| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 14, Number 8, August 2022, pages 300-308
U-Shaped Relationship Between Proteinuria and High-Density Lipoprotein Cholesterol: Results of Cross-Sectional and Six Years Cohort Studies (KITCHEN-10)
Manami Igataa, Kei Nakajimaa, b, c, d
aSchool of Nutrition and Dietetics, Faculty of Health and Social Services, Kanagawa University of Human Services, Yokosuka 238-8522, Japan
bSaitama Medical Center, Department of Endocrinology and Diabetes, Saitama Medical University, Kawagoe 350-8550, Japan
cFood and Nutrition, Faculty of Human Sciences and Design, Japan Women’s University, Bunkyo-ku, Tokyo 112-8681, Japan
dCorresponding Author: Kei Nakajima, Food and Nutrition, Faculty of Human Sciences and Design, Japan Women’s University, Bunkyo-ku, Tokyo 112-8681, Japan
Manuscript submitted July 11, 2022, accepted August 13, 2022, published online August 27, 2022
Short title: Proteinuria and HDL Cholesterol
doi: https://doi.org/10.14740/jocmr4762
| Abstract | ▴Top |
Background: Although a very high level of high-density lipoprotein cholesterol (HDL-C) may be a potential cardiovascular disease risk factor, the detail and underlying mechanism remain unclear. Therefore, we examined the associations of serum HDL-C with the incidence of proteinuria, a predictor for cardiovascular disease, in a community-based study.
Methods: We investigated clinical parameters, including serum HDL-C and proteinuria, among 1,191,409 people aged 40 - 74 years who underwent a health checkup in a cross-sectional study. In the cohort study, the incidence of proteinuria after 6 years was investigated in 451,987 participants without proteinuria at baseline, who were simultaneously enrolled in the cross-sectional study.
Results: The prevalence of proteinuria showed a U-shaped relationship with 10 HDL-C categories, with a minimum of 60 - 89 mg/dL in the cross-sectional study. Logistic regression analysis showed similar U-shaped relationships between odds ratios for proteinuria and HDL-C categories, with a minimum of 70 - 79 mg/dL. The associations between very high HDL-C (≥ 90 mg/dL) and proteinuria were strengthened after adjustment for body mass index (BMI). In the cohort study, a crude L-shaped relationship was observed between the incidence of proteinuria and baseline HDL-C, which turned into U-shaped relationship after adjustment for baseline BMI and HDL-C after 6 years.
Conclusions: Low and very high levels of HDL-C may be associated with the incidence of proteinuria, and BMI may be a potent contributing factor to the underlying mechanism.
Keywords: High-density lipoprotein cholesterol; Proteinuria; Low-density lipoprotein cholesterol; Extremely high HDL-C; Body mass index; Big data
| Introduction | ▴Top |
In addition to kidney disease, proteinuria is considered a potent predictor for cardiovascular disease [1-3]. In the past two decades, the causes of proteinuria have been attributed to diabetes and hypertension [1, 4], which have been increasing worldwide. However, the association between proteinuria and dyslipidemia, especially in relation to high-density lipoprotein (HDL), which is a potential protector against atherosclerotic disease [5, 6], has been scarcely reported, although a low serum HDL cholesterol (HDL-C) level has been proposed as a risk factor for the incidence of proteinuria and kidney disease [7-9]. Reducing low-density lipoprotein cholesterol (LDL-C) levels with pharmacotherapy, a primary management for dyslipidemia, decreases cardiovascular disease risk, whereas a substantial residual risk remains after such treatment in most patients [10]. In line of this, low level of HDL-C is considered as a therapeutic target for the residual risk management [11].
On the other hand, a very high level of HDL-C may be also a potential cardiovascular disease and increased mortality risk factor [12-16]. Although the association between low HDL-C levels and proteinuria is reasonable, the association between very or extremely high levels of HDL-C and proteinuria has not been explored.
In recent years, we conducted large community-based studies [17, 18] showing that very or extremely high HDL-C levels, such as levels over 100 mg/dL or below 50 mg/dL, may be associated with diabetes and hypertension. These two diseases can impair kidney function over the time and eventually elicit proteinuria, mostly involving albuminuria [1, 4]. Therefore, we hypothesized that people with very high HDL-C may be at increased risk for the incident of proteinuria. In this study, to confirm this hypothesis and to elucidate the underlying mechanism, we investigated the association between levels of serum HDL-C and the prevalence of proteinuria in a community-based cross-sectional study as well as the association between the incidence of proteinuria after 6 years and baseline HDL-C levels in a cohort study (sub-analysis).
| Materials and Methods | ▴Top |
Study design and participants
We conducted a composite multidisciplinary study involving secondary health check data in Japan (Kanagawa Investigation of the Total Checkup Data from the National Database; KICHEN) to elucidate the factors primarily associated with cardiometabolic diseases. The overall study concept and design are described elsewhere [19]. The present study included all individuals who underwent a health check, which has been mandatory for Japan people aged 40 - 74 years old, and were living in Kanagawa Prefecture, the second most populated prefecture in Japan after Tokyo. This study was conducted in accordance with the Declaration of Helsinki, and the study protocol was approved by the Ethics Committee of Kanagawa University of Human Services (10-43) and the Ministry of Health, Labor, and Welfare of Japan (No. 121).
In the cross-sectional study, we retrospectively reviewed electronic record data for clinical parameters, including HDL-C and proteinuria assessed using dipstick urinalysis, in 1,209,118 people aged 40 - 74 years who underwent a health checkup between April 2008 and March 2009. In the 6-year cohort study (sub-analysis), the incidence of proteinuria between April 2014 and March 2015 was investigated among participants who did not have proteinuria at baseline (April 2008 and March 2009) and who underwent the same health checkup after 6 years. All participants in the cohort study were simultaneously enrolled in the cross-sectional study.
After excluding individuals with incomplete available clinical and lifestyle data, the data for 1,191,409 and 451,987 individuals were included in the cross-sectional and cohort studies. Individuals at moderate to severe conditions were unlikely to be enrolled in our studies because healthcare stuff in checkup institutions advised such people to go a medical institution immediately. We retrieved digitally recorded anonymous data from the Ministry of Health, Labor and Welfare of Japan, as part of its nationwide program involving the provision of medical data to third parties [20]. To protect against the identification of specific individuals, individuals’ ages had been categorized as 40 - 44, 45 - 49, 50 - 54, 55 - 59, 60 - 64, 65 - 69, and 70 - 74 years. In this study, however, to evaluate participant age as a single numeric value, we transformed age groups into substituted ages (s-age), corresponding to the median for each age group (42, 47, 52, 57, 62, 67, and 72 years, respectively).
