| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Letter to the Editor
Volume 14, Number 3, March 2022, pages 142-145
Potential Biomarkers Associated With the Nature of Uterine Benign Mesenchymal Tumors
Saya Tamuraa, Takuma Hayashia, b, d, Kaoru Abikoa, Ikuo Konishia, c
aNational Hospital Organization, Kyoto Medical Centre, Kyoto, Japan
bSTART-Program, Japan Science and Technology Agency (JST), Tokyo, Japan
cKyoto University Graduate School of Medicine, Kyoto, Japan
dCorresponding Author: Takuma Hayashi, National Hospital Organization, Kyoto Medical Center, Mukaihatake-cho, Fushimi-ku, Kyoto 612-8555, Japan
Manuscript submitted February 9, 2022, accepted March 7, 2022, published online March 21, 2022
Short title: Molecular Approach of Uterine Benign Mesenchymal
doi: https://doi.org/10.14740/jocmr4683
| To the Editor | ▴Top |
Uterine leiomyomas are benign tumors that develop in the myometrium of the uterus. Estrogen is involved in their development and progression, and even in premenopausal patients who had strong symptoms, uterine leiomyomas significantly shrink after menopause. However, since uterine leiomyoma cells have a wide variety of properties, various pathological conditions of uterine leiomyomas are observed in clinical practice. Intravenous leiomyomatosis is a relatively rare benign tumor in which smooth muscle tissue grows and spreads intravenously. Intravenous leiomyomas are histologically benign, but these sometimes spread continuously to the inferior vena cava and heart and cause sudden death. A benign metastasizing leiomyoma is a condition in which metastatic lesions of benign leiomyoma are found in distant organs of women with a history of uterine leiomyoma. In metastasizing leiomyomas, lung metastasis is the most common. The causes of uterine leiomyomas include several gene or chromosomal abnormalities (mutations such as translocations and rearrangements, including 12q chromosomes and 7q gene deletion), but its pathogenic mechanism has not been clearly understood.
Uterine smooth muscle tumors are mesenchymal tumors that differentiate into smooth muscle cells and are the most common uterine tumors. Uterine smooth muscle tumors are classified as leiomyoma, a benign uterine smooth muscle, leiomyosarcoma, a malignant uterine smooth muscle tumor, and a smooth muscle tumor of uncertain malignant potential of unknown malignancy. The differential diagnosis of malignant uterine smooth muscle tumors is performed by histopathological observation based on nuclear atypia, number of fissions, and coagulation necrosis as indicators. The differentiation into uterine smooth muscle cells is characterized by a mutually orthogonal bundled proliferation of spindle-shaped cells consisting of blunt, elongated nuclei at both ends and acidophilic cytoplasm. Normal uterine leiomyomas and uterine leiomyosarcomas have the morphological characteristics of uterine smooth muscle cells, which are the basis for diagnosing smooth muscle tumors.
In uterine leiomyomas, in addition to typical smooth muscle tumors (normal type), there are smooth muscle tumors (special type) with special clinical findings [1]. In special uterine leiomyomas, a large number of increased fissions are observed. In clinical practice, as uterine leiomyomas, mitotically active leiomyomas, leiomyomas with increased mitotic activity, cellular leiomyomas, leiomyomas with bizarre nuclei, symplastic leiomyomas, epithelioid leiomyomas, myxoid leiomyomas, lipomatous variants, lipoleiomyomas, dissecting leiomyomas, intravenous leiomyomatosis, benign metastasizing leiomyomas, and disseminated peritoneal leiomyomatosis are observed. In uterine leiomyomas, in addition to a normal spindle-shaped type, special leiomyomas are also observed, such as epithelioid-leiomyosarcomas and myxoid leiomyosarcomas [1, 2].
