| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 14, Number 5, May 2022, pages 202-208
No Significant Association Between Plasma Endosialin Levels and the Presence or Severity of Coronary Artery Disease
Yoshimi Kishimotoa, Masayuki Aoyamab, Emi Saitac, Reiko Ohmorid, Kojiro Tanimotob, Kazuo Kondoe, Yukihiko Momiyamab, f
aDepartment of Food Science and Human Nutrition, Setsunan University, Osaka, Japan
bDepartment of Cardiology, National Hospital Organization Tokyo Medical Center, Tokyo, Japan
cResearch Institute of Environmental Medicine, Nagoya University, Aichi, Japan
dFaculty of Regional Design, Utsunomiya University, Tochigi, Japan
eOchanomizu University, Tokyo, Japan
fCorresponding Author: Yukihiko Momiyama, Department of Cardiology, National Hospital Organization Tokyo Medical Center, Meguro-ku, Tokyo 152-8902, Japan
Manuscript submitted April 25, 2022, accepted May 25, 2022, published online May 31, 2022
Short title: Plasma Endosialin Levels in CAD
doi: https://doi.org/10.14740/jocmr4730
| Abstract | ▴Top |
Background: Endosialin, also called tumor endothelial marker-1 or CD248, is a transmembrane glycoprotein that is suggested to play a role in inflammation as well as tumor progression. Endosialin expression was also reported to be upregulated in human atherosclerotic lesions. However, no study has reported blood endosialin levels in patients with coronary artery disease (CAD).
Methods: We investigated the association between plasma endosialin levels and the presence or severity of CAD in 376 patients who underwent elective coronary angiography for suspected CAD. The severity of CAD was represented as the numbers of stenotic coronary vessels and segments.
Results: Of the 376 study patients, CAD was found in 210 patients (one-vessel disease (1-VD), n = 90; two-vessel disease (2-VD), n = 65; and three-vessel disease (3-VD), n = 55). Compared with 166 patients without CAD, 210 patients with CAD had higher C-reactive protein (CRP) levels (median 0.57 vs. 0.43 mg/L, P = 0.007). However, endosialin levels did not significantly differ between patients with and without CAD (0.91 vs. 0.92 ng/mL, P = 0.693). A stepwise increase in CRP levels was found depending on the number of > 50% stenotic vessels: 0.43 in CAD(-), 0.52 in 1-VD, 0.57 in 2-VD, and 0.58 mg/L in 3-VD (P = 0.019). No marked difference was found in endosialin levels among four groups of CAD(-), 1-VD, 2-VD, and 3-VD (0.92, 0.89, 0.98, and 0.87 ng/mL, respectively, P = 0.785). Moreover, no significant correlation was found between endosialin levels and the numbers of > 50% and > 25% stenotic segments or CRP levels. In multivariate analysis, endosialin levels were not a significant factor associated with CAD independent of atherosclerotic risk factors.
Conclusions: Plasma endosialin levels in patients with CAD were found to be not higher than in those without CAD and to be not significantly associated with the presence or severity of CAD.
Keywords: Atherosclerosis; Biomarker; Coronary artery disease; C-reactive protein; Endosialin; Inflammation
| Introduction | ▴Top |
Endosialin, also called tumor endothelial marker-1 (TEM-1) or CD248, is a 165-kDa transmembrane glycoprotein that is comprised of a N-terminal extracellular domain, a Sushi domain and three epidermal growth factor (EGF)-like domains and that was initially identified on the blood vessels of tumors and was recognized as an abnormally expressed marker of tumor vascular endothelial cells [1-3]. However, subsequent studies reported that endosialin is not expressed by endothelial cells but by perivascular cells, such as vascular smooth muscle cells, fibroblasts, and CD8 T cells [3-5]. Endosialin expression is also low in normal perivascular cells. During inflammation and tumor progression, endosialin was shown to be upregulated in perivascular cells, adipocytes and some tumor cells [6-8]. In colon cancer, silencing endosialin inhibited the migration and proliferation of tumor-associated fibroblasts [9]. Endosialin-knockout mice showed reduced tumor growth and invasion [10]. This suggests that endosialin plays a role in tumor progression and neovascularization. Regarding the association between endosialin and inflammation, strong expression of endosialin was reported in synovial tissues from patients with rheumatoid arthritis, and endosialin-knockout mice showed less severe arthritis with less leukocyte infiltration and circulating cytokines [11]. These findings thus indicate that endosialin is associated with inflammation, accompanied by a proinflammatory state with increased proinflammatory cytokines [11-13].
