38(6):1262-1270.
doi pubmed - Rasmussen BA, Bush K, Keeney D, Yang Y, Hare R, O'Gara C, Medeiros AA. Characterization of IMI-1 beta-lactamase, a class A carbapenem-hydrolyzing enzyme from Enterobacter cloacae. Antimicrob Agents Chemother. 1996;40(9):2080-2086.
doi pubmed - Naas T, Nordmann P. Analysis of a carbapenem-hydrolyzing class A beta-lactamase from Enterobacter cloacae and of its LysR-type regulatory protein. Proc Natl Acad Sci U S A. 1994;91(16):7693-7697.
doi pubmed - Nordmann P, Naas T, Poirel L. Global spread of carbapenemase-producing enterobacteriaceae. Emerg Infect Dis. 2011;17(10):1791-1798.
doi pubmed - Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis. 2009;9(4):228-236.
doi - Queenan AM, Bush K. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev. 2007;20(3):440-458.
doi pubmed - Concha NO, Rasmussen BA, Bush K, Herzberg O. Crystal structure of the wide-spectrum binuclear zinc beta-lactamase from Bacteroides fragilis. Structure. 1996;4(7):823-836.
doi - Yang Y, Rasmussen BA, Bush K. Biochemical characterization of the metallo-beta-lactamase CcrA from Bacteroides fragilis TAL3636. Antimicrob Agents Chemother. 1992;36(5):1155-1157.
doi pubmed - Boyd SE, Holmes A, Peck R, Livermore DM, Hope W. OXA-48-like beta-lactamases: global epidemiology, treatment options, and development pipeline. Antimicrob Agents Chemother. 2022:e0021622.
doi pubmed - Philippon A, Arlet G, Jacoby GA. Plasmid-determined AmpC-type beta-lactamases. Antimicrob Agents Chemother. 2002;46(1):1-11.
doi pubmed - Lyon JA. Imipenem/cilastatin: the first carbapenem antibiotic. Drug Intell Clin Pharm. 1985;19(12):895-899.
doi - Poirel L, Heritier C, Tolun V, Nordmann P. Emergence of oxacillinase-mediated resistance to imipenem in Klebsiella pneumoniae. Antimicrob Agents Chemother. 2004;48(1):15-22.
doi pubmed - Figueiredo S, Poirel L, Seifert H, Mugnier P, Benhamou D, Nordmann P. OXA-134, a naturally occurring carbapenem-hydrolyzing class D beta-lactamase from Acinetobacter lwoffii. Antimicrob Agents Chemother. 2010;54(12):5372-5375.
doi pubmed - Higgins PG, Poirel L, Lehmann M, Nordmann P, Seifert H. OXA-143, a novel carbapenem-hydrolyzing class D beta-lactamase in Acinetobacter baumannii. Antimicrob Agents Chemother. 2009;53(12):5035-5038.
doi pubmed - Figueiredo S, Bonnin RA, Poirel L, Duranteau J, Nordmann P. Identification of the naturally occurring genes encoding carbapenem-hydrolysing oxacillinases from Acinetobacter haemolyticus, Acinetobacter johnsonii, and Acinetobacter calcoaceticus. Clin Microbiol Infect. 2012;18(9):907-913.
doi pubmed - Bonnin RA, Ocampo-Sosa AA, Poirel L, Guet-Revillet H, Nordmann P. Biochemical and genetic characterization of carbapenem-hydrolyzing beta-lactamase OXA-229 from Acinetobacter bereziniae. Antimicrob Agents Chemother. 2012;56(7):3923-3927.
doi pubmed - Higgins PG, Perez-Llarena FJ, Zander E, Fernandez A, Bou G, Seifert H. OXA-235, a novel class D beta-lactamase involved in resistance to carbapenems in Acinetobacter baumannii. Antimicrob Agents Chemother. 2013;57(5):2121-2126.
doi pubmed - Bouchet F, Atze H, Fonvielle M, Edoo Z, Arthur M, Etheve-Quelquejeu M, Iannazzo L. Diazabicyclooctane functionalization for inhibition of beta-lactamases from enterobacteria. J Med Chem. 2020;63(10):5257-5273.
doi pubmed - Coleman K. Diazabicyclooctanes (DBOs): a potent new class of non-beta-lactam beta-lactamase inhibitors. Curr Opin Microbiol. 2011;14(5):550-555.
doi pubmed - Shields RK, Nguyen MH, Chen L, Press EG, Potoski BA, Marini RV, Doi Y, et al. Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother. 2017;61(8):e00883-17.
