| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 14, Number 5, May 2022, pages 188-195
Analysis of Risk Factors of Soft Tissue Bleeding in COVID-19 Patients: A Point of View After Two Years of Pandemic
Eliodoro Faiellaa, b, Gennaro Castielloa, Domiziana Santuccia, b, d, Giuseppina Pacellaa, Caterina Bernettia, Moises Muley Villamuc, Raffaele Antonelli Incalzic, Bruno Beomonte Zobela, Carlo Cosimo Quattrocchia, Rosario Francesco Grassoa
aDepartment of Radiology, Campus Bio-Medico University, Via Alvaro del Portillo, 21-00128 Rome, Italy
bDepartment of Radiology, Sant’Anna Hospital, Via Ravona, 22042 San Fermo della Battaglia CO, Italy
cDepartment of Geriatrics, Campus Bio-Medico University, Via Alvaro del Portillo, 21-00128 Rome, Italy
dCorresponding Author: Domiziana Santucci, Department of Radiology, Campus Bio-Medico University, Via Alvaro del Portillo, 21-00128 Rome, Italy
Manuscript submitted March 25, 2022, accepted April 29, 2022, published online May 31, 2022
Short title: Soft Tissue Bleeding in COVID-19 Patients
doi: https://doi.org/10.14740/jocmr4708
| Abstract | ▴Top |
Background: The aim of the study was to analyze the relationship between patient characteristics, including anagraphic and laboratoristic data and amount of adipose tissue measured in computed tomography (CT) scans in coronavirus disease 2019 (COVID-19) patients, and incidence of soft tissue bleeding requiring medical and/or interventional radiology management.
Methods: A total of 132 patients hospitalized for COVID-19 pathology from October 2020 to May 2021 were included in the study and divided into two groups: a bleeding group of 70 cases with soft tissue bleeding occurring during hospitalization, and a control group of 62 hospitalized COVID-19 patients without bleeding events. In the bleeding group, two subgroups were considered: an embolization group including soft tissue bleeding cases requiring interventional radiology with transarterial embolization (TAE) (16/70; 22.9%) and a non-embolization group, clinically managed without TAE (54/70; 77.1%). Demographics and clinical data, visceral adipose tissue (VAT) area and subcutaneous adipose tissue (SAT) area measured on CT images and VAT/SAT ratio were compared between bleeding and control groups and between embolization and non-embolization subgroups.
Results: Bleeding and control groups did not significantly differ for sex distribution, COVID-19, platelet (PLT) count, international normalized ratio (INR), SAT area, VAT area, and VAT/SAT ratio. Embolization and non-embolization groups did not significantly differ for age, COVID-19, PLT count, INR, SAT area, and VAT/SAT ratio. Bleeding group had lower body mass index (BMI) than control group as well as embolization group compared to non-embolization group. A statistically significant difference was observed between embolization and non-embolization groups for VAT area, with smaller values in embolization group (mean difference: 64.2 cm2, 95% confidence interval: 8.3 - 120.1; P < 0.05).
Conclusion: Soft tissue bleeding in COVID-19 is more frequent and severe in patients with low amount of VAT, demonstrating that fat mass may have a containing function on bleeding, limiting its progression in surrounding structures. There are some other factors that influence the risk of bleeding, such as age, thromboprophylaxis therapy and BMI.