Atherosclerotic diseases, such as coronary artery disease (CAD), are recognized to be a chronic inflammatory disease [14, 15]. Hasanov et al [4] reported that endosialin expression was upregulated in human atherosclerotic lesions and that endosialin-knockout mice showed reduced atherosclerosis. These findings therefore suggest that endosialin may play a role in the pathogenesis of atherosclerosis. Recently, serum endosialin levels in patients with colon cancer were reported to be high [7]. However, no study has reported blood endosialin levels in patients with CAD. Our study was done to elucidate the association between plasma endosialin levels and the presence or severity of CAD in 376 patients undergoing elective coronary angiography.
| Materials and Methods | ▴Top |
Study patients
In 2008, we started our study to prospectively collect blood samples and clinical and angiographic data from patients undergoing coronary angiography at Tokyo Medical Center. Our study was conducted in compliance with the Helsinki Declaration for research on human beings. Our study was approved by the institutional ethics committee of the hospital (R08-050/R21-037). After written informed consent was obtained, overnight-fasting blood samples were taken on the morning of the day of angiography. A total of 376 consecutive patients who underwent elective coronary angiography for suspected CAD were enrolled in this study to measure plasma endosialin levels. Any patients with acute coronary syndrome (ACS), such as acute myocardial infarction and unstable angina, were excluded from this study. Patients with a history of percutaneous coronary intervention or cardiac surgery, or those with heart failure or aortic diseases were also excluded. Since blood endosialin levels in patients with cancer were reported to be high [8], patients with any cancer were excluded. Hypertension was defined as blood pressures of ≥ 140/90 mm Hg or on drugs; 223 (59%) patients were taking anti-hypertensive drugs. Hyperlipidemia was defined as a low-density lipoprotein (LDL)-cholesterol level of > 140 mg/dL or on drugs; 148 (39%) patients were taking statin. Diabetes mellitus (DM) (a fasting glucose level of ≥ 126 mg/dL or on treatment) was present in 103 (27%) patients, and 134 (38%) patients were smokers (≥ 10 pack-years).
Measurements of plasma endosialin and C-reactive protein (CRP) levels
Blood samples were collected in ethylenediaminetetraacetic acid (EDTA)-containing tubes and then were centrifuged at 2,000 g for 15 min at 4 °C. The plasma was frozen and stored at -80 °C until analyzed. Plasma endosialin levels were measured by an enzyme-linked immunosorbent assay (ELISA) with a commercially available kit (Human Endosialin ELISA kit, CUSABIO, Wuhan, China). According to the data supplied by the manufacturer, this kit was developed to measure endosialin levels in serum, plasma, tissue homogenates, or cell lysates using a mouse monoclonal antibody against the extracelluar domain of endosialin and was shown to have no cross-reactivity with analogues, i.e., CD23, CD14 and CD163. The range of values detected by this assay was from 0.31 to 20 ng/mL, and the intra-assay and inter-assay coefficients of variation were < 8% and < 10%, respectively. Plasma high-sensitivity CRP (hsCRP) levels were also measured by a BNII nephelometer (Dade Behring, Tokyo, Japan).
Coronary angiography
Coronary angiograms were recorded on a cineangiogram system (Philips Electronics, Tokyo, Japan). CAD was defined as at least one coronary artery having > 50% luminal diameter stenosis on angiograms. The severity of CAD was represented as the numbers of > 50% stenotic vessels and > 50% and > 25% stenotic segments and the severity score of stenosis. The degree of stenosis in each segment was scored from 0 to 4 points (0, ≤ 25%; 1, 26-50%; 2, 51-75%; 3, 76-90%; 4, > 90% stenosis), and then the severity score was defined as the sum of scores of all segments. Coronary artery segments were defined as 29 segments according to the Coronary Artery Surgery Study (CASS) classification. All angiograms were evaluated by a single cardiologist (YM), blinded to the clinical and laboratory data.