Uterine leiomyoma is the most frequently occurring gynecologic tumor in females. Uterine leiomyomas are usually found in women in their 40s and 50s [1]. Uterine leiomyomas develop in 20-30% of females in their 30s and in 40% of females in their 40s [2, 3]. Including the tiny tumors found under a microscope, about 70-80% of women in their 50s develop uterine leiomyomas [2, 3]. The development of clinically problematic uterine leiomyomas is more common in women during sexual maturity and rarely in women under the age of 18 [2, 3]. Moreover, many premenopausal uterine leiomyomas shrink after menopause. Uterine leiomyomas also grow rapidly during pregnancy and while taking oral contraceptives, and these shrink with gonadotropin-releasing hormone analog administration [2]. Therefore, uterine leiomyomas are understood to be female hormone-dependent. Many women with uterine leiomyomas are asymptomatic, but some have vaginal bleeding, epidemics, and compression. Abdominal symptoms are present during progesterone treatment and pregnancy. Unpleasant symptoms depend on the size, number, and location of the onset of mesenchymal tumors. A torsion of pedunculated subarachnoid leiomyoma and acute intramyoma hemorrhage during pregnancy cause pain. When a leiomyoma grows, it causes pressure on adjacent organs and low back pain. Intravenous leiomyoma may rarely adhere to the heart or large blood vessels [4]. In benign metastasizing leiomyoma, lung metastases are most commonly observed. About 15 years after hysterectomy, metastatic lesions to multiple organs are observed.
The incidence of uterine leiomyosarcomas is approximately 37%, accounting for 1-2% of all uterine malignancies and 0.1-0.3% of all smooth muscle tumors [1, 5, 6]. The incidence of uterine leiomyosarcomas is significantly higher in the elderly (usually over 40 years) compared to the age of onset of uterine leiomyomas. In addition, postmenopausal women also have a high incidence of uterine leiomyosarcoma. There is no association between the risk factors for uterine leiomyosarcoma and those for endometrial cancer (i.e., pregnancy, obesity, and diabetes). Symptoms of uterine leiomyosarcoma include irregular bleeding, lower abdominal pain, pelvic pain, and intrapelvic mass [7]. In its early stages, tumor rupture and metastases may be rarely observed. It is not uncommon for uterine leiomyosarcomas to adhere to the gastrointestinal tract and bladder. The frequency of hematogenous metastases to the lungs is the highest. Although a rapid tumor growth is not a medical evidence for the development of sarcoma; if there is an increase in uterine tumors in postmenopausal women who have not received a hormone replacement therapy, malignant tumors should be suspected. The prognosis of uterine leiomyosarcoma is poor (5-year survival rate is about 25%), and its recurrence rate is high (45-75%), even at stage I [5, 6].
Intravenous leiomyoma is a rare clinical condition in which a histologically benign leiomyoma invades the uterus and extrauterine veins (Fig. 1). Uterine leiomyomas can reach the inferior vena cava and right atrium, but rarely the lungs. Macroscopically complex coiled or nodular intramuscular lesions develop into the veins inside and outside the uterus in a spiral or worm-like manner [3]. Intramuscular lesions do not extend into the arteries. Intravenous leiomyomas are histologically an image of normal leiomyomas, but there are also cases in which marked edema-like changes, fibrosis, and vitrification are observed. There are cases where abundant large and small blood vessel-like tumors are observed, and there are cases where pathological findings of original smooth muscle tumors are poor [8]. Intravenous leiomyomas are covered with endothelial cells. Intravenous smooth muscle tumors are also thought to have originated from venous smooth muscles. In clinical practice, it is difficult to distinguish the growth pattern of intravenous leiomyosarcomas from that of endometrial stromal sarcomas or leiomyosarcomas. The differential diagnosis is performed based on the histopathological findings of the tumors. Endometrial stromal sarcomas have a dense proliferation of small, oval, endometrial stromal cells and numerous spiral arterial microvessels. Uterine leiomyosarcomas have an atypical, fission, and neoplastic coagulative necrosis. Although benign leiomyomas are found in intravenous leiomyomas, cases of intravenous leiomyomas that reach the atrium may have a poor prognosis. An intravenous leiomyoma is basically a benign tumor.
