| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 14, Number 3, March 2022, pages 111-118
Impact of Change in Allocation Score Methodology on Post Kidney Transplant Average Length of Stay
Hanadi Y. Hamadia, e , Hani M. Wadeib
, Jing Xua
, Dayana Martineza, Aaron Spauldingc
, Shehzad K. Niazid
, Tambi Jarmib
aDepartment of Health Administration, Brooks College of Health, University of North Florida, Jacksonville, FL, USA
bDepartment of Transplant, Mayo Clinic, Jacksonville, FL, USA
cDivision of Health Care Delivery Research, Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery, Mayo Clinic, Jacksonville, FL, USA
dDepartment of Psychiatry and Psychology, Mayo Clinic, Jacksonville, FL, USA
eCorresponding Author: Hanadi Y. Hamadi, Department of Health Administration, University of North Florida, Jacksonville, FL 32224, USA
Manuscript submitted January 26, 2022, accepted March 12, 2022, published online March 25, 2022
Short title: Kidney Transplant Average Length of Stay
doi: https://doi.org/10.14740/jocmr4673
| Abstract | ▴Top |
Background: In December 2014, a new Kidney Allocation System (KAS) was implemented nationwide to improve access and quality of care to historically disadvantaged patients. However, no study to date has examined the relationship between the KAS and potential changes in hospital length of stay (LOS). This study aimed to examine the relationship between the KAS implemented in December 2014 and potential changes in hospital LOS.
Methods: We used data from the Florida Agency for Health Care Administration on kidney transplant surgeries completed between 2011 and 2018. A cross-sectional cohort study design included seven hospitals that performed kidney transplants for the duration of the study. A propensity score matching approach was used to examine the relationship between KAS and LOS. All acute general medical and surgical hospitals in Florida that performed kidney transplant surgery were included in the analysis.
Results: We included 7,795 patients, 6,119 discharged to home, and 1,676 discharged to home with home health services after transplant. The average LOS prior to KAS was 6.52 days and 6.08 days post KAS. Propensity matched results show that patients transferred to home experienced a decrease in the LOS (coefficient (β) = -0.68; 95% confidence interval (CI): -0.95, -0.42) after the new allocation score was implemented. Similarly, patients transferred to home with home health experienced a decrease in the LOS (β = -1.90; 95% CI: -2.69, -1.11) after the new allocation was implemented.
Conclusion: In conclusion, results indicate that KAS implementation did not add a burden on the health system by increasing LOS when considering patients with similar characteristics before and after KAS implementation. KAS is an important policy change that appears to not negatively affect the LOS when sicker patients could receive a kidney transplant. Our findings improve our understanding of the KAS policy and its influence on the health system.
Keywords: Kidney transplantation; Inpatient stay; Organ procurement; Patient discharge; Propensity score; Hospitals
| Introduction | ▴Top |
Over the last decade, there has been a significant uptrend in kidney transplantation in the United States [1]. In addition to improved survival, a kidney transplant can save up to approximately $1.45 million per kidney recipient [2]. However, the increased success of transplantation has created high demands for kidney transplants and resulted in significant organ shortages [3, 4]. In 1984, to help regulate organ donation management, Congress passed the National Organ Transplant Act, which established the Organ Procurement and Transplantation Network under which the United Network for Organ Sharing (UNOS) allocation system began to operate [5]. In 1999, an organ allocation system platform was launched by UNOS to manage organs for potential recipients [5]. Since the development of the kidney allocation systems, physicians and policymakers alike have sought to balance patient demand and the best utilization of kidney organs. However, equal access and kidney organ utilization were not fully achieved [6].