Statistical analysis
Any differences between two groups were evaluated by unpaired t-test for parametric variables, by Mann-Whitney U test for nonparametric variables, and by Chi-squared test for categorical variables. Differences among ≥ 3 groups were evaluated by an analysis of variance with Scheffe’s test for parametric variables, by Kruskal-Wallis test for nonparametric variables, and by Chi-squared test for categorical variables. Since the distributions of the measured endosialin and hsCRP levels were considered to be highly skewed and to be nonparametric variables by Shapiro-Wilk test, these results were presented as the median value. The correlations between endosialin levels and hemoglobin A1c (HbA1c) or hsCRP levels or the severity of CAD were evaluated by the Spearman’s rank correlation test. A multiple logistic regression analysis was performed to determine the independent association between endosialin levels and CAD. All statistical analyses were performed using the SPSS software package (IBM SPSS ver. 25, Tokyo, Japan). A P value of < 0.05 was considered to be statistically significant. Results are presented as the mean ± standard deviation (SD) or the median value and interquartile range.
| Results | ▴Top |
Of the 376 study patients, CAD was found in 210 patients (56%) (one-vessel disease (1-VD), n = 90; two-vessel disease (2-VD), n = 65; three-vessel disease (3-VD), n = 55). Compared with 166 patients without CAD, 210 patients with CAD were older and had a male predominance; higher prevalence of hypertension, hyperlipidemia and DM; and lower high-density lipoprotein (HDL)-cholesterol levels (Table 1). Plasma hsCRP levels were higher in patients with CAD than in those without CAD (median 0.57 vs. 0.43 mg/L, P = 0.007). A stepwise increase in hsCRP levels was found depending on the number of > 50% stenotic coronary vessels: 0.43 in CAD(-), 0.52 in 1-VD, 0.57 in 2-VD, and 0.58 mg/L in 3-VD (P = 0.019) (Table 1). Moreover, hsCRP levels significantly correlated with the numbers of stenotic segments and the severity score (Table 2).
 Click to view | Table 1. Clinical Characteristics of Patients With and Without CAD |
 Click to view | Table 2. Correlations Between Plasma Endosialin or hsCRP Levels and the Severity of CAD |
Plasma endosialin levels did not significantly differ between patients with and without CAD (median 0.91 vs. 0.92 ng/mL, P = 0.693) (Fig. 1). Moreover, no marked difference was found in endosialin levels among the four groups of CAD(-), 1-VD, 2-VD, and 3-VD (0.92, 0.89, 0.98, and 0.87 ng/mL, respectively, P = 0.785) (Fig. 1). No significant correlation was found between endosialin levels and the numbers of stenotic segments or the severity score (Table 2). Moreover, no correlation was found between endosialin levels and LDL-cholesterol or hsCRP levels. However, endosialin levels significantly but weakly correlated with HbA1c levels (rs = 0.12, P = 0.023) (Fig. 2). In a multivariate analysis, endosialin levels were found to be not a significant factor associated with CAD independent of atherosclerotic risk factors (Table 3).