Keywords: SARS-CoV-2 infection; Visceral fat; Soft tissue bleeding; Embolization
| Introduction | ▴Top |
Coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was declared a pandemic by the World Health Organization (WHO) for its huge impact on health systems [1]. Respiratory system involvement is considered the principal cause of death in patients with COVID-19 [2, 3], although changes in coagulation function have been also commonly associated with SARS-CoV-2 infection as one of the most important adverse prognostic signs [4, 5]. Dysregulation of coagulation caused by host response to viral replication produces a hypercoagulability state often resulting in venous and arterial thrombosis and multiorgan dysfunction [6]. Consequently, heparin-based therapies were introduced [7] which, together with factors such as thrombocytopenia, hyperfibrinolytic state and consumption of coagulation factors, produce a higher risk of bleedings [8-10] in patients affected by COVID-19, including soft tissue hematomas requiring medical or interventional radiology management through transarterial embolization (TAE) [11]. Several studies showed a strong association between obesity and COVID-19 severity [12-17]. Obese patients affected by COVID-19 demonstrated an overall worse disease outcome with more severe infection forms, higher hospitalization and mechanical ventilation rates [18] compared to non-obese subjects. COVID-19 coagulopathy is also aggravated by obesity due to its intrinsic coagulation alterations that are in addition to those with those related to the SARS-CoV-2 infection [19]. In particular, excess visceral fat is considered an important risk factor for COVID-19 severity due to its viral receptor and cytokine expression [20]. The aim of this study was to analyze the relationship between patient characteristics, including anagraphic data and laboratoristic data and the amount of adipose tissue measured in computed tomography (CT) scans in COVID-19 patients, and the incidence of soft tissue bleeding requiring medical and/or interventional radiology management.