In 2014, the new Kidney Allocation System (KAS) was introduced to balance the utility and equity of kidney allocation for historically disadvantaged recipients. The main goals of KAS were to eliminate age mismatching [7], increase access to recipients that are highly sensitized to human leukocyte antigens (HLAs) [8] and improve access to disadvantaged minorities, specifically African American population [9]. Historically disadvantaged recipients were considered difficult to match and accumulate the longest wait time on the transplant waiting list [10]. These patients were sensitized against the HLA and had developed high levels of autoantibodies which limit the number of donors they could match with [11]. KAS increased the opportunity for patients who had the probability of surviving the longest, receiving the longest-lasting kidneys “longevity matching,” and improving matching for HLA-sensitized candidates who had historically lower transplantation rates [7, 12]. The new system created a new metric, estimated post-transplant survival (EPTS), which sought to simplify the recipient criteria [5]. Following the implementation of KAS, there was an access increase for highly sensitized patients who were transplanted [8]. However, while KAS aimed to improve equitable kidney transplantation, it may have led to an increase in cold ischemia time, associated adverse effects, and financial burden [8, 13, 14]. KAS aimed to allocate the best kidneys to the best recipient considering the highly sensitized patients. This process had required more resources to mobilize the available kidney to potential recipients far away from the donor hospital and ultimately add more cost on the system and cold ischemia time on the organ [15]. A study by Serrano et al using retrospective data and a Poisson regression model showed a 4% increase in the average length of stay (LOS) was caused by a 10-h increase in cold ischemia time while delayed graft function showed an increase of 60% in average LOS [16].
Despite various unequivocal improvements in the KAS donor ranking metric system, the potential impact on the average LOS has not been considered. Increased LOS is often associated with a greater burden of disease or organ-related complications, causing reimbursement and regulatory implications [17]. Five days has been the average LOS for a kidney transplant [17]. However, a study by Lin et al showed that both LOS < 4 days post-kidney transplant and > 2 weeks were associated with significant adverse effects for recipient survival [18], and a study by McAdams-DeMarco et al showed that non-frail and frail recipients with a longer LOS had increased mortality [17]. Our study investigated the impact of change in the allocation score methodology on post kidney transplant average LOS.
| Materials and Methods | ▴Top |
Data source
Data of kidney transplants between January 1, 2011 and December 31, 2018 were obtained from the Florida Hospital Inpatient Data File collected by the Florida Agency for Health Care Administration (AHCA). The dataset contains administrative records of patient clinical and demographic information from over 13 million inpatient hospital discharges for over 147 inpatient hospitals across Florida.
Participants
Patients receiving a whole kidney transplant were included in the study, but patients receiving other organs simultaneously were excluded. Kidney transplantation International Classification of Diseases (ICD) Clinical Modification (CM) codes were utilized to identify our sample. The ICD-9-CM indication of kidney transplant codes is Transplant of Kidney (55.6) and Transplanted Kidney (55.69). ICD-10-CM indication of kidney transplant codes is Transplantation of Right Kidney, Allogeneic, Open Approach Transplantation of Right Kidney, Syngeneic, Open Approach (0TY00Z1), Transplantation of Right Kidney, Zooplastic, Open Approach (0TY00Z2), Transplantation of Left Kidney, Allogeneic, Open Approach (0TY10Z0), Transplantation of Left Kidney, Syngeneic, Open Approach (0TY10Z1), and Transplantation of Left Kidney, Zooplastic, Open Approach (0TY10Z2). ICD-9-CM to ICD-10-CM conversion occurred at the same time that the KAS was implemented; therefore, to ensure their ICD coding was accounted for, all identified kidney transplant counts were cross-checked with the Organ Procurement and Transplantation Network database.
Measures
We investigated the difference in kidney transplant patients’ hospital LOS before and after the change in allocation score in December 2014. Our outcome variable was patients’ LOS. LOS was standardized by measuring the total number of days from the day of the procedure (kidney transplant surgery) until the day of discharge to either home or home with home health services. Patients discharged to another facility were excluded from this study because the sample size was small (n = 59). Our primary independent variable was the change in kidney allocation score and the implantation of the KAS, which was operationalized as before the change in allocation score (January 1, 2011 - December 4, 2014) and after the change in allocation score (December 5, 2014 - December 31, 2018). In this study, we included patient gender (male/female), race and ethnicity (non-Hispanic white, non-Hispanic black, Hispanic, and other), diabetes diagnosis (yes/no), Elixhauser Comorbidity Index, and dialysis encounter (yes/no).