Measurement of clinical parameters
Measurements were conducted in the morning after participants had completed an overnight fast. Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m2). Clinical parameters including proteinuria were measured using internal and external standards, as instructed to all health checkup institutes by the Ministry of Health, Labor and Welfare [19, 20]. Serum LDL-C, HDL-C, and triglyceride concentrations were measured automatically, mainly spectrophotometrically (using a direct, non-precipitation method) following rigorous instructions from the MHLW throughout the 6 years [21, 22].
Proteinuria with dipstick urinalysis was classified as one of five grades: none (-), trace (±), +1, +2, and ≥ +3. We defined proteinuria as ≥ +1. Dipstick urinalysis for midstream urine was conducted with visual reading by automated reading using a machine reader with reflectance photometry or by trained medical staff. Over half of dipstick urinalysis (around 57%) was interpreted with automated reading [19].
Data of estimated glomerular filtration rate (eGFR) and measurements related to urine specimens were unavailable in our database because the checkups were initially conducted to screen for metabolic syndrome [19, 20]; however, it is well known that metabolic syndrome and cardiovascular disease are closely associated with impaired kidney function [23, 24].
Statistical analysis
Data are expressed as mean ± standard deviation or median (interquartile range). Continuous and categorical variables were analyzed using analysis of variance (ANOVA) and the χ2 or Cochran-Armitage test. Logistic regression models were used to examine the associations between proteinuria and levels of HDL-C, with adjustment for relevant confounding factors including age, sex, pharmacotherapy (for hypertension, diabetes, and dyslipidemia), current smoking, alcohol consumption, regular exercise, serum triglyceride level, and BMI, yielding odds ratios (ORs) and 95% confidence intervals (CIs). Because alcohol consumption raises serum HDL-C levels [25, 26], we conducted the same analysis by restricting participants to those who consumed very small amounts of alcohol or no alcohol. Among all participants in the cross-sectional study, participants who did not have proteinuria at baseline were enrolled in the cohort study. In the cohort study, ORs for the incident of proteinuria were replaced with relative risks (RRs) when the percentage incidence of proteinuria was < 5% [27]. As in the cross-sectional study, confounding factors were chosen based on biological plausibility, but we did not follow a stepwise procedure to automatically select confounding factors. In the cohort study, the analysis was conducted for the whole study population, but it was not conducted according to sex because the number of participants was lower than the number in the cross-sectional study.
To consider the changes in the levels HDL-C during 6 years, which can be influenced by the regression towards the mean [28], the HDL-C after 6 years was finally included as a confounding factor in the multivariate logistic regression analysis. Statistical analyses were performed using SAS-Enterprise Guide (SAS-EG 7.1) in the SAS system, version 9.4 (SAS Institute, Cary, NC, USA). A two-tailed P < 0.05 was considered significant.