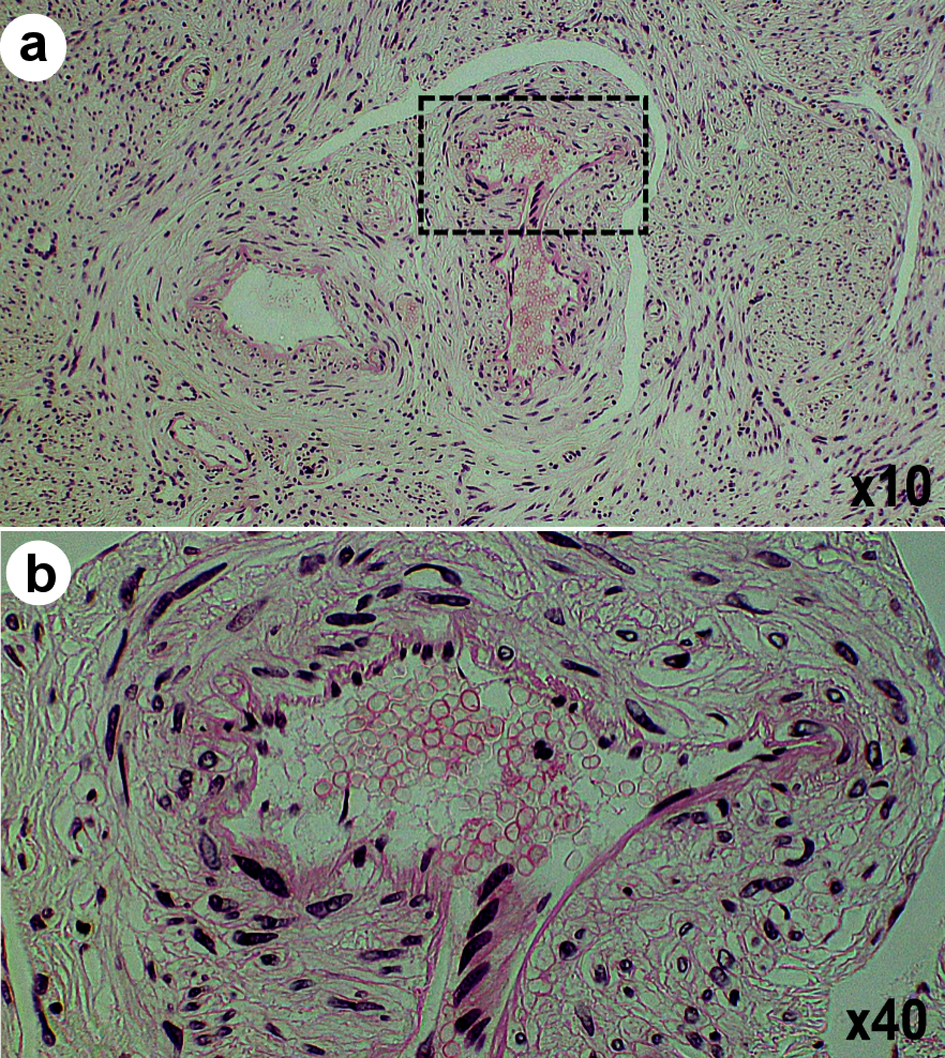 Click for large image | Figure 1. Intravenous leiomyomatosis. (a) Photograph obtained from a histopathological study of a tissue section shows leiomyoma with abundant blood vessels in dilated veins. (b) An enlarged image of the dotted rectangle area shown in (a). |
Histopathological findings of hematogenous metastases in malignant tumors have shown that tumor stem cells, which have differentiated from epithelial cells to mesenchymal cells, can infiltrate intravenously [9]. Based on clinical studies, in intravenous leiomyomatosis, more smooth muscle tumor stem-like cells are present in intravenous smooth muscle tumors as compared with extravenous smooth muscle tumors. It is considered that markers for smooth muscle tumor stem-like cells (i.e., CD34, CD133, etc.) may be applied to distinguish intravenous leiomyomas from other uterine mesenchymal tumors (Table 1) [9-17]. The expression of low-molecular weight protein 2 (LMP2)/b1i, which is one of immunoproteasome subunits, is observed in uterine leiomyomas, but its expression is not clearly detected in intravenous leiomyomatosis as in uterine leiomyosarcoma (Table 1) [9, 18]. Previous clinical studies have shown that intravenous leiomyomatosis may have the oncological properties of uterine leiomyosarcoma, where hematogenous metastases occur frequently [9, 18].