Traditionally, the Elixhauser Comorbidity Index includes diabetes as a comorbidity; however, we excluded diabetes from the Elixhauser Comorbidity Index and recalculated the index [19]. We limited the analysis to the 4 years before the change and 4 years after the change date and to only hospitals that performed kidney transplant surgery for the entire study period. Our final sample consisted of a total of seven hospitals and 7,795 patients who had received a kidney transplant. Of the 7,795, 6,119 patients were discharged to home, and 1, 676 were discharged to home with home health services. Of the 6,119 patients discharged to home, 2,698 had undergone their procedure before the change in allocation score and 3,421 after the change in allocation score. Of the 1,676 patients discharged to home with home health services, 823 had undergone their procedure before the change in allocation score and 852 after the change in allocation score.
Statistical analysis
We used a cross-sectional design to examine the association between hospitals’ LOS and KAS implantation. All analyses were executed using Stata version 17MP. Frequencies and percentages were used to summarize categorical variables, while means and standard deviations (SDs) were used to summarize continuous variables. Propensity scores were calculated as the probability of receiving a transplant after the allocation score has changed and matched on patient characteristics. Our matched and balanced sample of patients discharged to home consisted of 1,592 patients before the change in allocation score, and 3,421 patients post the change in allocation score. Our matched and balanced sample of patients discharged to home with home health services consisted of 437 patients prior to the change in allocation score, and 852 patients post the change in allocation score. Patient characteristics were compared between prior and post allocation scores using the standardized mean difference for an effect size of < 0.01 or a bias percent of < 10%. Yang and Dalton’s [20, 21] methodology of calculating the standardized mean difference for categorical variables was used. To ensure rigor, the matching procedure was repeated 100 times using a random nearest neighbor matching from 1 to 3. The matching sample with the minimal Euclidean distance was used for the analysis [22]. In our best-fitting model, we used a 1 prior to 2 post allocation score (1:2) matching with a caliper of 0.02. The university’s Institutional Review Board categorized the research as exempt. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
| Results | ▴Top |
Patient characteristics by allocation score change (2011 - 2014 vs. 2015 - 2018) are shown in Table 1. Results demonstrate that most patients in each group were transferred to home (2011 - 2014: 75.6% and 2015 - 2018: 80.0%, P < 0.0001) compared to home with home health. Between the two time periods, there was a slight increase in the percentage of males receiving a transplant (59.8% to 62.4%, P = 0.016), and there was an increase in the percentage of patients receiving dialysis (16.44% to 24.05, P < 0.0001). Further, the percentage of non-Hispanic black (28.8% to 32.2%), Hispanic (18.3% to 21.9%), and “other patients” (6.3% to 7.2%) all increased after the allocation score change. In comparison, the percentage of non-Hispanic white patients (46.6% to 38.7%) decreased despite maintaining a majority status. Additionally, the results show that the average LOS reduced from 6.52 (SD: 4.36) to 6.08 (SD: 4.17) days (P < 0.0001), and the Elixhauser Comorbidity Index increased from 3.89 (SD: 1.60) to 4.00 (SD 1.56) (P = 0.006). Finally, the percentage of diabetic patients increased from 31.8% to 34.8% (P = 0.005) from pre- to post-KAS. Figure 1 shows the discharged status across the study period. Results show that for patients discharged to home, there was a decline in the LOS after the implementation of the KAS at the end of 2015; however, for patients discharged to home with home health services, there was a sharp increase immediately after the implementation of the KAS.
