| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Short Communication
Volume 14, Number 4, April 2022, pages 165-169
Participant Perspectives Concerning Resuming Clinical Research in the Era of COVID-19
Daniel S. Hsiaa, d, Karlie M. Williamsb, Robbie A. Beylc
aClinical Trials Unit, Pennington Biomedical Research Center, Baton Rouge, LA, USA
bDepartment of Biology, Xavier University of Louisiana, New Orleans, LA, USA
cDepartment of Biostatistics, Pennington Biomedical Research Center, Baton Rouge, LA, USA
dCorresponding Author: Daniel S. Hsia, Clinical Trials Unit, Pennington Biomedical Research Center, Baton Rouge, LA 70808, USA
Manuscript submitted January 25, 2022, accepted March 24, 2022, published online April 23, 2022
Short title: Perspectives on Resuming Research
doi: https://doi.org/10.14740/jocmr4670
| Abstract | ▴Top |
Background: The coronavirus disease 2019 (COVID-19) pandemic caused a shutdown of clinical research but offered a unique opportunity to understand attitudes and motivations around contributing to clinical research and resuming in-person visits during a pandemic.
Methods: We conducted an anonymous survey study at Pennington Biomedical Research Center (PBRC) in participants returning for in-person visits from May 26, 2020 to August 11, 2020 and in people who previously expressed interest in research via an online Research Electronic Data Capture (REDCap) survey from August 6, 2020 to September 11, 2020. The survey gathered demographic information and presented statements that required answers on a scale of 1 (absolutely disagree) to 10 (absolutely agree). Two hundred fifty-one people completed paper surveys in-person while 1,537 people completed the survey online.
Results: Online participants were more likely to be female (75.2% vs. 56.8%), more likely to have had COVID-19 symptoms (19.6% vs. 5.2%), and more likely to know someone with COVID-19 (72.7% vs. 49.4%). More people who came in-person thought they were low risk for severe COVID-19 compared to those who filled out the survey online (52.2% vs. 38.4%, P = 0.0002). More people who completed the survey online preferred to do as many study visits over the phone or internet as possible (37.8% vs. 22.7%, P < 0.0001). More people who came in-person agreed that clinical research is even more important than before COVID-19 (54.2% vs. 44.3%, P = 0.0035).
Conclusions: The majority of people felt that clinical research is important because of the health benefits received and because it may help others. These data may provide important considerations in the planning of future studies in the era of COVID-19.
Keywords: Survey; Participation; Clinical research; COVID-19
| Introduction | ▴Top |
On March 11, the World Health Organization (WHO) declared coronavirus disease 2019 (COVID-19) a pandemic after more than 100,000 people in over 100 countries were infected [1]. To help limit the spread of COVID-19, the majority of states (including Louisiana on March 22, 2020) enacted stay at home orders directing residents to shelter at home and limit movements outside of their homes except for essential needs [2]. This also led to the immediate suspension of face-to-face research activity at a number of sites across the world including at LSU’s Pennington Biomedical Research Center (PBRC), which conducts basic, clinical and population research in the areas of nutrition, obesity, diabetes, cardiovascular disease, cancer and dementia. Reopening of the state occurred in staged phases with phase one having occurred on May 15, 2020 and followed by phase two having occurred on June 5, 2020. Phase one allowed certain types of businesses to reopen at 25% of occupancy with strict social distancing while phase two expanded that to 50% of occupancy [3, 4]. As of May 18, 2020, clinical research operations at PBRC resumed with limited in-person visits in accordance with the Governor’s order. This unique situation was an opportunity to better understand attitudes and motivations around contributing to clinical research and resuming in-person visits during a pandemic through a survey. There may be important domains to consider as research activities are restarted since not all studies provide direct participant benefit. Therefore, the objective of this survey study was to improve the understanding of the characteristics and perspectives of participants who return for in-person clinical research visits compared to others who were not involved in research at the time of the survey. The information obtained may help with the planning of future clinical research studies in the era of COVID-19.
| Materials and Methods | ▴Top |
Study data were collected and managed using Research Electronic Data Capture (REDCap) electronic data capture tools hosted at Pennington Biomedical Research Center. REDCap is a secure, web-based software platform designed to support data capture for research studies, providing: 1) an intuitive interface for validated data capture; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for seamless data downloads to common statistical packages; and 4) procedures for data integration and interoperability with external sources.