| Materials and Methods | ▴Top |
Ethical review and approval were waived for this study, due to retrospective nature of the study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Study population
Patients admitted to our institution with more than 18 years of age and patients who were tested positive to COVID-19 were included. Patients admitted to our institution with less than 18 years of age or patients who were tested negative to COVID-19 were excluded.
A total of 132 patients hospitalized at out center for COVID-19 pathology from October 2020 to May 2021 were included in the study and divided into two groups: a bleeding group, including 70 cases of soft tissue bleeding occurring during hospitalization, and a control group, with 62 hospitalized COVID-19 patients without bleeding events. In the bleeding group, two subgroups were considered: an embolization group including soft tissue bleeding cases which required interventional radiology treatment with TAE (16/70; 22.9%) and a non-embolization group of soft tissue bleeding cases which were clinically managed without TAE (54/70; 77.1%).
Soft tissue bleeding management
In cases of soft tissue bleeding, conservative management was the main strategy for hemodynamically stable patients. TAE was performed in cases of progressive, uncontrolled bleeding (confirmed by CT angiography) leading to hemodynamic instability. Embolizing techniques included coils alone, glue alone, gelfoam alone and combinations of microparticles with coils, gelfoam and coils, and glue and coils (Figs. 1 and 2). Bleeding sites and embolization techniques are listed in Table 1.
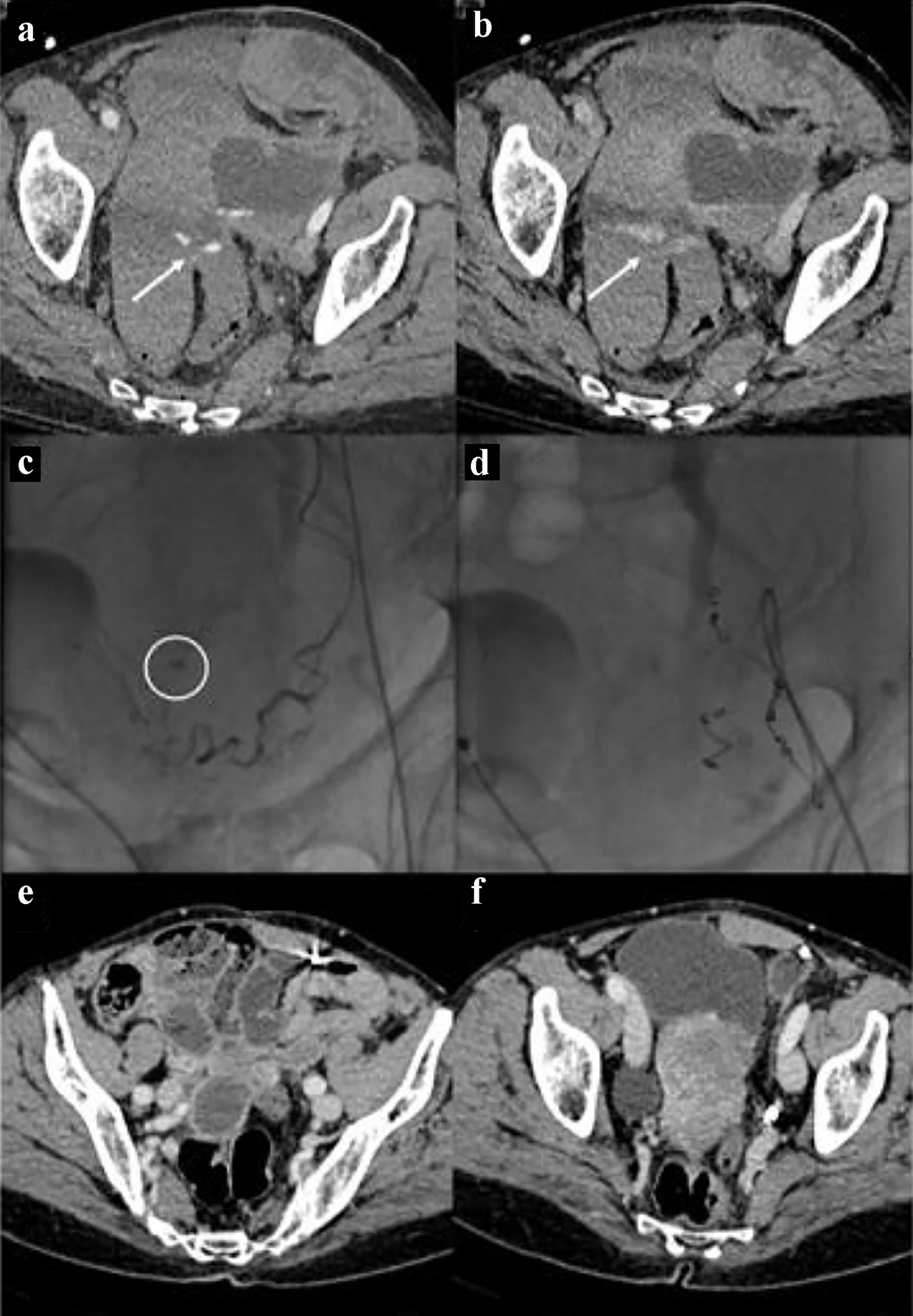 Click for large image | Figure 1. Bleeding in the context of the left uterine broad ligament. The CT images in (a) and (b) show the spots of active bleeding (arrows) with progressive spread of contrast medium. The angiographic image in (c) shows the active bleeding spot adjacent to the left uterine artery. The angiographic image in (d) shows the complete embolization, by means of metallic micro-spirals, of the arterial afferents at the bleeding point. CT images in (e) and (f) show complete reabsorption of hematomas after 3 months. CT: computed tomography. |