 Click to view | Table 1. Patient Characteristics |
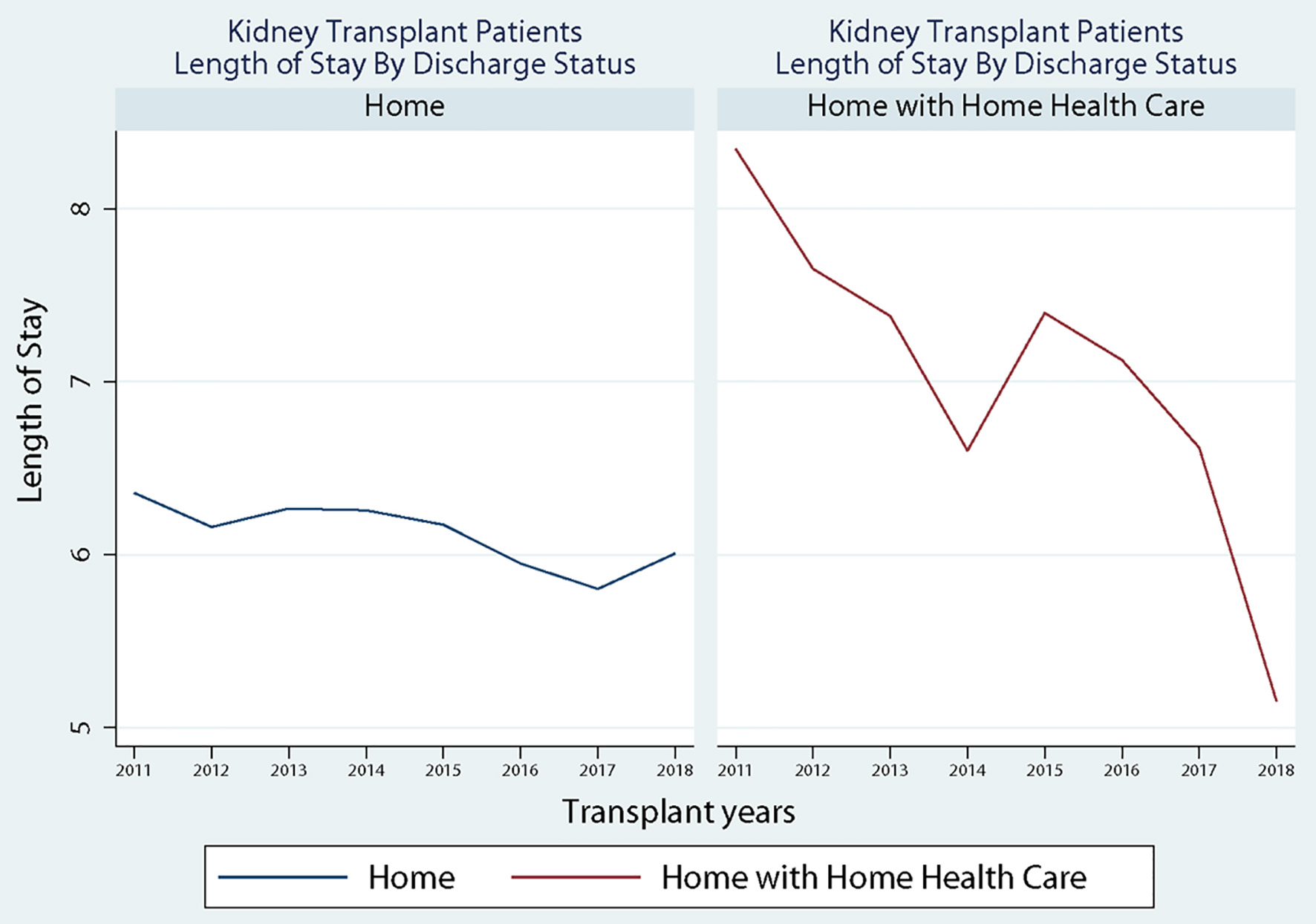 Click for large image | Figure 1. Discharged status across study period. |
Pre- and post-matched samples for patients transferred to home are displayed in Table 2. Prior to matching, the sample was unbalanced with regard to patient Elixhauser Comorbidity Index, gender, race and ethnicity, patient on dialysis, and the location where patients received their surgery. After matching, all variables were appropriately balanced as indicated by an effect size of less than 0.1 and a non-significant P-value between the allocation score years. Similarly, Table 3 demonstrates the pre- and post-matched sample for patients transferred to home with home health. Prior to matching, the sample was unbalanced with regard to patient age, patients on dialysis, and patients with diabetes. After matching, all variables were appropriately balanced as indicated by an effect size of less than 0.1 and a non-significant P-value between the allocation score years.