| Results | ▴Top |
Cross-sectional study
Mean HDL-C levels were 59.0 mg/dL in men and 70.6 mg/dL in women, and 37,640 (3.2%) participants had HDL-C ≥ 100 mg/dL. Overall, 45,959 (3.9%) participants had proteinuria, which was higher in men (4.7%) than women (2.8%; P < 0.0001, χ2 test).
Table 1 shows the clinical characteristics of participants according to serum HDL-C levels. Most parameters, including BMI, systolic blood pressure, and LDL-C, as well as the prevalence of current smokers and pharmacotherapy for hypertension, diabetes, or dyslipidemia, decreased with increasing HDL-C level whereas age increased (ANOVA and Cochran-Armitage test, all P < 0.0001). However, female sex, daily alcohol consumption, and the amount of alcohol consumed per session increased with increasing HDL-C level (Cochran-Armitage and χ2 test, all P < 0.001). In the total participants, the incidence of proteinuria showed a U-shaped relationship with 10 HDL-C categories, with a minimum of 80 - 89 mg/dL (2.7%).
 Click to view | Table 1. Clinical Characteristics of Participants |
Figure 1 shows the percentages of proteinuria in 10 HDL-C categories according to sex. A U-shaped relationship was more clearly visible in men than in women.
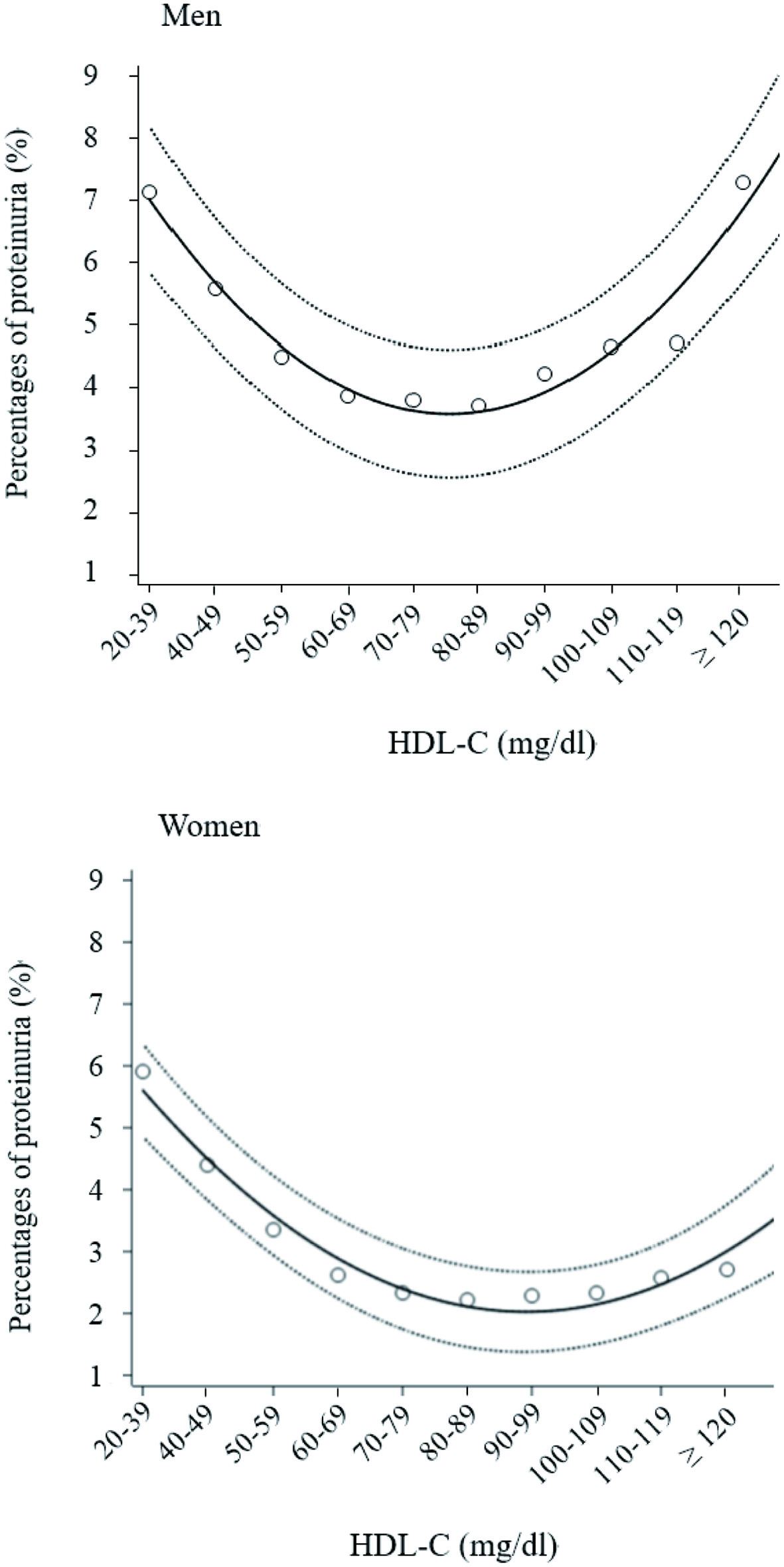 Click for large image | Figure 1. Percentages of proteinuria according to 10 high-density lipoprotein cholesterol (HDL-C) categories. Open circles indicate mean percentage of proteinuria in each HDL-C category, with proteinuria +1 and no proteinuria 0. Solid and dashed lines express quadratic regression curves and 95% confidence intervals, respectively. |
Multivariate logistic regression analysis showed a similar U-shaped relationship between ORs for proteinuria and HDL-C categories, with a minimum of 60 - 89 mg/dL (Fig. 2). Particularly in men, the associations between very high HDL-C (≥ 90 mg/dL) and proteinuria were strengthened after adjustment for confounding factors including serum triglyceride level and BMI, whereas the association between low HDL-C and proteinuria was attenuated.
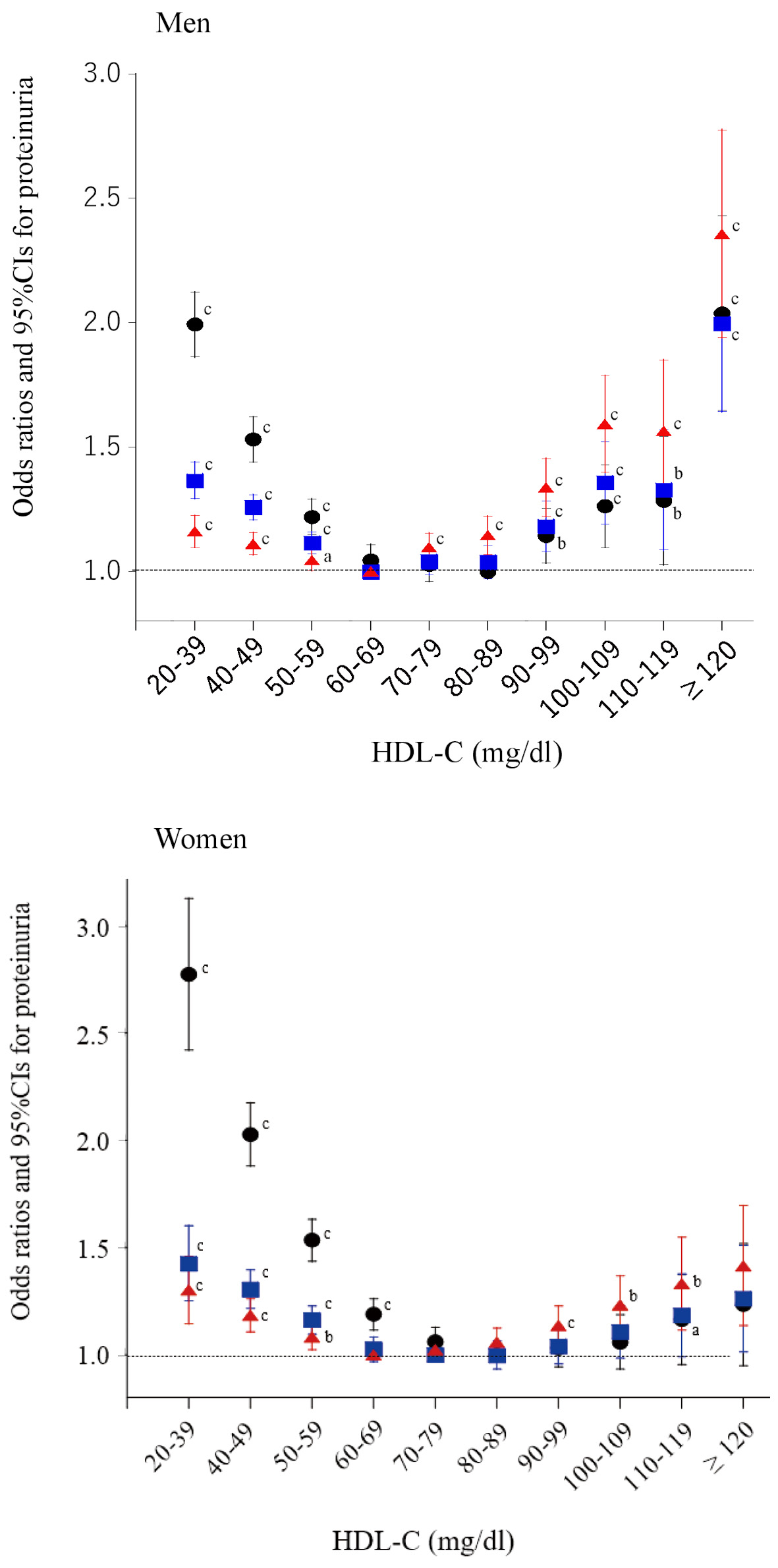 Click for large image | Figure 2. Odds ratios and 95% confidence intervals (CIs) for each category of high-density lipoprotein cholesterol (HDL-C) for proteinuria. Black circles: unadjusted; blue squares: adjusted for age, smoking, pharmacotherapy (hypertension, diabetes, and dyslipidemia), regular exercise, and serum triglyceride level; red triangles: further adjusted for body mass index. aP < 0.05, bP < 0.01, cP < 0.001. |
When we restricted subjects to those who drank < 23 g alcohol per session or who rarely drank alcohol, the percentages of proteinuria were decreased across the increasing HDL-C in both men and women (Table 2) (Cochran-Armitage test, both P < 0.0001). As Figure 3 shows the results of logistic regression analysis, the association between very high HDL-C (≥ 100 mg/dL) and proteinuria remained statistically significant, regardless of whether participants consumed less alcohol or infrequently drank alcohol. Overall, U-shaped associations were observed between proteinuria and HDL-C categories. Further sub-analysis after classification according to sex was not conducted because of the small sample size in the two highest HDL-C categories (n = 372 and 273).