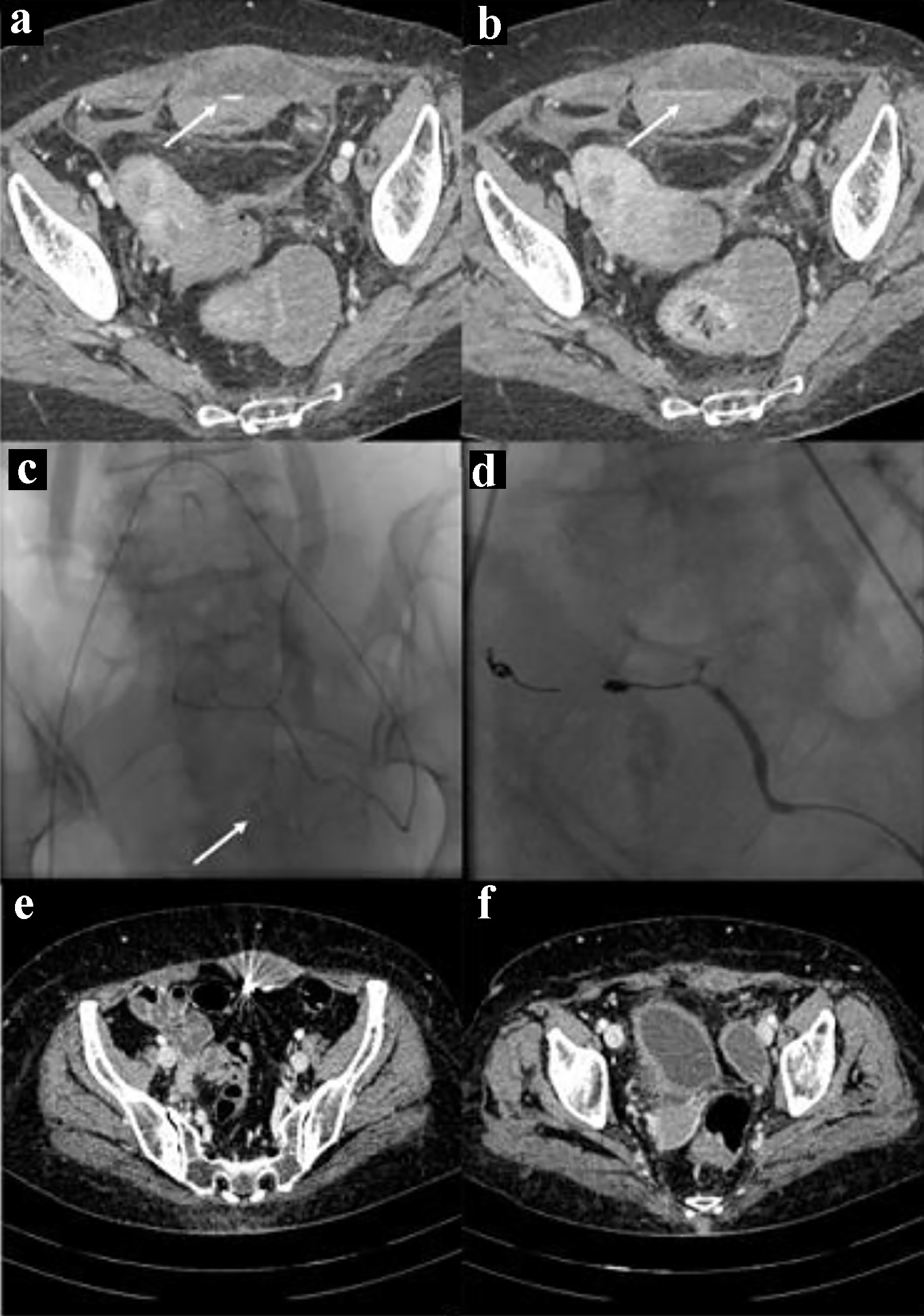 Click for large image | Figure 2. Bleeding in the context of the left rectus abdominis muscle. The CT images in (a) and (b) show the spots of active bleeding (arrows) with progressive spread of contrast medium. The angiographic image in (c) shows the active bleeding spot (arrow) adjacent to the left inferior epigastric artery. The angiographic image in (d) shows the complete embolization, by means of glue and metallic micro-spirals, of the arterial afferents at the bleeding point. CT images in (e) and (f) show complete reabsorption of hematomas after 3 months. CT: computed tomography. |
 Click to view | Table 1. Soft Tissue Bleeding Features and Embolization Techniques |
Data collection
Demographics and clinical data were collected including body mass index (BMI), platelet (PLT) count, international normalized ratio (INR) and hemoglobin levels (Hb) at the patient admission and pulmonary involvement severity score (SS) [21, 22]. We also counted how many days after admission bleeding occurred, estimating the time of admission to the hospital with the start of anticoagulant therapy. At least one CT examination was performed in all patients during hospitalization, with contrast medium administration, using a dual source 384-slice (2 × 192) CT (Siemens SOMATOM Force, Erlangen, Germany) with real-time voltage modulation (70 - 150 kV), real-time dose modulation (CARE Dose4D™; 80 - 250 mAs), spiral pitch factor of 1.8 and collimation width of 0.6 mm. Measurements of subcutaneous adipose tissue (SAT) and visceral adipose tissue (VAT) areas in cm2 were obtained by analyzing axial CT images at the level of the third lumbar vertebra with an image processing application (OsiriX, Pixmeo, Bernex, Switzerland) (Fig. 3) [23].