 Click to view | Table 2. Matching and Balancing Procedure Sample of Patients Transferred to Home |
 Click to view | Table 3. Matching and Balancing Procedure Sample of Patients Transferred to Home With Home Health Services |
Propensity score matching results for patients transferred to home and those transferred to home health are displayed in Table 4. For patients transferred to home, there was a decrease in the LOS (coefficient (β) = -0.68; 95% confidence interval (CI): -0.95, -0.42) after the new allocation score was implemented. Similarly, for patients transferred to home with home health, there was a decrease in the LOS (β = -1.90; 95% CI: -2.69, -1.11) after the new allocation was implemented.
 Click to view | Table 4. Propensity Score Model Results |
| Discussion | ▴Top |
The motivation for implementing a new KAS was to make kidney transplants more available to populations who were disadvantaged under the old allocation system because of their sensitization and dialysis time [23, 24]. Patients with panel-reactive antibodies (PRAs) 99% and higher had a significant increase in transplant rate from 2.4% pre-KAS to 17.7% post-KAS [25]. Patients with greater than 10 years on dialysis had an increased transplant rate from 4.3% pre-KAS to 18.6% post-KAS [7]. The KAS also included a strategy to favorably allocate the highest quality kidneys to recipients in the top 20th percentile of estimated post-transplantation survival [8]. With these multiple positive changes related to KAS, the direct impact of KAS on post-transplant LOS has not been extensively evaluated. Our study analyzed LOS data for kidney transplant recipients between 2011 and 2018 in the state of Florida. A total of 3,521 patients were transplanted before the KAS implementation and 4,274 after. Our results indicate that after matching, patients receiving a transplant after the KAS change may have a reduced LOS. This reduction was not dependent upon whether they were discharged home or home with home healthcare. To reiterate, discharge to a location other than home was not evaluated in this study due to the limited number of patients experiencing a non-home discharge.
While KAS has sought to create a more equitable sharing system for kidneys, there are concerns the system creates adverse effects due to increased cold ischemia time (CIT), resulting in delayed graft function (DGF) [26, 27]. Previous work by Serrano and colleagues indicated that increases in LOS can be attributed to increases in CIT and DGF. However, Taber et al found an increase in the incidence of DGF rates (5.4%, 95% CI: 23.3% to 7.4%) after kidney transplant following the implementation of KAS with no impact on LOS (0.12 days, 95% CI: -0.11 to 0.35) [28]. Despite the limited studies to connect DGF and LOS, it is acceptable to consider DGF as one of the factors that could impact LOS after kidney transplantation. This paradoxical pattern, after KAS implementation, with initial increased DGF and no change in LOS could be explained by our findings. When assessing the discharged status by discharged to home vs. discharged to home with home health, we identified an initial increase in home health utilization after KAS implementation that did not persist beyond 2015 (Fig. 1). We have shown the overall discharge home with home health status to decrease from 2011 until 2018 except the temporary increase in 2015 after KAS implementation. This could arguably be related to the increased number of more ill patients transplanted right after the KAS implementation and could have impacted the LOS but was counteracted by discharging patients with home health [29]. When we matched the patients’ comorbidities before and after KAS implementation, the LOS continued to be better among patients discharged to home and patients discharged home with home health.