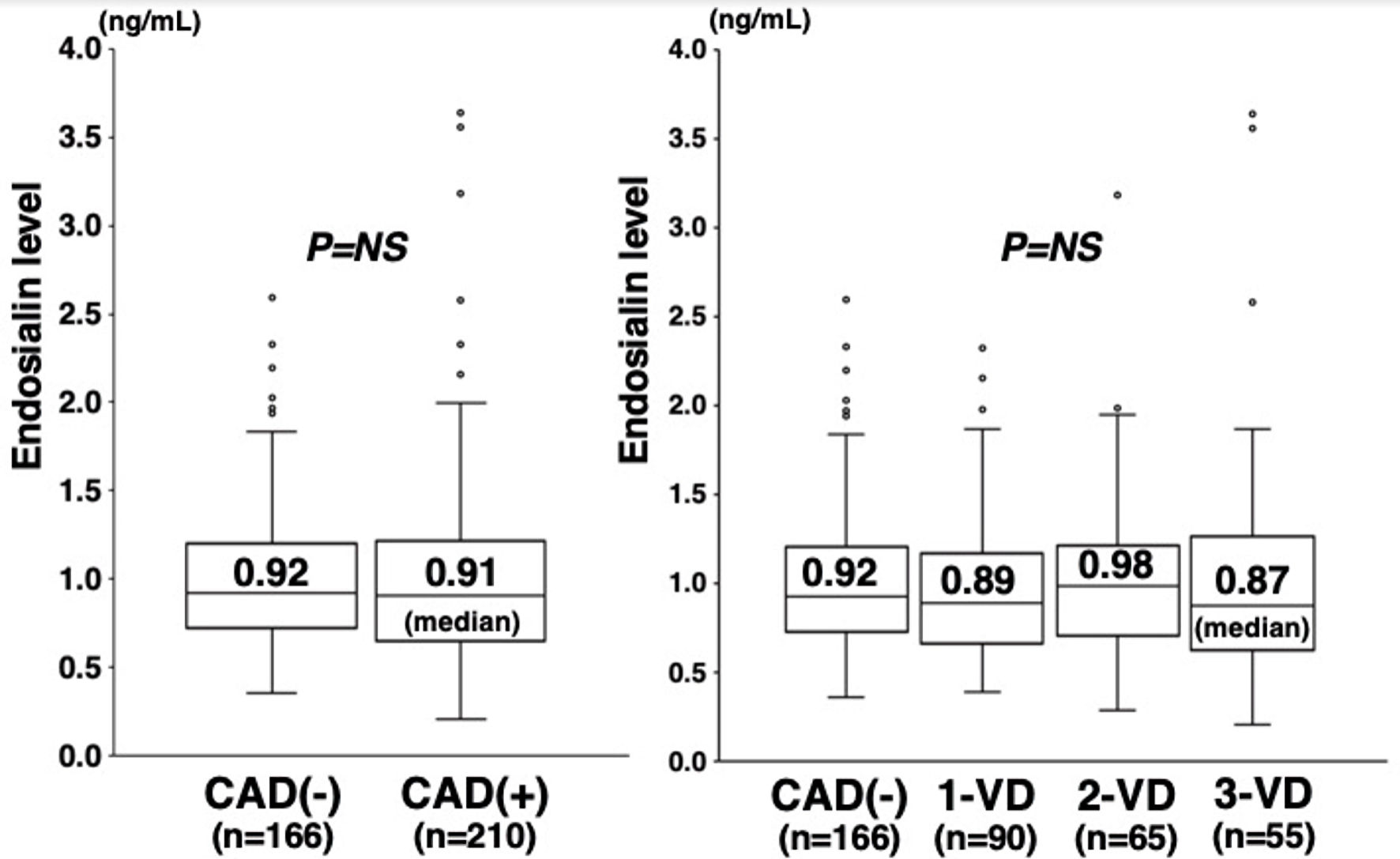 Click for large image | Figure 1. Plasma endosialin levels and the presence of CAD or the number of stenotic coronary vessels. Plasma endosialin levels did not significantly differ between patients with and without CAD (0.91 vs. 0.92 ng/mL) (left). No significant difference was found in endosialin levels among the four groups of CAD(-), 1-VD, 2-VD, and 3-VD (0.92, 0.89, 0.98, and 0.87 ng/mL) (right). The central line represents the median, and the box represents the 25th to 75th percentiles. The whiskers represent the lowest and highest value in the 25th percentile minus 1.5 IQR and 75th percentile plus 1.5 IQR, respectively. NS: not significant; CAD: coronary artery disease; 1-VD: one-vessel disease; 2-VD: two-vessel disease; 3-VD: three-vessel disease; IQR: interquartile range. |