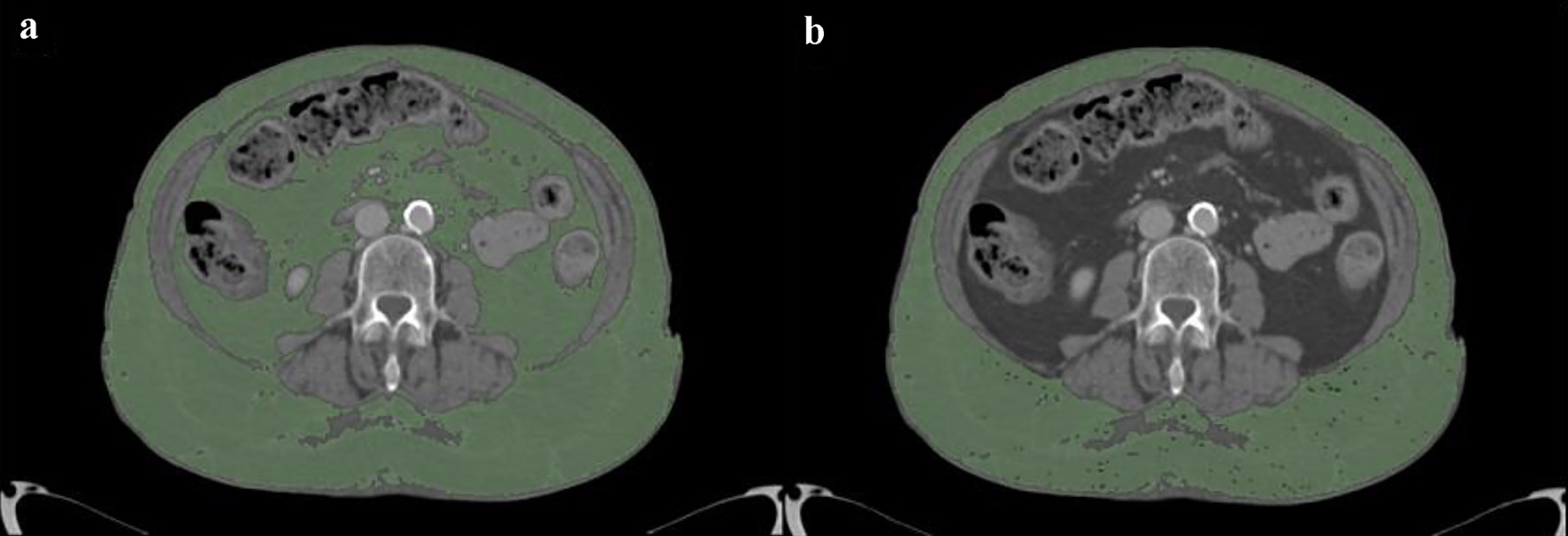 Click for large image | Figure 3. Measurement of adipose tissue areas in cm2 obtained by analyzing axial CT images at the level of the third lumbar vertebra with an image processing application (OsiriX, Pixmeo, Bernex, Switzerland): TAT (a) and SAT (b). VAT is calculated by difference between TAT and SAT. CT: computed tomography; TAT: total adipose tissue; SAT: subcutaneous adipose tissue; VAT: visceral adipose tissue. |
Statistical analysis
Demographics and clinical data, VAT and SAT areas and the VAT/SAT ratio were compared between the bleeding and the control groups using Chi-square and Student’s t-test. The same variables were then compared between the embolization and non-embolization subgroups using Chi-square and Student’s t-test.
To explore the risk factors associated with bleeding event and with TAE procedure, a multivariate logistic regression analysis was conducted. The following continuous variables were included in the analysis: sex, age, BMI, PLT, INR and Hb.
Statistical significance was considered as P < 0.05. Statistical analysis was performed using IBM SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, NY, USA).
| Results | ▴Top |
The bleeding group consisted of 70 cases while the control group included 62 cases. Bleeding never occurred before 4 days after admission, with a mean of onset of 8 days. All patient received heparin therapy for thromboprophylaxis during hospitalization and none had known pre-existing coagulation disorders. Bleeding and control groups did not significantly differ for sex distribution, COVID-19 SS, PLT count, INR, SAT area, VAT area and VAT/SAT ratio, although SAT and VAT areas tend to be lower in the bleeding group, although in a non-significant way. There was a slightly significant difference in age between bleeding group and control group, with older patients in the former and a mean difference of 6 years (95% confidence interval (CI): 0.4 - 11.6; P < 0.05). There was a slight significant difference in BMI between bleeding group and control group, with lower values in the former.