 Click to view | Table 1. Differential Expressions of SMA, CAV1, CCNB, CCNE, LMP2, NT5DC2, CD133, and Ki-67 in Human Uterine Mesenchymal Tumors and Uterine LANT-Like Tumor |
A histologically benign leiomyoma that metastasizes to distant organs, such as the lungs and lymph nodes, is called benign metastasizing leiomyoma, which most commonly metastasizes to the lungs [19, 20]. In benign metastatic leiomyoma, a tumor consisting of single or multiple non-atypical smooth muscle cells develops while adhering to the lung parenchyma and bronchioles and extruding clearly the surrounding tissue. Multiorgan metastases are often found by accident over a long period of time after surgery; therefore, a uterine leiomyoma of the primary lesion is often not sufficiently histologically investigated. It cannot be ruled out that such cases could be low-grade leiomyosarcomas. A benign metastasizing leiomyoma is estrogen receptor/progesterone receptor (ER/PgR)-positive and shrinks due to pregnancy and menopause. Therefore, benign metastasizing leiomyomas are considered hormone-dependent. A careful differentiation between benign metastasizing leiomyomas and lung metastases of endometrial stromal sarcomas is required.
Chromosomal abnormalities were found in about 40% cases of uterine leiomyoma. It was reported that t(12:14)(q15; p23-24), gene rearrangement including the short arm of chromosome 6, and deletion of the long arm of chromosome 7 are detected in uterine leiomyomas [21]. It was recently reported that such chromosomal abnormalities are due to genetic abnormalities similar to chromothripsis [22]. In addition, pathogenic variants of MED12 gene mutations were found in 70% of uterine leiomyoma cases [23, 24].
Compared to those in uterine leiomyomas, the genetic abnormalities in uterine leiomyomas are more complicated. Although various chromosomal abnormalities were reported in cases of uterine leiomyosarcomas, genetic abnormalities specific to uterine leiomyosarcomas have not been clarified. Chromosomal abnormalities that are frequently found in uterine leiomyomas are not found in uterine leiomyomas. MED12 gene mutations are extremely rare in uterine leiomyosarcomas. Therefore, it is considered that uterine leiomyosarcoma, which are caused by a malignant transformation of uterine leiomyomas, are extremely rare [25]. There is no oncological evidence for LMP2/b1i-deficient mice that spontaneously develop uterine leiomyomas [26]. Previous clinical studies reported gene deletions and mutations in uterine leiomyosarcomas, and no response to antitumor agents targeting these gene mutations in uterine leiomyosarcomas was observed [27, 28]. Further clinical research on pharmacogenomics must be conducted to develop targeted drugs for uterine mesenchymal tumors, including leiomyomas and uterine leiomyosarcomas.
The diagnosis of a mass extending from the inferior vena cava to the right heart system in middle-aged and older women should be done with caution. In addition, in uterine leiomyoma, it is necessary to pay attention to its accumulation in distant organs by imaging examination.