 Click to view | Table 2. Prevalence of Proteinuria in Participants Without Habitual Alcohol Consumption |
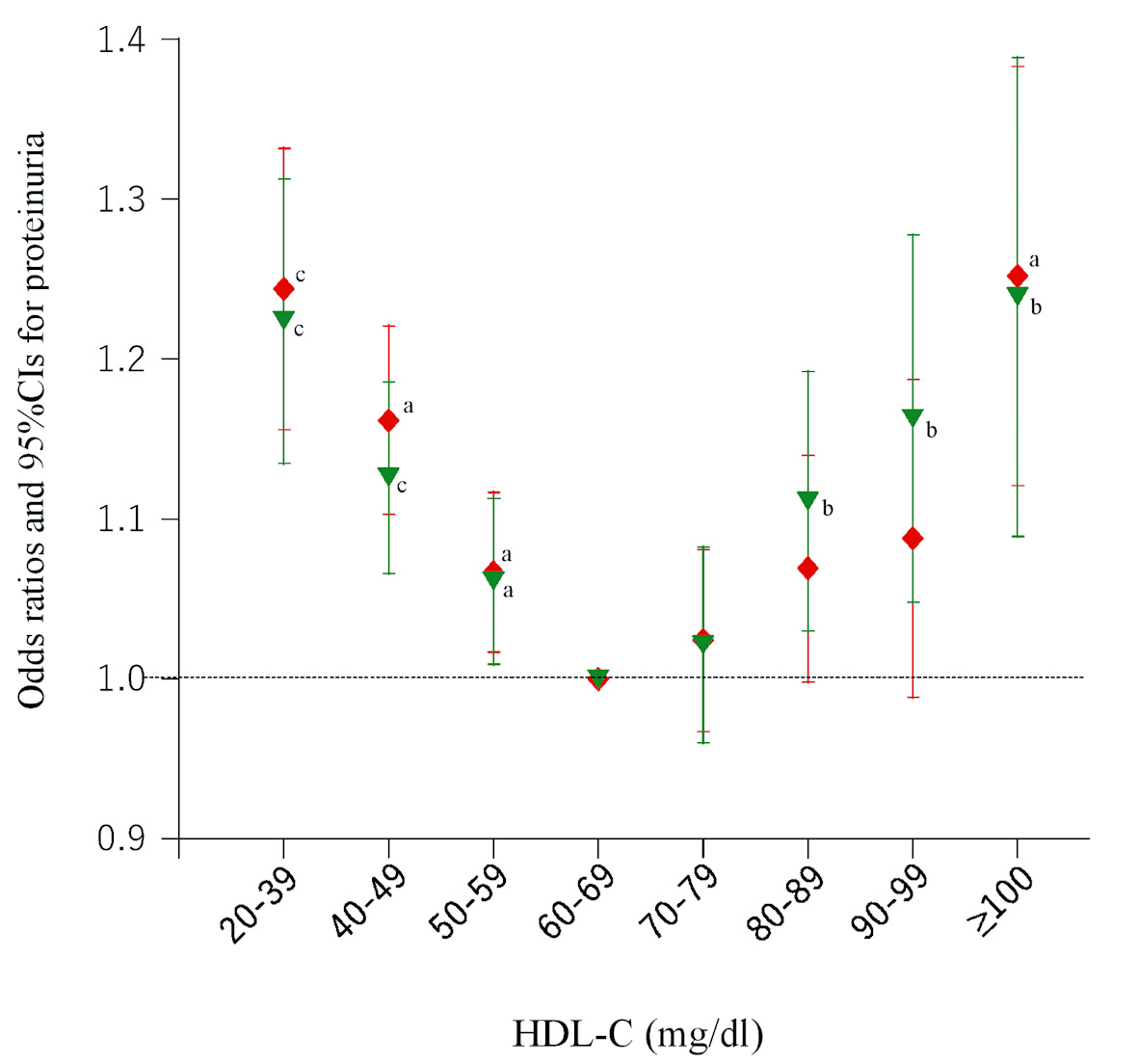 Click for large image | Figure 3. Odds ratios and 95% confidence intervals (CIs) for each category of high-density lipoprotein cholesterol (HDL-C) for proteinuria in participants without regular alcohol consumption. Red diamonds: participants who consumed low amounts of alcohol (< 23 g ethanol per session); green inverted triangles: participants who rarely or never drank alcohol. All odds ratios were adjusted for age, sex, smoking, pharmacotherapy (hypertension, diabetes, and dyslipidemia), regular exercise, serum triglyceride level, and body mass index. aP < 0.05, bP < 0.01, cP < 0.001. |
Cohort study
The incidence of proteinuria after 6 years was determined in 11,497 (2.5%) participants. An L-shaped relationship was observed between the incidence of proteinuria and the 10 baseline HDL-C categories (Table 3).
 Click to view | Table 3. Incidence of Proteinuria After 6 Years in 451,987 Participants Without Proteinuria at Baseline |
As shown in Figure 4, HDL-C increased over 6 years in men with baseline HDL-C < 60 mg/dL and in women with HDL-C < 70 mg/dL; HDL-C decreased in men with baseline HDL-C ≥ 70 mg/dL and in women with HDL-C ≥ 80 mg/dL. Actual levels of HDL-C after 6 years are shown according to baseline HDL-C categories here (Supplementary Material 1, www.jocmr.org). In the group of very high HDL-C (HDL-C ≥ 100 mg/dL) at baseline, 48.7% of male subjects and 55.1% of female subjects remained in the same HDL-C group.