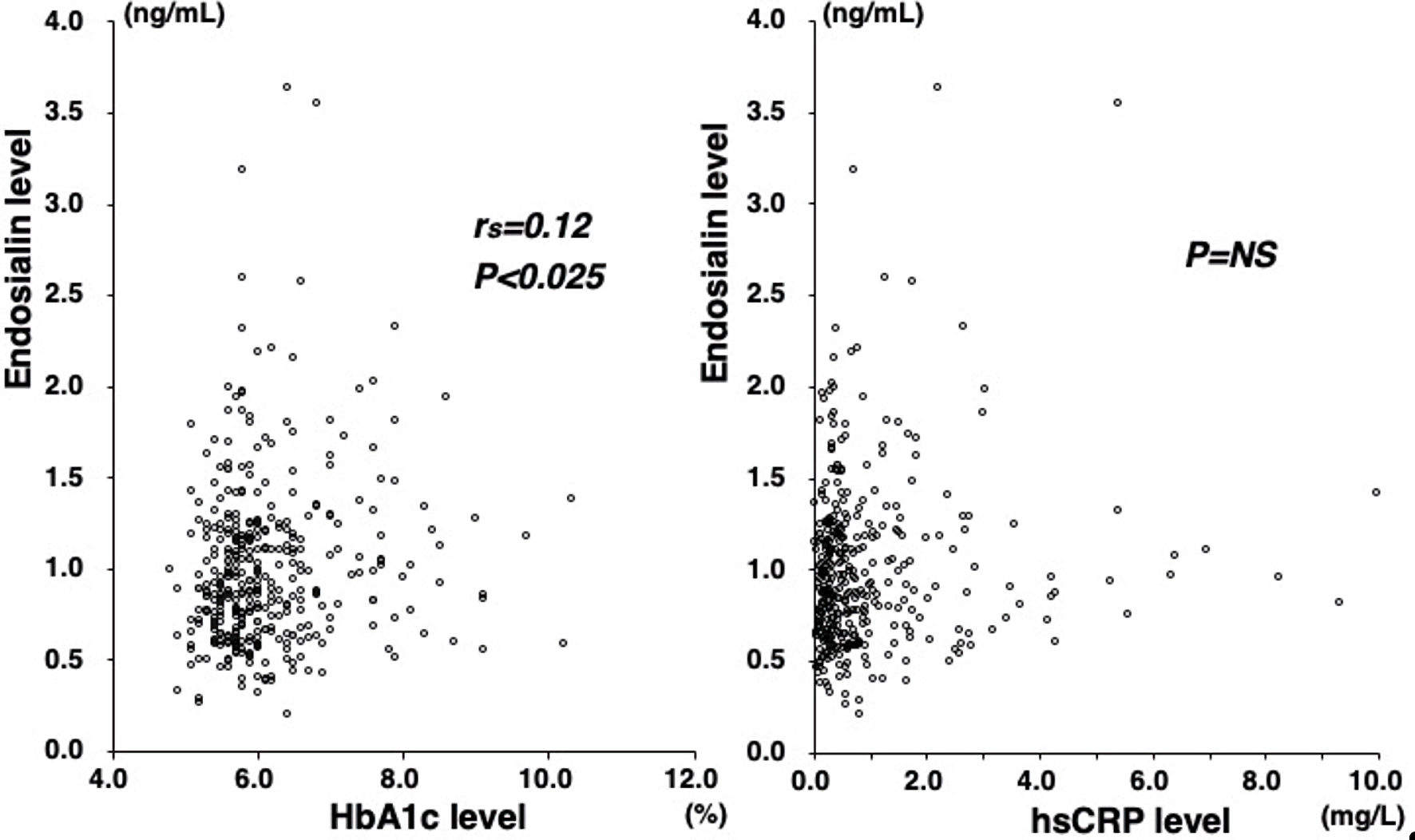 Click for large image | Figure 2. Correlations between plasma endosialin levels and HbA1c or hsCRP levels. Plasma endosialin levels signficantly but weakly correlated with HbA1c levels (rs = 0.12, P < 0.025) (left). However, no significant correlation was found between endosialin and hsCRP levels (right). HbA1c: hemoglobin A1c; hsCRP: high-sensitivity CRP; NS: not significant. |
 Click to view | Table 3. Factors Associated With CAD (Multiple Logistic Regression Analysis of the 376 Study Patients) |
| Discussion | ▴Top |
In the present study, plasma endosialin levels in patients with CAD were found to be not significantly higher than in those without CAD and did not significantly correlate with the severity of CAD. In contrast, hsCRP levels in patients with CAD were high and correlated with the severity of CAD. No significant correlation was found between endosialin and hsCRP levels.