Significantly, these results could alleviate some concerns regarding the adverse effects of the KAS change. LOS is an indicator used to assess the efficiency of hospital quality of delivered care [30]. Specifically, Lingsma et al analyzed administrative data from the Global Comparators Project from 26 hospitals on patients discharged between 2007 and 2012. They found that mortality and LOS were correlated at the patient and the hospital level. Patients in the upper quartile LOS had higher odds of mortality than those in the lowest quartile [31]. Hospitals with high standardized mortality had higher proportions of prolonged LOS [30]. Finally, shorter LOS reduces the burden of medical fees and increases the hospital bed turnover, which in turn increases the profit margin while reducing overall social costs [32]. The health care system has adopted a goal of resources optimization without harming patients in order to reduce the cost of kidney transplants related to surgical intervention and hospitalization [33].
Limitations
This study is limited by the data source and data points available. The administrative dataset used for this analysis is limited to the hospital encounter and does not contain data associated with events occurring outside of that encounter. As a result, outcomes such as post-hospital survival, readmissions, or graft failure are not identifiable. The dataset also does not provide information regarding the number of dialysis treatments, medications, or CIT or graft function measures. As a result, the conclusions drawn from this study are limited by these data limitations. Additionally, results of the analysis are limited to the State of Florida, and as such limits generalizability to centers outside of Florida. Furthermore, there is a chance that by matching on age and diabetes status, which are components of the new KAS, effects could be partially mediated as it may have masked pre- to post-KAS differences. However, we do not feel the propensity match likely hid the effect as a 1 prior to 2 post allocation score match was used to better assess differences. Further, the objective was to determine if LOS differed before vs. after the change. By matching on the identified characteristics, we are better able to assess if the identified crude unmatched increase in LOS when considering patients with similar characteristics. Future studies should further explore these components and seek to assess how specific KAS evaluated characteristics might influence differences in LOS. Finally, LOS could be continuing to decline for reasons separate from KAS or in combination with KAS, like changes in center behavior, that cannot be ascertained for in data. Future studies should seek to evaluate associations with LOS and post-transplant survival, graft failure, and CIT both before and after the KAS.
Conclusion
In conclusion, the optimal utilization of resources has become a standard goal in modern clinical practice. Our report indicates that KAS implementation may not have added a new burden on the health system by increasing LOS when considering similar patients receiving transplants before and after the KAS implementation.
Acknowledgments
The authors acknowledge the support of the University of North Florida, Mayo Clinic Jacksonville, and The Agency for Health Care Administration.
Financial Disclosure
Authors have no funding sources to disclose for the study.
Conflict of Interest
All authors declare no potential conflict of interest.
Informed Consent
Not applicable.
Author Contributions
Dr. Hanadi Y. Hamadi assisted in conceiving and designing the work, analyzing and interpreting the data, drafting the article, and providing intellectual content of critical importance to the work described. Hani M. Wadei assisted in interpreting data, providing intellectual content of critical importance to the work described. Dr. Jing Xu assisted in analyzing and interpreting the data and providing intellectual content of critical importance to the work described. Ms. Dayana Martinez assisted in drafting the article and literature search. Dr. Aaron Spaulding assisted in interpreting data and providing intellectual content of critical importance to the work described. Dr. Shehzad K. Niazi assisted in interpreting data, providing intellectual content of critical importance to the work described. Dr. Tambi Jarmi assisted in interpreting data, providing intellectual content of critical importance to the work described, and drafting the article.
Data Availability
The Florida Agency for Health Care Administration inpatient discharge data is available for purchase at https://www.floridahealthfinder.gov/Researchers/OrderData/order-data.aspx and restrictions apply to the availability of these data, which were used under license for this study.