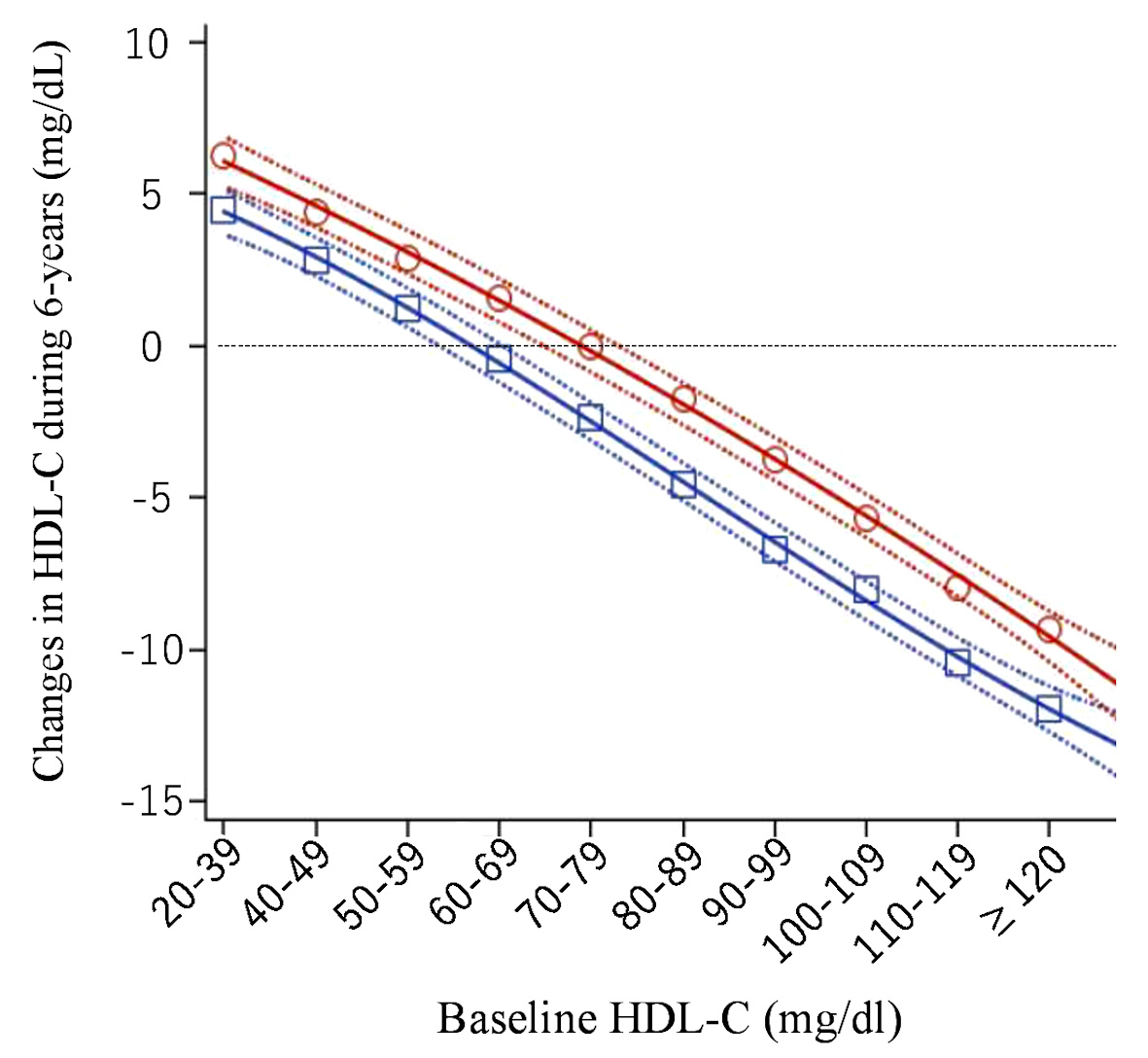 Click for large image | Figure 4. Changes in high-density lipoprotein cholesterol (HDL-C) over 6 years (mg/dL). Open red circles (women) and open blue squares (men) express mean change in HDL-C over 6 years in each baseline HDL-C category. Solid and dashed lines express quadratic regression curves and 95% confidence intervals for the averages, respectively. |
The RRs for incidence of proteinuria showed a similar L-shaped relationship with HDL-C categories (Fig. 5), which did not change after adjustment for confounding factors including serum triglyceride level. However, after further adjustment for BMI, the relationship changed to a blunt U-shaped relationship with HDL-C categories. Final model including HDL-C after 6 years as a confounding factor showed a small J-shaped relationship.
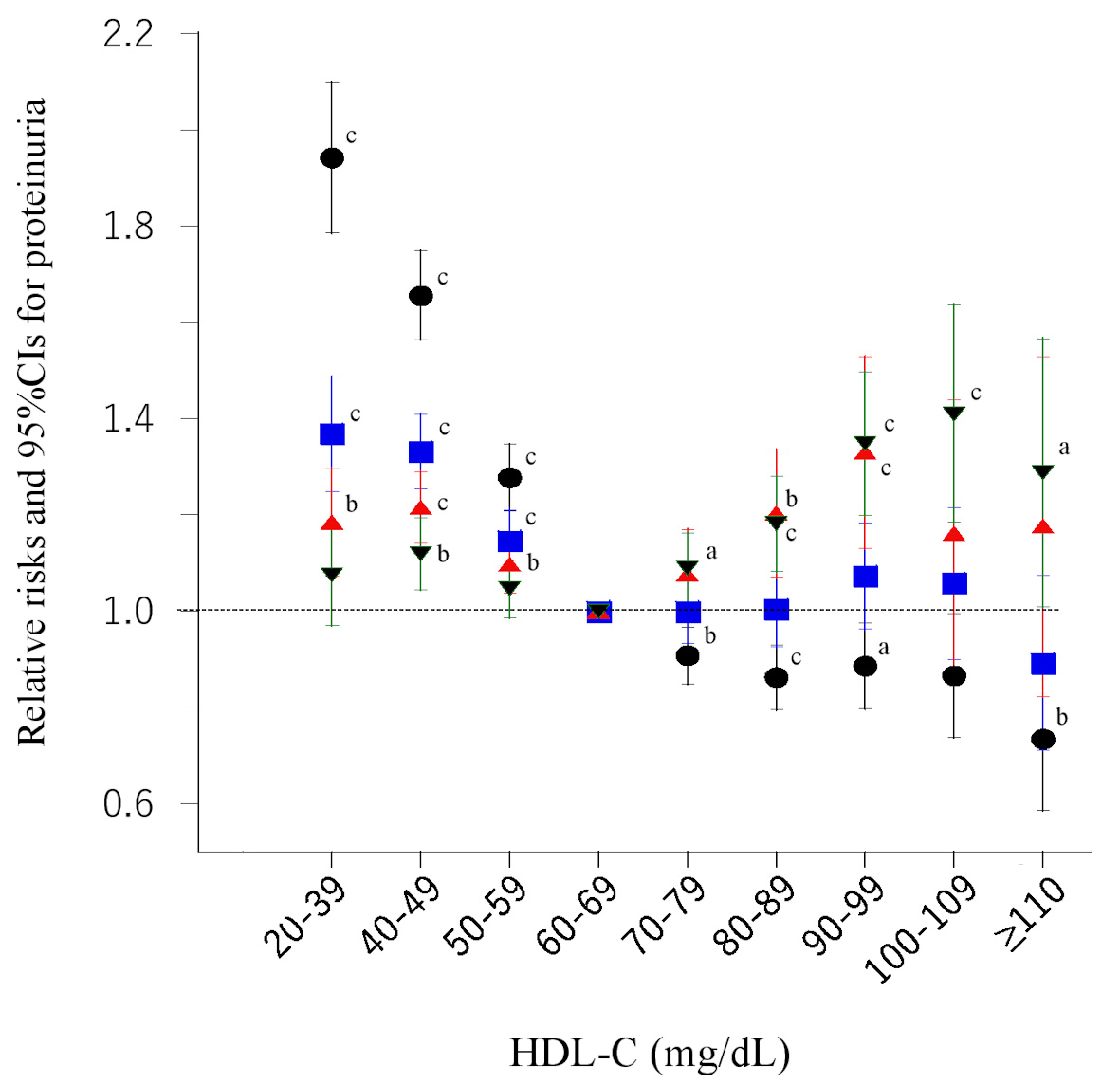 Click for large image | Figure 5. Risk ratios and 95% confidence intervals (CIs) for each category of baseline high-density lipoprotein cholesterol (HDL-C) for proteinuria. Black circles: unadjusted; blue squares: adjusted for age, smoking, pharmacotherapy (hypertension, diabetes, and dyslipidemia), regular exercise, and serum triglyceride level; red triangles: further adjusted for body mass index; green inverted triangles: further plus adjusted for HDL-C after 6 years. aP < 0.05, bP < 0.01, cP < 0.001. |
| Discussion | ▴Top |
It has been reported that microalbuminuria is observed in patients with diabetes who have low HDL-C levels [1, 7]. Additionally, higher levels of serum HDL-C have been shown to be associated with decreased rates of microalbuminuria in patients with type 2 diabetes [8]. However, the average HDL-C in the highest quartile group in that study was 56 mg/dL; this was not high in our study consisting of a mostly healthy general population with relatively high levels of HDL-C.
Our study is the first to show the relationship between a broad range of circulating HDL-C levels (20 to > 120 mg/dL) and proteinuria assessed using dipstick urinalysis. Our cross-sectional study consisted of 1 million people, which enabled us to classify participants into 10 HDL-C categories, namely, from very low levels to very or extremely high levels of HDL-C > 120 mg/dL, with adequate sample sizes and case (proteinuria) numbers even after further classification by sex.
Our results obtained from the cross-sectional study demonstrated a U-shaped relationship between the prevalence of proteinuria and HDL-C levels, which suggests that low (< 60 mg/dL) but also very high levels of HDL-C (≥ 90 or 100 mg/dL) are associated with proteinuria. These associations were not altered after adjustment for relevant confounding factors. Interestingly, the association between proteinuria and very high HDL-C was strengthened after adjustment for confounders particularly BMI, whereas the association was attenuated in cases of low HDL-C.
In our and other previous studies [29, 30], a J-shaped association was found between the incidence of proteinuria and BMI, suggesting that obesity, but also low body weight, can lead to incident proteinuria. In the current study, obesity accompanied with high serum triglyceride level was more prevalent in the categories of low HDL-C whereas low body weight accompanied with relatively low serum triglyceride level was more prevalent in the categories of very high HDL-C. Thus, the association between proteinuria and low HDL-C may be largely dependent on higher BMI, a potent background factor, whereas the observed association between proteinuria and very high HDL-C may be independent of lower BMI. Taken these together, it is possible that BMI is a potent contributing factor to the underlying mechanism. However, it is unknown whether this hypothesis is applicable to other populations with larger BMI such as Western populations.