Endosialin was reported to be upregulated in synovial tissues from patients with rheumatoid arthritis, and endosialin-knockout mice showed the amelioration of arthritis [11], thus suggesting a role of endosialin in inflammatory diseases. Endosialin upregulation was also reported in fibrotic diseases [16, 17]. Endosialin was upregulated in the fibrotic areas of lung samples from patients with pulmonary fibrosis [16]. Endosialin expression also increased in fibrotic liver of patients with liver cirrhosis, and endosialin-knockout mice showed less liver fibrosis in response to injury [17]. These findings thus suggest that endosialin may be involved in the progression of fibrosis. Fibrosis associated with inflammation is recognized to play a crucial role in atherosclerosis [14, 15, 18]. Vascular fibrosis involves smooth muscle cell proliferation and extracellular matrix accumulation, thereby leading to arterial wall thickening and stenosis [18]. Endosialin was reported to be abundantly expressed in human atherosclerotic lesions, and endosialin-knockout mice had less atherosclerosis with less macrophage recruitment [4]. Thus, endosialin may play a role in the progression of atherosclerosis associated with inflammation and fibrosis. Furthermore, endosialin-knockout mice were reported to be protected against arterial thrombosis [6]. An immunofluorescence analysis also showed increased endosialin expression and infiltrating CD8 T cells in aortic aneurysm specimens, suggesting a role of endosialin in vascular remodeling [3].
Endosialin expression in adipose tissues was shown to increase with obesity [13]. Endosialin-knockout mice showed less inflammation with improvement in insulin sensitivity and glucose tolerance [13]. Furthermore, endosialin-knockout mice had a reduction in fat pad size and serum cholesterol levels [19]. These findings thus implicate endosialin in obesity, glucose tolerance and lipid metabolism, and these effects of endosialin may affect the progression of atherosclerosis. In our study, plasma endosialin levels did not correlate with body mass index (BMI) or LDL-cholesterol levels. However, endosialin levels significantly but weakly correlated with HbA1c levels, suggesting that endosialin may affect glucose tolerance.
Using the originally developed anti-endosialin monoclonal antibody, O’Shannessy et al. [20] measured serum endosialin levels in 117 patients with colorectal cancer and 100 healthy subjects and reported no difference in endosialin levels between the two groups. In contrast, Pietrzyk et al [7] recently measured serum endosialin levels by ELISA with a commercially available kit in 45 patients with colorectal cancer and 35 healthy subjects and reported endosialin levels to be higher in patients with colorectal cancer than in healthy subjects. Endosialin is recognized to be a soluble protein that can be cleaved and detected in blood [20]. In blood, endosialin was shown to be present as 120 - 150 kDa molecules representing glycosylated extracellular domain fragments [20]. However, no study has shown blood endosialin levels in patients with CAD. Since endosialin is suggested to play a role in the progression of atherosclerosis [3, 4, 6], we hypothesized that plasma endosialin levels in patients with CAD would be high. Our present study is the first to report plasma endosialin levels in patients with CAD. Unfortunately, endosialin levels did not significantly differ between patients with and without CAD and did not correlate with the severity of CAD. In a multivariate analysis, endosialin levels were not a significant factor for CAD. As previously reported [21], hsCRP levels were higher in patients with CAD than in those without CAD and correlated with the severity of CAD. Therefore, plasma endosialin levels were not significantly associated with the presence or severity of CAD, and they are unlikely to be a better biomarker for CAD than hsCRP levels. Our results suggest that plasma endosialin levels do not closely reflect the degree of coronary atherosclerosis and inflammation and that endosialin is unlikely to play a major role in the progression of coronary atherosclerosis.