doi pubmed - Shirley M. Ceftazidime-avibactam: a review in the treatment of serious gram-negative bacterial infections. Drugs. 2018;78(6):675-692.
doi pubmed - Ehmann DE, Jahic H, Ross PL, Gu RF, Hu J, Durand-Reville TF, Lahiri S, et al. Kinetics of avibactam inhibition against Class A, C, and D beta-lactamases. J Biol Chem. 2013;288(39):27960-27971.
doi pubmed - Niu S, Wei J, Zou C, Chavda KD, Lv J, Zhang H, Du H, et al. In vitro selection of aztreonam/avibactam resistance in dual-carbapenemase-producing Klebsiella pneumoniae. J Antimicrob Chemother. 2020;75(3):559-565.
doi pubmed - Livermore DM, Meunier D, Hopkins KL, Doumith M, Hill R, Pike R, Staves P, et al. Activity of ceftazidime/avibactam against problem Enterobacteriaceae and Pseudomonas aeruginosa in the UK, 2015-16. J Antimicrob Chemother. 2018;73(3):648-657.
doi pubmed - Lee M, Abbey T, Biagi M, Wenzler E. Activity of aztreonam in combination with ceftazidime-avibactam against serine- and metallo-beta-lactamase-producing Pseudomonas aeruginosa. Diagn Microbiol Infect Dis. 2021;99(1):115227.
doi pubmed - Biagi M, Lamm D, Meyer K, Vialichka A, Jurkovic M, Patel S, Mendes RE, et al. Activity of aztreonam in combination with avibactam, clavulanate, relebactam, and vaborbactam against multidrug-resistant stenotrophomonas maltophilia. Antimicrob Agents Chemother. 2020;64(12):e00297-20.
doi pubmed - Moya B, Barcelo IM, Bhagwat S, Patel M, Bou G, Papp-Wallace KM, Bonomo RA, et al. Potent beta-lactam enhancer activity of zidebactam and WCK 5153 against acinetobacter baumannii, including carbapenemase-producing clinical isolates. Antimicrob Agents Chemother. 2017;61(11):e01238-17.
doi pubmed - Moya B, Barcelo IM, Cabot G, Torrens G, Palwe S, Joshi P, Umarkar K, et al. In vitro and in vivo activities of beta-lactams in combination with the novel beta-lactam enhancers zidebactam and WCK 5153 against multidrug-resistant metallo-beta-lactamase-producing klebsiella pneumoniae. Antimicrob Agents Chemother. 2019;63(5):e00128-19.
doi pubmed - Moya B, Barcelo IM, Bhagwat S, Patel M, Bou G, Papp-Wallace KM, Bonomo RA, et al. WCK 5107 (Zidebactam) and WCK 5153 are novel inhibitors of PBP2 showing potent "beta-Lactam Enhancer" activity against pseudomonas aeruginosa, including multidrug-resistant metallo-beta-lactamase-producing high-risk clones. Antimicrob Agents Chemother. 2017;61(6):e02529-16.
doi pubmed - McLeod SM, Moussa SH, Hackel MA, Miller AA. In vitro activity of sulbactam-durlobactam against acinetobacter baumannii-calcoaceticus complex isolates collected globally in 2016 and 2017. Antimicrob Agents Chemother. 2020;64(4):e02534-19.
doi pubmed - Shapiro AB, Moussa SH, McLeod SM, Durand-Reville T, Miller AA. Durlobactam, a new diazabicyclooctane beta-lactamase inhibitor for the treatment of acinetobacter infections in combination with sulbactam. Front Microbiol. 2021;12:709974.
doi pubmed - Seifert H, Muller C, Stefanik D, Higgins PG, Miller A, Kresken M. In vitro activity of sulbactam/durlobactam against global isolates of carbapenem-resistant Acinetobacter baumannii. J Antimicrob Chemother. 2020;75(9):2616-2621.
doi pubmed - Mushtaq S, Vickers A, Woodford N, Haldimann A, Livermore DM. Activity of nacubactam (RG6080/OP0595) combinations against MBL-producing Enterobacteriaceae. J Antimicrob Chemother. 2019;74(4):953-960.
doi pubmed - Barnes MD, Taracila MA, Good CE, Bajaksouzian S, Rojas LJ, van Duin D, Kreiswirth BN, et al. Nacubactam enhances meropenem activity against carbapenem-resistant klebsiella pneumoniae producing KPC. Antimicrob Agents Chemother. 2019;63(8):e00432-19.