Abbreviations
AHCA: Florida Agency for Health Care Administration; CI: confidence interval; CIT: cold ischemia time; CM: Clinical Modification; EPTS: estimated post-transplant survival; KAS: Kidney Allocation System; ICD: International Classification of Diseases; LOS: length of stay; UNOS: United Network for Organ Sharing
| References | ▴Top |
- Hart A, Smith JM, Skeans MA, Gustafson SK, Wilk AR, Castro S, Foutz J, et al. OPTN/SRTR 2018 annual data report: kidney. Am J Transplant. 2020;20(Suppl s1):20-130.
doi pubmed - Held PJ, McCormick F, Ojo A, Roberts JP. A cost-benefit analysis of government compensation of kidney donors. Am J Transplant. 2016;16(3):877-885.
doi pubmed - Bastani B. The present and future of transplant organ shortage: some potential remedies. J Nephrol. 2020;33(2):277-288.
doi pubmed - McCormick F, Held PJ, Chertow GM. The terrible toll of the kidney shortage. J Am Soc Nephrol. 2018;29(12):2775-2776.
doi pubmed - Stegall MD, Stock PG, Andreoni K, Friedewald JJ, Leichtman AB. Why do we have the kidney allocation system we have today? A history of the 2014 kidney allocation system. Hum Immunol. 2017;78(1):4-8.
doi pubmed - Wang CJ, Wetmore JB, Israni AK. Old versus new: Progress in reaching the goals of the new kidney allocation system. Hum Immunol. 2017;78(1):9-15.
doi pubmed - Stewart DE, Kucheryavaya AY, Klassen DK, Turgeon NA, Formica RN, Aeder MI. Changes in Deceased Donor Kidney Transplantation One Year After KAS Implementation. Am J Transplant. 2016;16(6):1834-1847.
doi pubmed - Sethi S, Najjar R, Peng A, Mirocha J, Vo A, Bunnapradist S, Jordan SC, et al. Allocation of the highest quality kidneys and transplant outcomes under the new kidney allocation system. Am J Kidney Dis. 2019;73(5):605-614.
doi pubmed - Harding K, Mersha TB, Pham PT, Waterman AD, Webb FA, Vassalotti JA, Nicholas SB. Health disparities in kidney transplantation for African Americans. Am J Nephrol. 2017;46(2):165-175.
doi pubmed - Parsons RF, Locke JE, Redfield RR, 3rd, Roll GR, Levine MH. Kidney transplantation of highly sensitized recipients under the new kidney allocation system: A reflection from five different transplant centers across the United States. Hum Immunol. 2017;78(1):30-36.
doi pubmed - Baxter-Lowe LA, Kucheryavaya A, Tyan D, Reinsmoen N. CPRA for allocation of kidneys in the US: More candidates >/=98% CPRA, lower positive crossmatch rates and improved transplant rates for sensitized patients. Hum Immunol. 2016;77(5):395-402.
doi pubmed - Friedewald JJ, Turgeon N. Early experience with the new kidney allocation system: a perspective from a transplant center. Clin J Am Soc Nephrol. 2017;12(12):2060-2062.
doi pubmed - Formica RN, Jr. A critical assessment on kidney allocation systems. Transplant Rev (Orlando). 2017;31(1):61-67.
doi pubmed - Senanayake S, Graves N, Healy H, Baboolal K, Barnett A, Sypek MP, Kularatna S. Deceased donor kidney allocation: an economic evaluation of contemporary longevity matching practices. BMC Health Serv Res. 2020;20(1):931.
doi pubmed - Hart A, Gustafson SK, Skeans MA, Stock P, Stewart D, Kasiske BL, Israni AK. OPTN/SRTR 2015 Annual Data Report: Early effects of the new kidney allocation system. Am J Transplant. 2017;17(Suppl 1):543-564.
doi pubmed - Serrano OK, Vock DM, Chinnakotla S, Dunn TB, Kandaswamy R, Pruett TL, Feldman R, et al. The relationships between cold ischemia time, kidney transplant length of stay, and transplant-related costs. Transplantation. 2019;103(2):401-411.
doi pubmed - McAdams-DeMarco MA, King EA, Luo X, Haugen C, DiBrito S, Shaffer A, Kucirka LM, et al. Frailty, length of stay, and mortality in kidney transplant recipients: a national registry and prospective cohort study. Ann Surg. 2017;266(6):1084-1090.