Our study has several limitations. First, angiography was used to assess coronary atherosclerosis. It cannot visualize plaques and only shows the lumen characteristics. Second, our study was cross-sectional in nature and was unable to establish causality, since it only depicted some associations. Third, no patients with ACS, who usually need emergent angiography, were included in our study. Further study in patients with ACS is needed to clarify the potential role of endosialin in this syndrome. Fourth, we did not measure endosialin levels in the coronary sinus. Endosialin levels at this site may be higher than in peripheral blood. Fifth, as we previously reported that plasma hsCRP levels were more closely associated with aortic atherosclerosis than coronary atherosclerosis [21], endosialin levels in patients with CAD may more closely reflect atherosclerosis in other vascular beds or inflammation in non-vascular tissues than coronary atherosclerosis. Finally, we compared patients with and without CAD and had no healthy controls. Even patients without CAD may have had some atherosclerosis in other vascular beds, as well as in the coronary arteries. Pietrzyk et al [7] measured serum endosialin levels using a kit (Human Endosialin ELISA kit, MyBiosource, USA) in patients with cancer vs. healthy controls (1.31 vs. 0.92 ng/mL). The kit used in their study was different from that in our study (Human Endosialin ELISA kit, CUSABIO, China), but the detection limit was similar (0.31 to 20 ng/mL). In spite of different ELISA kits, their reported levels of healthy controls seem to be similar to those of our patients without CAD (0.92 ng/mL). However, our study was in Japanese patients undergoing angiography, who are generally considered to be a highly select population at high risk for CAD. Our results may not be applicable to the general or other ethnic populations.
Conclusions
Plasma endosialin levels in patients with CAD were found to be not higher than in those without CAD and were not associated with CAD. Our results suggest that plasma endosialin levels cannot be a good biomarker reflecting the presence or severity of CAD and that endosialin is unlikely to play a major role in the progression of coronary atherosclerosis.
Acknowledgments
The authors have none to declare.
Financial Disclosure
This study was supported in part by a grant from Tanuma Green House Foundation. Financial funding was also provided by Bayer Yakuhin Ltd., Daiichi Sankyo Co., and Pfizer Japan Inc.; however, these sponsors had no role in the design, analysis, or interpretation of the study.
Conflict of Interest
The authors have no conflict of interest to declare.
Informed Consent
Written informed consent was obtained from all the study patients.
Author Contributions
YK, RO and YM conceived and designed the study. MA, and ES acquired the data. YK and YM analyzed and interpreted the data and drafted the paper. RO, KT and KK critically reviewed and supervised the manuscript. All authors read and approved the final manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
CAD: coronary artery disease; ACS: acute coronary syndrome; DM: diabetes mellitus; CRP: C-reactive protein; hsCRP: high-sensitivity CRP; ELISA: enzyme-linked immunosorbent assay; CASS: Coronary Artery Surgery Study; BMI: body mass index; SBP: systolic blood pressure; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol
| References | ▴Top |
- St Croix B, Rago C, Velculescu V, Traverso G, Romans KE, Montgomery E, Lal A, et al. Genes expressed in human tumor endothelium. Science. 2000;289(5482):1197-1202.
doi pubmed - Rettig WJ, Garin-Chesa P, Healey JH, Su SL, Jaffe EA, Old LJ. Identification of endosialin, a cell surface glycoprotein of vascular endothelial cells in human cancer. Proc Natl Acad Sci U S A. 1992;89(22):10832-10836.
doi pubmed - Hu X, Wu T, Wang C, Li J, Ying C. CD248+CD8+ T lymphocytes suppress pathological vascular remodeling in human thoracic aortic aneurysms. Exp Biol Med (Maywood). 2021;246(2):121-129.
doi pubmed - Hasanov Z, Ruckdeschel T, Konig C, Mogler C, Kapel SS, Korn C, Spegg C, et al. Endosialin Promotes Atherosclerosis Through Phenotypic Remodeling of Vascular Smooth Muscle Cells. Arterioscler Thromb Vasc Biol. 2017;37(3):495-505.
doi pubmed - MacFadyen JR, Haworth O, Roberston D, Hardie D, Webster MT, Morris HR, Panico M, et al. Endosialin (TEM1, CD248) is a marker of stromal fibroblasts and is not selectively expressed on tumour endothelium. FEBS Lett. 2005;579(12):2569-2575.