doi pubmed - Asempa TE, Motos A, Abdelraouf K, Bissantz C, Zampaloni C, Nicolau DP. Meropenem-nacubactam activity against AmpC-overproducing and KPC-expressing Pseudomonas aeruginosa in a neutropenic murine lung infection model. Int J Antimicrob Agents. 2020;55(2):105838.
doi pubmed - Miller AA, Shapiro AB, McLeod SM, Carter NM, Moussa SH, Tommasi R, Mueller JP. In vitro characterization of ETX1317, a broad-spectrum beta-lactamase inhibitor that restores and enhances beta-lactam activity against multi-drug-resistant enterobacteriales, including carbapenem-resistant strains. ACS Infect Dis. 2020;6(6):1389-1397.
doi pubmed - Durand-Reville TF, Comita-Prevoir J, Zhang J, Wu X, May-Dracka TL, Romero JAC, Wu F, et al. Discovery of an orally available diazabicyclooctane inhibitor (ETX0282) of class A, C, and D serine beta-lactamases. J Med Chem. 2020;63(21):12511-12525.
doi pubmed - Mushtaq S, Vickers A, Woodford N, Livermore DM. WCK 4234, a novel diazabicyclooctane potentiating carbapenems against enterobacteriaceae, pseudomonas and acinetobacter with class A, C and D beta-lactamases. J Antimicrob Chemother. 2017;72(6):1688-1695.
doi pubmed - Iregui A, Khan Z, Landman D, Quale J. Activity of meropenem with a novel broader-spectrum beta-lactamase inhibitor, WCK 4234, against gram-negative pathogens endemic to New York city. Antimicrob Agents Chemother. 2019;64(1):e01666-19.
doi pubmed - Papp-Wallace KM, Nguyen NQ, Jacobs MR, Bethel CR, Barnes MD, Kumar V, Bajaksouzian S, et al. Strategic approaches to overcome resistance against gram-negative pathogens using beta-lactamase inhibitors and beta-lactam enhancers: activity of three novel diazabicyclooctanes WCK 5153, zidebactam (WCK 5107), and WCK 4234. J Med Chem. 2018;61(9):4067-4086.
doi pubmed - Tooke CL, Hinchliffe P, Bragginton EC, Colenso CK, Hirvonen VHA, Takebayashi Y, Spencer J. beta-Lactamases and beta-Lactamase inhibitors in the 21st century. J Mol Biol. 2019;431(18):3472-3500.
doi pubmed - Nguyen LP, Pinto NA, Vu TN, Lee H, Cho YL, Byun JH, D'Souza R, et al. In vitro activity of a novel siderophore-cephalosporin, GT-1 and serine-type beta-lactamase inhibitor, GT-055, against escherichia coli, klebsiella pneumoniae and acinetobacter spp. Panel Strains. Antibiotics (Basel). 2020;9(5):267.
doi pubmed - Hamrick JC, Docquier JD, Uehara T, Myers CL, Six DA, Chatwin CL, John KJ, et al. VNRX-5133 (Taniborbactam), a broad-spectrum inhibitor of serine- and metallo-beta-lactamases, restores activity of cefepime in enterobacterales and pseudomonas aeruginosa. Antimicrob Agents Chemother. 2020;64(3):e01963-19.
doi pubmed - Wang X, Zhao C, Wang Q, Wang Z, Liang X, Zhang F, Zhang Y, et al. In vitro activity of the novel beta-lactamase inhibitor taniborbactam (VNRX-5133), in combination with cefepime or meropenem, against MDR Gram-negative bacterial isolates from China. J Antimicrob Chemother. 2020;75(7):1850-1858.
doi pubmed - Krajnc A, Brem J, Hinchliffe P, Calvopina K, Panduwawala TD, Lang PA, Kamps J, et al. Bicyclic boronate VNRX-5133 inhibits metallo- and serine-beta-lactamases. J Med Chem. 2019;62(18):8544-8556.
doi pubmed - Kloezen W, Melchers RJ, Georgiou PC, Mouton JW, Meletiadis J. Activity of cefepime in combination with the novel beta-lactamase inhibitor taniborbactam (VNRX-5133) against extended-spectrum-beta-lactamase-producing isolates in in vitro checkerboard assays. Antimicrob Agents Chemother. 2021;65(4):e02338-20.
doi pubmed - Piccirilli A, Segatore B, Brisdelli F, Amicosante G, Perilli M. Potent inhibitory activity of taniborbactam towards NDM-1 and NDM-1(Q119X) mutants, and in vitro activity of cefepime/taniborbactam against MBLs producing Enterobacterales. Int J Antimicrob Agents. 2021;57(1):106228.