doi pubmed - Lin SJ, Koford JK, Baird BC, Habib AN, Reznik I, Chelamcharla M, Shihab FS, et al. The association between length of post-kidney transplant hospitalization and long-term graft and recipient survival. Clin Transplant. 2006;20(2):245-252.
doi pubmed - Thompson NR, Fan Y, Dalton JE, Jehi L, Rosenbaum BP, Vadera S, Griffith SD. A new Elixhauser-based comorbidity summary measure to predict in-hospital mortality. Med Care. 2015;53(4):374-379.
doi pubmed - Yang D, Dalton JE, editors. A unified approach to measuring the effect size between two groups using SAS®. SAS global forum 2012; Cleveland, OH, U.S.A.
- Bayoumi AM. STDDIFF: Stata module to compute standardized differences for continuous and categorical variables. Statistical Software Components S458275. Chestnut Hill, MA, U.S.A: Boston College Department of Economics; 2016.
- Fullerton B, Pohlmann B, Krohn R, Adams JL, Gerlach FM, Erler A. The comparison of matching methods using different measures of balance: benefits and risks exemplified within a study to evaluate the effects of German disease management programs on long-term outcomes of patients with type 2 diabetes. Health Serv Res. 2016;51(5):1960-1980.
doi pubmed - Israni AK, Salkowski N, Gustafson S, Snyder JJ, Friedewald JJ, Formica RN, Wang X, et al. New national allocation policy for deceased donor kidneys in the United States and possible effect on patient outcomes. J Am Soc Nephrol. 2014;25(8):1842-1848.
doi pubmed - Patzer RE, Basu M, Smith KD, Plantinga L, Mohan S, Escoffery C, Kim JJ, et al. Awareness of the new kidney allocation system among United States dialysis providers with low waitlisting. Am J Nephrol. 2018;47(2):115-119.
doi pubmed - Gebel HM, Kasiske BL, Gustafson SK, Pyke J, Shteyn E, Israni AK, Bray RA, et al. Allocating deceased donor kidneys to candidates with high panel-reactive antibodies. Clin J Am Soc Nephrol. 2016;11(3):505-511.
doi pubmed - Samoylova ML, Shaw BI, Irish W, McElroy LM, Connor AA, Barbas AS, Sanoff S, et al. Decreased graft loss following implementation of the kidney allocation score (KAS). Am J Surg. 2020;220(5):1278-1283.
doi pubmed - Wainright JL, Kucheryavaya AY, Klassen DK, Stewart DE. The impact of the new kidney allocation system on prior living kidney donors' access to deceased donor kidney transplants: an early look. Am J Transplant. 2017;17(4):1103-1111.
doi pubmed - Taber DJ, DuBay D, McGillicuddy JW, Nadig S, Bratton CF, Chavin KD, Baliga PK. Impact of the new kidney allocation system on perioperative outcomes and costs in kidney transplantation. J Am Coll Surg. 2017;224(4):585-592.
doi pubmed - Watanabe Y, Takeda H, Kobayashi B. Inorganic sulfate-induced swelling in isolated rat-liver mitochondria. J Biochem. 1969;65(3):435-441.
doi pubmed - Shulan M, Gao K. Revisiting hospital length of stay: what matters? Am J Manag Care. 2015;21(1):e71-77.
- Lingsma HF, Bottle A, Middleton S, Kievit J, Steyerberg EW, Marang-van de Mheen PJ. Evaluation of hospital outcomes: the relation between length-of-stay, readmission, and mortality in a large international administrative database. BMC Health Serv Res. 2018;18(1):116.
doi pubmed - Baek H, Cho M, Kim S, Hwang H, Song M, Yoo S. Analysis of length of hospital stay using electronic health records: A statistical and data mining approach. PLoS One. 2018;13(4):e0195901.
doi pubmed - Villa M, Siskind E, Sameyah E, Alex A, Blum M, Tyrell R, Fana M, et al. Shortened length of stay improves financial outcomes in living donor kidney transplantation. Int J Angiol. 2013;22(2):101-104.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