For this study, we conducted surveys in two different ways. From May 26, 2020 to August 11, 2020, paper surveys were provided to participants who returned for in-person study visits at PBRC. Surveys were filled out anonymously and placed in a designated box. On August 6, 2020, a link to a REDCap survey was sent to 29,574 subscribers on a standing e-mail list consisting of previous research participants and others interested in joining research studies to serve as a comparison group to those filling out the survey in-person. The REDCap survey was closed on September 11, 2020 while the state remained in phase two of reopening. The study was reviewed by the PBRC Institutional Review Board and was determined to be exempt.
Survey
The paper survey had 15 questions total and was divided into two parts (participant characteristics and participant attitudes). The online survey had one additional question asking whether the person had an in-person visit between May 22 and July 31, 2020 to help eliminate duplicate responses. The survey can be viewed in the Supplementary Material 1 (www.jocmr.org). The first part gathered information about demographic characteristics of the participants and the second part presented statements about research and in-person visits that required participants to answer on a scale of 1 (absolutely disagree) to 10 (absolutely agree).
Study population and data collection
Adults 18 years and older were allowed to fill out the survey. Participants and/or their legally authorized representative or parent/guardian who attended a study visit in-person with their child were allowed to fill out the paper survey. Information concerning age, sex, race, and personal experience with COVID-19 was collected.
Statistical analysis
Descriptive statistics were used to compare demographic characteristics between those who filled out the survey in-person versus online. Based on the distribution of the survey responses, three categories of responses were created: disagree (1 - 3), neutral (4 - 7), and agree (8 - 10). Chi-square tests were used to compare the percentages in these categories between the in-person and online groups.
| Results | ▴Top |
A total of 251 people completed paper surveys in-person at PBRC out of a total of 512 visits that occurred from May 26, 2020 to August 11, 2020. In addition, a total of 1,537 people completed the survey online via REDCap and indicated that they had not had an in-person visit during the time when paper surveys were administered. Demographic characteristics of both groups are presented in Table 1. The highest proportion of people who filled out the survey in-person were participating in healthy aging/Alzheimer’s disease studies (36.1%). Those who completed the survey online were slightly older, were more likely to be female, more likely to have had symptoms of COVID-19, and more likely to know someone who became ill due to COVID-19.
 Click to view | Table 1. Participant Characteristics* |
In the second part of the survey, more than half of participants (52.2%) who came for in-person visits thought they were low risk for severe illness related to COVID-19 compared to 38.4% of people who filled out the survey online (P = 0.0002, Fig. 1a). In terms of attending in-person visits, 88.4% of those who attended an in-person visit vs. 62.7% who filled out the survey online agreed with the statement that they felt safe doing an in-person visit at PBRC (P < 0.0001). More people who completed the survey online preferred to do as many study visits over the phone or internet as possible (37.8% vs. 22.7%, P < 0.0001, Fig. 1b). The majority of people in both groups (in-person vs. online) agreed with the statement that clinical research is important because of the health benefits received (73.5% vs. 68.1%, P = 0.12) and with the statement that clinical research helps other people (92.2% vs. 88.4%, P = 0.18). As seen in Figure 1c, more people who came to in-person visits disagreed with the statement that clinical research is important because of the financial incentives received compared to people who filled out the survey online (42.4% vs. 34.9%, P = 0.04). Finally, as seen in Figure 1d, more people who came to in-person visits agreed with the statement that clinical research is even more important than before COVID-19 (54.2% vs. 44.3%, P = 0.0035).