doi pubmed - Dowell JA, Dickerson D, Henkel T. Safety and pharmacokinetics in human volunteers of taniborbactam (VNRX-5133), a novel intravenous beta-lactamase inhibitor. Antimicrob Agents Chemother. 2021;65(11):e0105321.
doi pubmed - Liu B, Trout REL, Chu GH, McGarry D, Jackson RW, Hamrick JC, Daigle DM, et al. Discovery of taniborbactam (VNRX-5133): a broad-spectrum serine- and metallo-beta-lactamase inhibitor for carbapenem-resistant bacterial infections. J Med Chem. 2020;63(6):2789-2801.
doi pubmed - Karlowsky JA, Hackel MA, Sahm DF. In vitro activity of ceftibuten/VNRX-5236 against urinary tract infection isolates of antimicrobial-resistant enterobacterales. Antimicrob Agents Chemother. 2022;66(1):e0130421.
doi pubmed - Chatwin CL, Hamrick JC, Trout REL, Myers CL, Cusick SM, Weiss WJ, Pulse ME, et al. Microbiological characterization of VNRX-5236, a broad-spectrum beta-lactamase inhibitor for rescue of the orally bioavailable cephalosporin ceftibuten as a carbapenem-sparing agent against strains of enterobacterales expressing extended-spectrum beta-lactamases and serine carbapenemases. Antimicrob Agents Chemother. 2021;65(8):e0055221.
doi pubmed - Lomovskaya O, Tsivkovski R, Sun D, Reddy R, Totrov M, Hecker S, Griffith D, et al. QPX7728, an ultra-broad-spectrum B-Lactamase inhibitor for intravenous and oral therapy: overview of biochemical and microbiological characteristics. Front Microbiol. 2021;12:697180.
doi pubmed - Lomovskaya O, Tsivkovski R, Nelson K, Rubio-Aparicio D, Sun D, Totrov M, Dudley MN. Spectrum of beta-lactamase inhibition by the cyclic boronate QPX7728, an ultrabroad-spectrum beta-lactamase inhibitor of serine and metallo-beta-lactamases: enhancement of activity of multiple antibiotics against isogenic strains expressing single beta-lactamases. Antimicrob Agents Chemother. 2020;64(6):e00212-20.
doi pubmed - Tsivkovski R, Totrov M, Lomovskaya O. Biochemical characterization of QPX7728, a new ultrabroad-spectrum beta-lactamase inhibitor of serine and metallo-beta-lactamases. Antimicrob Agents Chemother. 2020;64(6):e00130-20.
doi pubmed - Mushtaq S, Chaudhry A, Adkin R, Woodford N, Benedict N, Pypstra R, et al. In-vitro activity of diverse beta-lactam/aai101 combinations vs. Multidrug-resistant gram-negative clinical strains. Proceedings of the ECCMID, 2014. Available from: https://allecra.com/allecra2019/images/images/news/poster-18-2014-ECCMID_Poster-BLI-combinations-MDR-gram-negative.pdf.
- Papp-Wallace KM, Bethel CR, Caillon J, Barnes MD, Potel G, Bajaksouzian S, Rutter JD, et al. Beyond piperacillin-tazobactam: cefepime and AAI101 as a potent beta-lactam-beta-lactamase inhibitor combination. Antimicrob Agents Chemother. 2019;63(5):.
doi pubmed - Crandon JL, Nicolau DP. In vitro activity of cefepime/AAI101 and comparators against cefepime non-susceptible enterobacteriaceae. Pathogens. 2015;4(3):620-625.
doi pubmed - Rodriguez D, Maneiro M, Vazquez-Ucha JC, Beceiro A, Gonzalez-Bello C. 6-arylmethylidene penicillin-based sulfone inhibitors for repurposing antibiotic efficiency in priority pathogens. J Med Chem. 2020;63(7):3737-3755.
doi pubmed - Meletis G. Carbapenem resistance: overview of the problem and future perspectives. Ther Adv Infect Dis. 2016;3(1):15-21.
doi pubmed - Sader HS, Farrell DJ, Flamm RK, Jones RN. Variation in potency and spectrum of tigecycline activity against bacterial strains from U.S. medical centers since its approval for clinical use (2006 to 2012). Antimicrob Agents Chemother. 2014;58(4):2274-2280.
doi pubmed - Corcione S, Lupia T, Maraolo AE, Mornese Pinna S, Gentile I, De Rosa FG. Carbapenem-sparing strategy: carbapenemase, treatment, and stewardship. Curr Opin Infect Dis. 2019;32(6):663-673.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