Acknowledgments
We thank the staff (Yukie Kawamura, Keiko Nakamura, Harue Aoki and Ayano Sakakibara) of the Division of Research Support, National Center for Global Health and Medicine, Kohnodai Hospital. We sincerely appreciate the technical assistance of the research staff at National Hospital Organization Kyoto Medical Center.
Financial Disclosure
This work was supported by the following research funding: Japan Society for the Promotion of Science for TH (grant No. 19K09840), START-program Japan Science and Technology Agency (JST) for TH (grant No. STSC20001), and National Hospital Organization Multicenter clinical study for TH (grant No. 2019- Cancer in general-02).
Conflict of Interest
The authors declare that they have no conflict of interest concerning this article.
Informed Consent
Not applicable.
Author Contributions
ST designed the research, and ST and TH collected and analyzed data. KA and IK wrote and approved the final paper.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Uterine leiomyoma. Female genital tumours, WHO classification of tumours, 5th ed., Vol.4. WHO classification of tumours editorial board. World Health Organization. 2020; p. 283-285.
- Zaloudek CJ, Hendrickson MR, Soslow RA. Mesenchymal tumors of uterus. In: Kurman RJ, Ellenson LH, Ronnett BM, eds. Blaustein's pathology of female genital tract. New York, NY: Springer; 2011:453.
doi - Weiss SW, Goldblum JR. Benign tumors of smooth muscle. In: Weiss SW, Goldblum JR, eds. Enzinger and Weiss's soft tissue tumors. Philadelphia, PA: Mosby Elsevier; 2008:520-521.
- Ip PP, Tse KY, Tam KF. Uterine smooth muscle tumors other than the ordinary leiomyomas and leiomyosarcomas: a review of selected variants with emphasis on recent advances and unusual morphology that may cause concern for malignancy. Adv Anat Pathol. 2010;17(2):91-112.
doi pubmed - Toledo G, Oliva E. Smooth muscle tumors of the uterus: a practical approach. Arch Pathol Lab Med. 2008;132(4):595-605.
doi pubmed - Uterine leiomyosarcoma. Female genital tumours WHO classification of tumours, 5th ed., Vol.4. WHO classification of tumours editorial board. World Health Organization. 2020; p. 272-276.
- Hayashi T, Yaegashi N, Tonegawa S, Konishi I. Potential biomarkers associated with malignancy in uterine mesenchymal tumors. European Journal of Gynaecological Oncology. 2021;42(5):824-828.
doi - Garg G, Mohanty SK. Uterine angioleiomyoma: a rare variant of uterine leiomyoma. Arch Pathol Lab Med. 2014;138(8):1115-1118.
doi pubmed - Tamura S, Hayashi T, Tokunaga H, Yaegashi N, Abiko K, Konishi I. Oncological properties of intravenous leiomyomatosis: involvement of mesenchymal tumor stem-like cells. Curr Issues Mol Biol. 2021;43(2):1188-1202.
doi pubmed - Hayashi T, Kawano M, Sano K, Ichimura T, Gur G, Yaish P, Zharhary D, et al. A novel diagnostic biomarker for human uterine leiomyosarcoma: PSMB9/beta1i. Chin Clin Oncol. 2017;6(2):22.
doi pubmed - Ravegnini G, Marino-Enriquez A, Slater J, Eilers G, Wang Y, Zhu M, Nucci MR, et al. MED12 mutations in leiomyosarcoma and extrauterine leiomyoma. Mod Pathol. 2013;26(5):743-749.
doi pubmed - Hayashi T, Sano K, Ichimura T, Kanai Y, Zharhary D, Aburatani H, Yaegashi N, et al. Characteristics of leiomyosarcoma: induction of hematogenous metastasis by isolated uterine mesenchymal tumor stem-like cells. Anticancer Res. 2020;40(3):1255-1265.
doi pubmed - Detection of uterine leiomyosarcoma using LMP2. Application JP2007548042A events. https://upload.umin.ac.jp/cgi-open-bin/ctr/ctr_view.cgi?recptno=R000044182.