In sub-analysis in the 6 year-cohort study, we found that very high and extremely high HDL-C at baseline decreased over 6 years (up to -12 mg/dL in men) whereas HDL-C increased with low HDL-C (up to 6 mg/dL in women) mostly due to the phenomenon of regression towards the mean [28].
Because the effect of alcohol on HDL-C appears to be reversible [25], elevated HDL-C owing to transient high alcohol consumption at baseline may be reduced by an improved lifestyle, including reduced alcohol consumption. However, this was not confirmed in this study.
Under crude conditions, an L-shaped relationship between proteinuria and HDL-C was observed, which transformed into a blunt U-shaped after adjustment for BMI. This change suggests that BMI may be a pivotal contributor to the underlying mechanism. The most plausible reason for the L-shaped and blunt U-shaped association in the cohort study, rather than the clear U-shaped association in the cross-sectional study, is that the 6-year study period may have been too short to evaluate the incidence of proteinuria in participants with very high HDL-C. Additionally, the decrease in HDL-C in the categories of very high HDL-C may attenuate the effect of very high HDL-C. Nevertheless, final adjustment for HDL-C after 6 years showed relatively distinct U-shaped relationship, which may attribute to the attenuation of changes in HDL-C during 6 years particularly in the high HDL-C groups.
In recent years, we have demonstrated the association of extremely high HDL-C, mostly defined as > 100 mg/dL, with diabetes [17], high blood pressure [18], and hypertensive retinopathy [31]. All of these are well-known risk factors for the incidence of kidney disease and proteinuria [1, 2, 4]. Considering these previous studies, the current results are not surprising and were somewhat expected. Furthermore, in the past decade, several studies have shown that extremely high HDL-C is associated with elevated mortality [12-16]. Additionally, increased HDL-C owing to the use of cholesteryl ester transfer protein (CETP) inhibitors does not protect against the incidence of cardiovascular disease or mortality [32-35]. Although genetic variation in CETP is a major determination for high HDL-C in Japan [36, 37], such genetic information was not investigated in this study.
In contrast, habitual alcohol consumption, an acquired lifestyle, raises serum HDL-C levels [25, 26]. The amount and frequency of alcohol consumption were higher in groups with higher HDL-C levels in this study. However, the association between proteinuria and very high HDL-C remained statistically significant in participants who drank lower amounts of alcohol or who drank infrequently, although the association of very high level of HDL-C with proteinuria was attenuated. Therefore, the current results suggest that alcohol consumption cannot fully explain the mechanism underlying the association of very high HDL-C levels with proteinuria.
Taken together, our results indicate that the most plausible explanation for the association between very high HDL-C and proteinuria is that the quality of HDL (in other words, a favorable function of HDL for cardiometabolic conditions [38-40]) rather than circulating HDL-C concentration [41] may be impaired in some people with very high levels of HDL-C, although there are no standardized assays to evaluate HDL function. Alternatively, very high HDL-C may be a marker of specific conditions related to cardiovascular diseases, which warrants further study.