doi pubmed - Kapopara PR, Safikhan NS, Huang JL, Meixner SC, Gonzalez K, Loghmani H, Ruf W, et al. CD248 enhances tissue factor procoagulant function, promoting arterial and venous thrombosis in mouse models. J Thromb Haemost. 2021;19(8):1932-1947.
doi pubmed - Pietrzyk L, Wdowiak P. Endosialin (TEM1) as a Diagnostic, Progression, and Prognostic Serum Marker for Patients With Colorectal Cancer-A Preliminary Study. Cancer Control. 2020;27(1):1073274820903351.
doi pubmed - Khan KA, McMurray JL, Mohammed F, Bicknell R. C-type lectin domain group 14 proteins in vascular biology, cancer and inflammation. FEBS J. 2019;286(17):3299-3332.
doi pubmed - Christian S, Winkler R, Helfrich I, Boos AM, Besemfelder E, Schadendorf D, Augustin HG. Endosialin (Tem1) is a marker of tumor-associated myofibroblasts and tumor vessel-associated mural cells. Am J Pathol. 2008;172(2):486-494.
doi pubmed - Tomkowicz B, Rybinski K, Foley B, Ebel W, Kline B, Routhier E, Sass P, et al. Interaction of endosialin/TEM1 with extracellular matrix proteins mediates cell adhesion and migration. Proc Natl Acad Sci U S A. 2007;104(46):17965-17970.
doi pubmed - Maia M, de Vriese A, Janssens T, Moons M, van Landuyt K, Tavernier J, Lories RJ, et al. CD248 and its cytoplasmic domain: a therapeutic target for arthritis. Arthritis Rheum. 2010;62(12):3595-3606.
doi pubmed - Teicher BA. CD248: A therapeutic target in cancer and fibrotic diseases. Oncotarget. 2019;10(9):993-1009.
doi pubmed - Petrus P, Fernandez TL, Kwon MM, Huang JL, Lei V, Safikhan NS, Karunakaran S, et al. Specific loss of adipocyte CD248 improves metabolic health via reduced white adipose tissue hypoxia, fibrosis and inflammation. EBioMedicine. 2019;44:489-501.
doi pubmed - Ross R. Atherosclerosis - an inflammatory disease. N Engl J Med. 1999;340(2):115-126.
doi pubmed - Wu MY, Li CJ, Hou MF, Chu PY. New insights into the role of inflammation in the pathogenesis of atherosclerosis. Int J Mol Sci. 2017;18(10):2034.
doi pubmed - Bartis D, Crowley LE, D'Souza VK, Borthwick L, Fisher AJ, Croft AP, Pongracz JE, et al. Role of CD248 as a potential severity marker in idiopathic pulmonary fibrosis. BMC Pulm Med. 2016;16(1):51.
doi pubmed - Wilhelm A, Aldridge V, Haldar D, Naylor AJ, Weston CJ, Hedegaard D, Garg A, et al. CD248/endosialin critically regulates hepatic stellate cell proliferation during chronic liver injury via a PDGF-regulated mechanism. Gut. 2016;65(7):1175-1185.
doi pubmed - Lan TH, Huang XQ, Tan HM. Vascular fibrosis in atherosclerosis. Cardiovasc Pathol. 2013;22(5):401-407.
doi pubmed - Armitage EG, Barnes A, Patrick K, Bechar J, Harrison MJ, Lavery GG, Rainger GE, et al. Metabolic consequences for mice lacking Endosialin: LC-MS/MS-based metabolic phenotyping of serum from C56Bl/6J Control and CD248 knock-out mice. Metabolomics. 2021;17(2):14.
doi pubmed - O'Shannessy DJ, Smith MF, Somers EB, Jackson SM, Albone E, Tomkowicz B, Cheng X, et al. Novel antibody probes for the characterization of endosialin/TEM-1. Oncotarget. 2016;7(43):69420-69435.
doi pubmed - Momiyama Y, Ohmori R, Fayad ZA, Kihara T, Tanaka N, Kato R, Taniguchi H, et al. Associations between plasma C-reactive protein levels and the severities of coronary and aortic atherosclerosis. J Atheroscler Thromb. 2010;17(5):460-467.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