- Hayashi T, Ichimura T, Yaegashi N, Shiozawa T, Konishi I. Expression of CAVEOLIN 1 in uterine mesenchymal tumors: No relationship between malignancy and CAVEOLIN 1 expression. Biochem Biophys Res Commun. 2015;463(4):982-987.
doi pubmed - Hayashi T, Sano K, Ichimura T, Tonegawa S, Yaegashi N, Konishi I. Diagnostic biomarker candidates including NT5DC2 for human uterine mesenchymal tumors. Intl J Trend in Sci Res and Dev (IJTSRD). 2021;5(3):604-606.
- Smooth muscle tumor of uncertain malignant potential of the uterine corpus. Female genital tumours WHO classification of tumours, 5th ed., Vol.4. WHO classification of tumours editorial board. World Health Organization. 2020; p. 279-280.
- Hayashi T, Ichimura T, Kasai M, Sano K, Zharhary D, Shiozawa T, Yaegashi N, et al. Characterization of Leiomyomatoid Angiomatous Neuroendocrine Tumour (LANT)-like Tumour in the Myometrium with Histopathological Examination. Anticancer Res. 2017;37(4):1765-1772.
doi pubmed - Hayashi T, Horiuchi A, Sano K, Hiraoka N, Kasai M, Ichimura T, Sudo T, et al. Potential role of LMP2 as tumor-suppressor defines new targets for uterine leiomyosarcoma therapy. Sci Rep. 2011;1:180.
doi pubmed - Biber R. [Benign metastasizing leiomyoma of the lung]. MMW Fortschr Med. 2009;151(26-29):46-47.
- Jiang H, Ma L, Qi XW, Yan LZ, Feng HX, Suo LJ, Liu B. Pulmonary benign metastasizing leiomyoma: a case report and literature review. Ann Palliat Med. 2021;10(5):5831-5838.
doi pubmed - Kurman RJ, Carcongiu ML, Herrington CS. WHO classification of tumours of female reproductive organs, 4th ed, IARC Press, Lyon. 2014.
- Mehine M, Kaasinen E, Makinen N, Katainen R, Kampjarvi K, Pitkanen E, Heinonen HR, et al. Characterization of uterine leiomyomas by whole-genome sequencing. N Engl J Med. 2013;369(1):43-53.
doi pubmed - Makinen N, Mehine M, Tolvanen J, Kaasinen E, Li Y, Lehtonen HJ, Gentile M, et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science. 2011;334(6053):252-255.
doi pubmed - Berta DG, Kuisma H, Valimaki N, Raisanen M, Jantti M, Pasanen A, Karhu A, et al. Deficient H2A.Z deposition is associated with genesis of uterine leiomyoma. Nature. 2021;596(7872):398-403.
doi pubmed - Matsubara A, Sekine S, Yoshida M, Yoshida A, Taniguchi H, Kushima R, Tsuda H, et al. Prevalence of MED12 mutations in uterine and extrauterine smooth muscle tumours. Histopathology. 2013;62(4):657-661.
doi pubmed - Hayashi T, Horiuchi A, Sano K, Yaegashi N, Konishi I. Uterine leiomyosarcoma tumorigenesis in Lmp2-deficient mice: involvement of impaired anti-oncogenic factor IRF1. Anticancer Res. 2015;35(9):4665-4679.
- Jonsson P, Bandlamudi C, Cheng ML, Srinivasan P, Chavan SS, Friedman ND, Rosen EY, et al. Tumour lineage shapes BRCA-mediated phenotypes. Nature. 2019;571(7766):576-579.
doi pubmed - Seligson ND, Kautto EA, Passen EN, Stets C, Toland AE, Millis SZ, Meyer CF, et al. BRCA1/2 functional loss defines a targetable subset in leiomyosarcoma. Oncologist. 2019;24(7):973-979.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