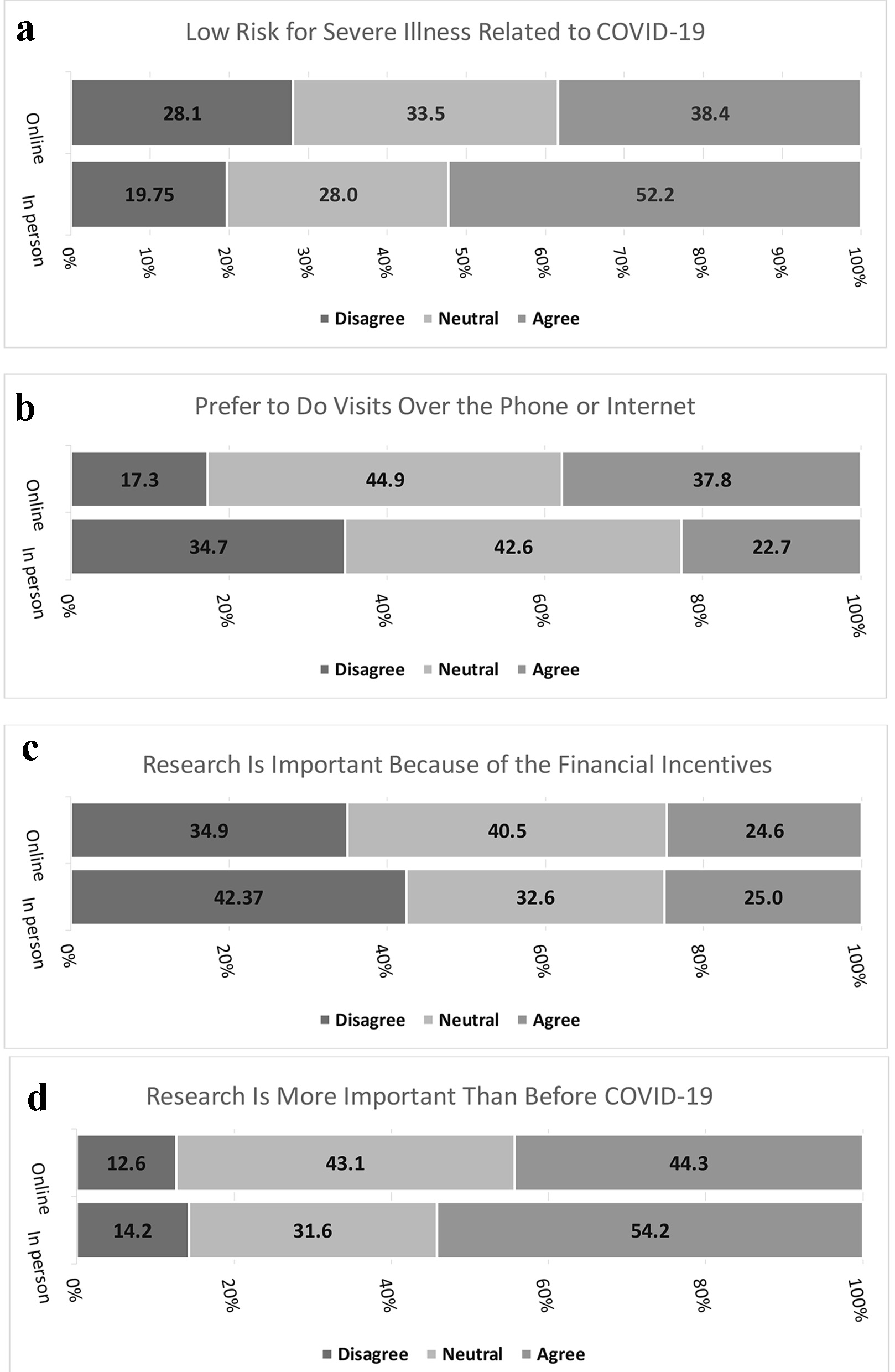 Click for large image | Figure 1. (a) Low risk for severe illness related to COVID-19. (b) Prefer to do visits over the phone or internet. (c) Research is important because of the financial incentives. (d) Research is more important than before COVID-19. COVID-19: coronavirus disease 2019. |
| Discussion | ▴Top |
The unique situation of stay-at-home orders to mitigate the spread of COVID-19 presented an opportunity to assess general attitudes around in-person clinical research visits and to better understand who would be willing to resume in-person research visits. This survey study attempted to capture information from people who returned to in-person visits at PBRC as well as from people who were not currently participating in a research study via an online platform. It has been previously reported that four in 10 adults in the USA reported avoiding medical care due to concerns related to COVID-19 [5]. Inevitably, this pandemic has also affected clinical research significantly and has caused disruptions to clinical trials worldwide with an estimated 80% of non-COVID-19 trials being stopped or interrupted [6]. Moreover, one report from May 2020 noted an average decline of 74% in new participant enrollment compared to the same time period in 2019 [7]. On the other hand, COVID-19 has helped to address some of the inefficiencies related to conducting clinical research as COVID-19 trials have been stream-lined and fast-tracked [6]. Thus, it is imperative to better understand the characteristics and perspectives of participants who are willing to return to in-person visits and to apply these findings to revising existing and upcoming studies to accommodate as many participants as possible. Finally, information collected through this survey may help to inform the resumption of clinical research activities after a prolonged closure of an institution and may provide some important considerations about what participants prioritize prior to reopening for in-person research visits.