The embolization group consisted of 16 cases, while the non-embolization group included 54 cases. Embolization and non-embolization groups did not significantly differ for age, COVID-19 SS, PLT count, INR, SAT area, and VAT/SAT ratio; INR tends to be higher in the embolization group, although in a non-significant way. There was a slightly significant difference in BMI between embolization and non-embolization groups, with lower values in the former. There was a significant difference in sex between embolization and non-embolization groups, with a higher number of females compared to males in the former group (P ≤ 0.05). A statistically significant difference was observed between the embolization and non-embolization groups for VAT area, with smaller values in the embolization group and a mean difference of 64.2 cm2 (95% CI: 8.3 - 120.1; P < 0.05). Results are listed in Tables 2 and 3.
 Click to view | Table 2. Variables for Bleeding Group and Control Group |
 Click to view | Table 3. Variables for Embolization Group and Non-Embolization Group |
The multivariate logistic regression analysis showed a statistical significance comparing TAE and non-TAE groups for sex (TAE was more frequently performed for male) and PLT (TAE was more frequently performed for patients with lower PLT count) (Table 4); the difference in values among bleeding and non-bleeding groups was significative only for BMI (Table 4).
 Click to view | Table 4. Multivariate Logistic Regression Analysis for Bleeding and Control Groups and for Embolization and Non-Embolization Groups |
| Discussion | ▴Top |
The bleeding phenomenon in patients affected by COVID-19 is of crucial importance for their clinical management and prognosis [6, 9, 10]. The bleeding events are the results of a series of clinical and therapeutic factors depending on the intrinsic characteristics of the infection and the standard therapeutic strategies implemented to manage the occurrence of thrombotic events [8]. Given the importance of the association between obesity and the clinical outcome in patients with SARS-CoV-2 infection [13, 14, 18], we aimed to analyze the relationship between the onset of soft tissue bleeding events and the amount of adipose tissue measured through CT scan analysis; measurements are not routinely performed in clinical practice and was our goal to assess their usefulness for predicting the bleeding risk in COVID-19 patients in an experimental setting. We considered the need for TAE treatment as an additional factor to determine the complexity of soft tissue bleeding [11]. Our results show that, although factors such as age are related to a higher risk of soft tissue bleeding events according to an overall increased risk of worse prognosis in elder people [3], bleeding in COVID-19 patients is not dependent on the amount of adipose tissue. In our population, a lower amount of VAT in patients with soft tissue bleeding who required TAE was observed, compared to patients undergoing medical treatment alone in the bleeding group. Even more, a tendency to a lower amount of VAT and SAT in patients with bleeding compared to the control group was counted. A lower BMI was also observed in the bleeding group compared to the control group and in the embolization group compared to the non-embolization group. Literature data show a worse overall prognosis of obese patients or those with greater amounts of visceral fat, presumably determined by inflammatory and metabolic factors [20, 24]. Our work shows that soft tissue bleeding phenomenon occurs most frequently in elderly patients [25] and that unstable bleedings requiring TAE are most frequent in patients with lower VAT. We hypothesize that complicated bleedings have a pathogenesis mechanism related to microtraumatic injuries to soft tissues rather than the patient’s fat mass. It is well known that non-self-limiting bleedings are more frequently associated with muscle lesions [26, 27], often caused by the only mobilization on COVID-19 patients, on which anticoagulant therapy is an additive risk factor [28, 29]. We assume that, most likely, a greater fat mass can have a containing function on bleeding by limiting its progression in surrounding structures. In conclusion, our data suggest that soft tissue hemorrhagic events in COVID-19 patients are not easily predictable but it may be more frequent to find them in elderly patients and with a major severity in patients with low VAT. This study has some limitations including poor patient population, poor comparison with literature data due to the relative recent spread of the disease, lack of subdivision of bleeding by site and contributing cause; for a better understanding of soft tissue bleeding in COVID patients, prospective studies and case-control analyzes with more detailed grouping are needed. Perhaps it might be interesting to know how long after the start of anticoagulant therapy and from the diagnosis of the pathology the bleeding phenomenon began.