Limitations
Several limitations should be mentioned in this study. First, proteinuria assessed using dipstick urinalysis may have limited usefulness in clinical settings [42, 43], particularly because the term proteinuria does not always reflect albuminuria, a major outcome of kidney disease. Additionally, other information concerning urine specimens including urine protein creatinine ratio, hematuria, and urine specific gravity, was unavailable. However, in this study, over half of dipstick urinalysis was performed with automated reading, which can eliminate potential sources of error commonly observed in manual performance [44]. Second, a lack of data for eGFR hampered our speculation about the mechanisms underlying the relationship between HDL-C and proteinuria. Third, factors related to the function of HDL, such as apolipoprotein (apo) A-I, apo A-II, HDL-2, and HDL-3 [41, 45, 46], were also unknown in this study. Therefore, it is unknown whether dysfunctional HDL exists in people with very high HDL-C. Finally, the cause-effective relationship between HDL-C and proteinuria remains unknown in this study, although a retrospective cohort study was also conducted. Particularly very high HDL-C might reflect some unfavorable conditions for the incident of proteinuria regardless of the direct causality. Taken these limitations into consideration, further studies are required to confirm current results.
Conclusions
Low and very high levels of HDL-C may be associated with a high proportion of proteinuria and the incidence of proteinuria. BMI may be a potent contributing factor to the underlying mechanism.
| Supplementary Material | ▴Top |
Suppl 1. HDL-C after 6 years according to baseline HDL-C categories.
Acknowledgments
None to declare.
Financial Disclosure
This research received no external funding.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Informed consent was not required because of anonymous data from the MHLW of Japan, as part of its nationwide program involving the provision of medical data to third parties. Instead, we have opened the study protocol online (https://www.kuhs.ac.jp/research/nationaldatabase/).
Author Contributions
KN and MI contributed to the overall study design, the interpretation of the initial analysis, and the discussion of the literature. KN prepared the first draft of the manuscript, and both authors read and approved the manuscript. Both authors agreed to the published version of the manuscript.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
| References | ▴Top |
- Haider MZ, Aslam A. Proteinuria. In: StatPearls. Treasure Island (FL), 2022.
- Kim YE, Lee M, Lee YH, Kang ES, Cha BS, Lee BW. Proteinuria as a significant predictive factor for the progression of carotid artery atherosclerosis in non-albuminuric type 2 diabetes. Diabetes Res Clin Pract. 2021;181:109082.
doi pubmed - Oeun B, Hikoso S, Nakatani D, Mizuno H, Suna S, Kitamura T, Okada K, et al. Prognostic significance of dipstick proteinuria in heart failure with preserved ejection fraction: insight from the PURSUIT-HFpEF registry. BMJ Open. 2021;11(9):e049371.
doi pubmed - Weinstock Brown W, Keane WF. Proteinuria and cardiovascular disease. Am J Kidney Dis. 2001;38(4 Suppl 1):S8-S13.
doi pubmed - Feig JE, Hewing B, Smith JD, Hazen SL, Fisher EA. High-density lipoprotein and atherosclerosis regression: evidence from preclinical and clinical studies. Circ Res. 2014;114(1):205-213.
doi pubmed - Bailey A, Mohiuddin SS. Biochemistry, High Density Lipoprotein. In: StatPearls. Treasure Island (FL), 2022.
- Bulum T, Duvnjak L, Prkacin I. [Lower levels of HDL2 cholesterol are associated with microalbuminuria in patients with type 1 diabetes]. Acta Med Croatica. 2011;65(3):243-250.
- Sun X, Xiao Y, Li PM, Ma XY, Sun XJ, Lv WS, Wu YL, et al. Association of serum high-density lipoprotein cholesterol with microalbuminuria in type 2 diabetes patients. Lipids Health Dis. 2018;17(1):229.
doi pubmed - Kawachi K, Kataoka H, Manabe S, Mochizuki T, Nitta K. Low HDL cholesterol as a predictor of chronic kidney disease progression: a cross-classification approach and matched cohort analysis. Heart Vessels. 2019;34(9):1440-1455.
doi pubmed - Kon V, Yang H, Fazio S. Residual cardiovascular risk in chronic kidney disease: role of high-density lipoprotein. Arch Med Res. 2015;46(5):379-391.
doi pubmed - Ozaki Y, Tanaka A, Nishiguchi T, Komukai K, Taruya A, Satogami K, Kashiwagi M, et al. High-density lipoprotein cholesterol as a therapeutic target for residual risk in patients with acute coronary syndrome. PLoS One. 2018;13(7):e0200383.
doi pubmed - Ko DT, Alter DA, Guo H, Koh M, Lau G, Austin PC, Booth GL, et al. High-density lipoprotein cholesterol and cause-specific mortality in individuals without previous cardiovascular conditions: the CANHEART study. J Am Coll Cardiol. 2016;68(19):2073-2083.
doi pubmed - Madsen CM, Varbo A, Nordestgaard BG. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: two prospective cohort studies. Eur Heart J. 2017;38(32):2478-2486.
doi pubmed - Hirata A, Sugiyama D, Watanabe M, Tamakoshi A, Iso H, Kotani K, Kiyama M, et al. Association of extremely high levels of high-density lipoprotein cholesterol with cardiovascular mortality in a pooled analysis of 9 cohort studies including 43,407 individuals: The EPOCH-JAPAN study. J Clin Lipidol. 2018;12(3):674-684.e675.
doi pubmed - Li X, Guan B, Wang Y, Tse G, Zou F, Khalid BW, Xia Y, et al. Association between high-density lipoprotein cholesterol and all-cause mortality in the general population of northern China. Sci Rep. 2019;9(1):14426.
doi pubmed - Huang YQ, Liu XC, Lo K, Liu L, Yu YL, Chen CL, Huang JY, et al. The U shaped relationship between high-density lipoprotein cholesterol and all-cause or cause-specific mortality in adult population. Clin Interv Aging. 2020;15:1883-1896.
doi pubmed - Nakajima K, Higuchi R, Iwane T, Shibata M, Takada K, Sugiyama M, Matsuda M, et al. High incidence of diabetes in people with extremely high high-density lipoprotein cholesterol: results of the Kanagawa investigation of total checkup data from the national database-1 (KITCHEN-1). J Clin Med. 2019;8(3):381.
doi pubmed - Nakajima K, Igata M, Higuchi R, Tanaka K, Mizusawa K, Nakamura T. Association of serum high-density lipoprotein cholesterol with high blood pressures at checkup: results of Kanagawa investigation of total checkup data from the national database-9 (KITCHEN-9). J Clin Med. 2021;10(21):5118.
doi pubmed - Nakajima K, Iwane T, Higuchi R, Shibata M, Takada K, Uda J, Anan M, et al. Kanagawa Investigation of the Total Check-up Data from the National database (KITCHEN): protocol for data-driven population-based repeated cross-sectional and 6-year cohort studies. BMJ Open. 2019;9(2):e023323.
doi pubmed - Ministry of Health, Labour and Welfare. Health examination and guidance program for Japanese adults. 2008. Available online: https://www.mhlw.go.jp/bunya/shakaihosho/iryouseido01/info02a.html. Accessed on April 8, 2022.