Limitations
There are several limitations to this survey study. The study population was self-selected whereby those people who were more comfortable with in-person visits came back to PBRC. The research participants who agreed to come back for in-person visits may have been skewed by the ongoing studies at the time. As an example, the age of the people surveyed may have been skewed by the high number of healthy aging/Alzheimer’s studies being conducted (36.1% of respondents were participating is this type of study). However, those who filled out the survey online were slightly older. In addition, those who answered the survey online may have previously participated in research; whereas, those who were less familiar with research or had not done a previous study may have been less likely to fill out the survey. Answers may change over time and could have been dependent on the prevalence of COVID-19 infection or other factors in a certain region. Therefore, the results may not be generalizable to the population at large.
Conclusions
The perception of being high risk for morbidity related to COVID-19 did not seem to impact those who attended in-person visits and nearly 90% of people felt safe during their visit. There does not appear to be a strong feeling either way for conducting study visits over the phone or online. For the most part, people feel that clinical research is important because of the health benefits they receive and because it may help others. These data may provide important considerations as more sites reopen for in-person clinical research visits and may also provide important considerations in the planning of future studies in the era of COVID-19.
| Supplementary Material | ▴Top |
Suppl 1. Survey of participant perspectives on resuming clinical research.
Acknowledgments
All authors would like to thank the online and in-person participants in this study.
Financial Disclosure
RAB is supported in part by U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health, which funds the Louisiana Clinical and Translational Science Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of Interest
The authors report no conflict of interest.
Informed Consent
A waiver of signed informed consent was also granted given that no identifiable information was collected, and a signed consent may have increased the risk for linking responses to participants filling out the survey in-person. Consent was implied if the participant filled out the survey.
Author Contributions
DSH designed study protocol and constructed initial draft of manuscript. KMW helped with data collection and survey design. RAB analyzed the survey results. All authors have approved the manuscript as submitted.
Data Availability
Any inquiries regarding supporting data availability of this study should be directed to the corresponding author.
Abbreviations
PBRC: Pennington Biomedical Research Center; WHO: World Health Organization; COVID-19: coronavirus disease 2019
| References | ▴Top |
- Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157-160.
- COVID-19 Stay at Home Order 2020. Available from: https://gov.louisiana.gov/home/.
- Gov. Edwards: Louisiana will move to phase one statewide on May 15, COVID-19 stay at home order will be lifted for Louisianans. 2020. updated May 11, 2020. Available from: https://gov.louisiana.gov/index.cfm/newsroom/detail/2488.
- Gov. Edwards signs order moving Louisiana to phase two of reopening on Friday 2020. updated June 4, 2020. Available from: https://gov.louisiana.gov/index.cfm/newsroom/detail/2532.
- Czeisler ME, Marynak K, Clarke KEN, Salah Z, Shakya I, Thierry JM, Ali N, et al. Delay or avoidance of medical care because of COVID-19-related concerns - United States, June 2020. MMWR Morb Mortal Wkly Rep. 2020;69(36):1250-1257.
doi pubmed - van Dorn A. COVID-19 and readjusting clinical trials. Lancet. 2020;396(10250):523-524.
doi - Sathian B, Asim M, Banerjee I, Pizarro AB, Roy B, van Teijlingen ER, do Nascimento IJB, et al. Impact of COVID-19 on clinical trials and clinical research: A systematic review. Nepal J Epidemiol. 2020;10(3):878-887.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