In conclusion, our results show that soft tissue bleeding in COVID-19 patients is more frequent and more severe in patients with low BMI and amount of VAT, demonstrating that the fat mass may have a containing function on bleeding by limiting its progression in surrounding structures. There are some other factors in our series that influence the risk of bleeding, such as age, thromboprophylaxis therapy and BMI. We should also keep in mind the concomitance of anticoagulant therapy in all COVID-19 patients and the complexity of hemodynamic system in these patients, which can affect the generalization of the results.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
All authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from all subjects involved in the study at the time of CT execution.
Author Contributions
Eliodoro Faiella: guarantor of integrity of the entire study, study concepts and design; Gennaro Castiello: study concepts and design, manuscript preparation; Domiziana Santucci: manuscript editing; Giuseppina Pacella: manuscript preparation; Caterina Bernetti: literature research, manuscript preparation; Moises Muley Villamu: clinical studies; Raffaele Antonelli Incalzi: supervision; Bruno Beomonte Zobel: data analysis, supervision; Carlo Cosimo Quattrocchi: English editing and revision; Rosario Francesco Grasso: supervision.
Data Availability
The datasets generated or analyzed during the study are available from the corresponding author on reasonable request.
| References | ▴Top |
- Fauci AS, Lane HC, Redfield RR. COVID-19 - Navigating the Uncharted. N Engl J Med. 2020;382(13):1268-1269.
doi pubmed - Yue H, Bai X, Wang J, Yu Q, Liu W, Pu J, Wang X, et al. Clinical characteristics of coronavirus disease 2019 in Gansu province, China. Ann Palliat Med. 2020;9(4):1404-1412.
doi pubmed - Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054-1062.
doi - Dorgalaleh A. Bleeding and bleeding risk in COVID-19. Semin Thromb Hemost. 2020;46(7):815-818.
doi pubmed - Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18(4):844-847.
doi pubmed - Chan NC, Weitz JI. COVID-19 coagulopathy, thrombosis, and bleeding. Blood. 2020;136(4):381-383.
doi pubmed - Hippensteel JA, LaRiviere WB, Colbert JF, Langouet-Astrie CJ, Schmidt EP. Heparin as a therapy for COVID-19: current evidence and future possibilities. Am J Physiol Lung Cell Mol Physiol. 2020;319(2):L211-L217.
doi pubmed - Al-Samkari H, Karp Leaf RS, Dzik WH, Carlson JCT, Fogerty AE, Waheed A, Goodarzi K, et al. COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. 2020;136(4):489-500.
doi pubmed - Musoke N, Lo KB, Albano J, Peterson E, Bhargav R, Gul F, DeJoy R, 3rd, et al. Anticoagulation and bleeding risk in patients with COVID-19. Thromb Res. 2020;196:227-230.
doi pubmed - Lucatelli P, De Rubeis G, Citone M, Lucarelli NM, Pasqualini V, Sturiale M, Giuliani S, et al. Heparin-related major bleeding in COVID-19-positive patient: perspective from the outbreak. Cardiovasc Intervent Radiol. 2020;43(8):1216-1217.
doi pubmed - Touma L, Cohen S, Cassinotto C, Reinhold C, Barkun A, Tran VT, Banon O, et al. Transcatheter arterial embolization of spontaneous soft tissue hematomas: a systematic review. Cardiovasc Intervent Radiol. 2019;42(3):335-343.
doi pubmed - Albashir AAD. The potential impacts of obesity on COVID-19. Clin Med (Lond). 2020;20(4):e109-e113.
doi pubmed - Huang Y, Lu Y, Huang YM, Wang M, Ling W, Sui Y, Zhao HL. Obesity in patients with COVID-19: a systematic review and meta-analysis. Metabolism. 2020;113:154378.