- Okada M, Matsui H, Ito Y, Fujiwara A. Direct measurement of HDL cholesterol: method eliminating apolipoprotein E-rich particles. J Clin Lab Anal. 2001;15(4):223-229.
doi pubmed - Miller WG, Myers GL, Sakurabayashi I, Bachmann LM, Caudill SP, Dziekonski A, Edwards S, et al. Seven direct methods for measuring HDL and LDL cholesterol compared with ultracentrifugation reference measurement procedures. Clin Chem. 2010;56(6):977-986.
doi pubmed - Nashar K, Egan BM. Relationship between chronic kidney disease and metabolic syndrome: current perspectives. Diabetes Metab Syndr Obes. 2014;7:421-435.
doi pubmed - Prasad GV. Metabolic syndrome and chronic kidney disease: Current status and future directions. World J Nephrol. 2014;3(4):210-219.
doi pubmed - Flegal KM, Cauley JA. Alcohol consumption and cardiovascular risk factors. Recent Dev Alcohol. 1985;3:165-180.
doi pubmed - Rosales C, Gillard BK, Gotto AM, Jr., Pownall HJ. The Alcohol-High-Density Lipoprotein Athero-Protective Axis. Biomolecules. 2020;10(7):987.
doi pubmed - Zocchetti C, Consonni D, Bertazzi PA. Relationship between prevalence rate ratios and odds ratios in cross-sectional studies. Int J Epidemiol. 1997;26(1):220-223.
doi pubmed - Bland JM, Altman DG. Some examples of regression towards the mean. BMJ. 1994;309(6957):780.
doi pubmed - Sato Y, Fujimoto S, Konta T, Iseki K, Moriyama T, Yamagata K, Tsuruya K, et al. U-shaped association between body mass index and proteinuria in a large Japanese general population sample. Clin Exp Nephrol. 2014;18(1):75-86.
doi pubmed - Muneyuki T, Sugawara H, Suwa K, Oshida H, Saito M, Hori Y, Seta S, et al. A community-based cross-sectional and longitudinal study uncovered asymptomatic proteinuria in Japanese adults with low body weight. Kidney Int. 2013;84(6):1254-1261.
doi pubmed - Nakajima K, Higuchi R, Mizusawa K, Nakamura T. Association between extremely high high-density lipoprotein-cholesterol and hypertensive retinopathy: results of a cross-sectional study from Kanagawa Investigation of Total Checkup Data from the National Database-6 (KITCHEN-6). BMJ Open. 2021;11(5):e043677.
doi pubmed - Riaz H, Khan SU, Rahman H, Shah NP, Kaluski E, Lincoff AM, Nissen SE. Effects of high-density lipoprotein targeting treatments on cardiovascular outcomes: A systematic review and meta-analysis. Eur J Prev Cardiol. 2019;26(5):533-543.
doi pubmed - Whyte MB. Is high-density lipoprotein a modifiable treatment target or just a biomarker for cardiovascular disease? JRSM Cardiovasc Dis. 2019;8:2048004019869736.
doi pubmed - Li C, Zhang W, Zhou F, Chen C, Zhou L, Li Y, Liu L, et al. Cholesteryl ester transfer protein inhibitors in the treatment of dyslipidemia: a systematic review and meta-analysis. PLoS One. 2013;8(10):e77049.
doi pubmed - Filippatos TD, Klouras E, Barkas F, Elisaf M. Cholesteryl ester transfer protein inhibitors: challenges and perspectives. Expert Rev Cardiovasc Ther. 2016;14(8):953-962.
doi pubmed - Nagano M, Yamashita S, Hirano K, Takano M, Maruyama T, Ishihara M, Sagehashi Y, et al. Molecular mechanisms of cholesteryl ester transfer protein deficiency in Japanese. J Atheroscler Thromb. 2004;11(3):110-121.
doi pubmed - Yokoyama S. Unique features of high-density lipoproteins in the Japanese: in population and in genetic factors. Nutrients. 2015;7(4):2359-2381.
doi pubmed - Ragbir S, Farmer JA. Dysfunctional high-density lipoprotein and atherosclerosis. Curr Atheroscler Rep. 2010;12(5):343-348.
doi pubmed - Imaizumi S, Navab M, Morgantini C, Charles-Schoeman C, Su F, Gao F, Kwon M, et al. Dysfunctional high-density lipoprotein and the potential of apolipoprotein A-1 mimetic peptides to normalize the composition and function of lipoproteins. Circ J. 2011;75(7):1533-1538.
doi pubmed - Salazar J, Olivar LC, Ramos E, Chavez-Castillo M, Rojas J, Bermudez V. Dysfunctional High-Density Lipoprotein: An Innovative Target for Proteomics and Lipidomics. Cholesterol. 2015;2015:296417.
doi pubmed - Fogacci F, Borghi C, Cicero AFG. New evidences on the association between high-density lipoprotein cholesterol and cardiovascular risk: a never ending research story. Eur J Prev Cardiol. 2022;29(5):842-843.
doi pubmed - Waugh JJ, Clark TJ, Divakaran TG, Khan KS, Kilby MD. Accuracy of urinalysis dipstick techniques in predicting significant proteinuria in pregnancy. Obstet Gynecol. 2004;103(4):769-777.
doi pubmed - Kavuru V, Vu T, Karageorge L, Choudhury D, Senger R, Robertson J. Dipstick analysis of urine chemistry: benefits and limitations of dry chemistry-based assays. Postgrad Med. 2020;132(3):225-233.
doi pubmed - Kight E, Hussain I, Bowden AK. Low-Cost, Volume-Controlled Dipstick Urinalysis for Home-Testing. J Vis Exp. 20211;71:e61406.
- Fruchart JC, Ailhaud G. Apolipoprotein A-containing lipoprotein particles: physiological role, quantification, and clinical significance. Clin Chem. 1992;38(6):793-797.
doi - Morgan J, Carey C, Lincoff A, Capuzzi D. High-density lipoprotein subfractions and risk of coronary artery disease. Curr Atheroscler Rep. 2004;6(5):359-365.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