doi pubmed - Petrakis D, Margina D, Tsarouhas K, Tekos F, Stan M, Nikitovic D, Kouretas D, et al. Obesity a risk factor for increased COVID19 prevalence, severity and lethality (Review). Mol Med Rep. 2020;22(1):9-19.
doi pubmed - Sanchis-Gomar F, Lavie CJ, Mehra MR, Henry BM, Lippi G. Obesity and outcomes in COVID-19: when an epidemic and pandemic collide. Mayo Clin Proc. 2020;95(7):1445-1453.
doi pubmed - Yang J, Hu J, Zhu C. Obesity aggravates COVID-19: A systematic review and meta-analysis. J Med Virol. 2021;93(1):257-261.
doi pubmed - Zhou Y, Chi J, Lv W, Wang Y. Obesity and diabetes as high-risk factors for severe coronavirus disease 2019 (Covid-19). Diabetes Metab Res Rev. 2021;37(2):e3377.
doi - Simonnet A, Chetboun M, Poissy J, Raverdy V, Noulette J, Duhamel A, Labreuche J, et al. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (Silver Spring). 2020;28(7):1195-1199.
doi pubmed - Pasquarelli-do-Nascimento G, Braz-de-Melo HA, Faria SS, Santos IO, Kobinger GP, Magalhaes KG. Hypercoagulopathy and adipose tissue exacerbated inflammation may explain higher mortality in COVID-19 patients with obesity. Front Endocrinol (Lausanne). 2020;11:530.
doi pubmed - Favre G, Legueult K, Pradier C, Raffaelli C, Ichai C, Iannelli A, Redheuil A, et al. Visceral fat is associated to the severity of COVID-19. Metabolism. 2021;115:154440.
doi pubmed - Pan F, Ye T, Sun P, Gui S, Liang B, Li L, Zheng D, et al. Time course of lung changes at chest CT during recovery from coronavirus disease 2019 (COVID-19). Radiology. 2020;295(3):715-721.
doi pubmed - Francone M, Iafrate F, Masci GM, Coco S, Cilia F, Manganaro L, Panebianco V, et al. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur Radiol. 2020;30(12):6808-6817.
doi pubmed - Noumura Y, Kamishima T, Sutherland K, Nishimura H. Visceral adipose tissue area measurement at a single level: can it represent visceral adipose tissue volume? Br J Radiol. 2017;90(1077):20170253.
doi pubmed - Pediconi F, Rizzo V, Schiaffino S, Cozzi A, Della Pepa G, Galati F, Catalano C, et al. Visceral adipose tissue area predicts intensive care unit admission in COVID-19 patients. Obes Res Clin Pract. 2021;15(1):89-92.
doi pubmed - Bonanad C, Garcia-Blas S, Tarazona-Santabalbina F, Sanchis J, Bertomeu-Gonzalez V, Facila L, Ariza A, et al. The effect of age on mortality in patients with COVID-19: a meta-analysis with 611,583 subjects. J Am Med Dir Assoc. 2020;21(7):915-918.
doi pubmed - Conti CB, Henchi S, Coppeta GP, Testa S, Grassia R. Bleeding in COVID-19 severe pneumonia: The other side of abnormal coagulation pattern? Eur J Intern Med. 2020;77:147-149.
doi pubmed - Bargellini I, Cervelli R, Lunardi A, Scandiffio R, Daviddi F, Giorgi L, Cicorelli A, et al. Spontaneous bleedings in COVID-19 patients: an emerging complication. Cardiovasc Intervent Radiol. 2020;43(7):1095-1096.
doi pubmed - Berger JS, Connors JM. Anticoagulation in COVID-19: reaction to the ACTION trial. Lancet. 2021;397(10291):2226-2228.
doi - INSPIRATION Investigators, Sadeghipour P, Talasaz AH, Rashidi F, Sharif-Kashani B, Beigmohammadi MT, Farrokhpour M, et al. Effect of intermediate-dose vs standard-dose prophylactic anticoagulation on thrombotic events, extracorporeal membrane oxygenation treatment, or mortality among patients with COVID-19 admitted to the intensive care unit: the INSPIRATION randomized clinical trial. JAMA. 2021;325(16):1620-1630.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


