| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Review
Volume 14, Number 2, February 2022, pages 80-87
False-Positive Causes in Serum Cardiac Troponin Levels
Aleksey Michailovich Chaulina, b, c
aDepartment of Cardiology and Cardiovascular Surgery, Faculty of Medicine, Samara State Medical University, Samara, Russia
bDepartment of Clinical Chemistry, Samara Regional Clinical Cardiological Dispensary, Samara, Russia
cEmail:
Manuscript submitted January 10, 2022, accepted February 14, 2022, published online February 24, 2022
Short title: False-Positive Causes in Serum cTn Levels
doi: https://doi.org/10.14740/jocmr4664
- Abstract
- Introduction
- Fibrin Clots
- Heterophile Antibodies
- Alkaline Phosphatase
- Rheumatoid Factor
- Cross-Reactions of Diagnostic (Anti-cTn) Antibodies With Troponin Molecules Released From Skeletal Muscle
- Conclusions
- References
| Abstract | ▴Top |
Cardiac troponins (cTns) are the most valuable and specific markers of cardiovascular diseases, including acute myocardial infarction. These biomarkers can also be used to assess the degree of myocardial damage in non-cardiac diseases that can negatively affect the cells of cardiac muscle tissue. However, in everyday clinical practice, doctors often encounter with false-positive cases of increased cTns. False-positive cases of increased cTns can contribute to incorrect diagnosis and subsequent inadequate treatment, which causes significant harm to the patient. This review discusses some common causes of a false-positive increase in the level of cTns in the blood serum. Such causes are fibrin clots, heterophilic antibodies, alkaline phosphatase, rheumatoid factor, and cross-reactions of diagnostic (anti-cTn) antibodies with skeletal troponins. Detailed attention is focused on the mechanisms of false-positive increase, and ways to identify and combat these false-positive causes of increased cTns. This has an important practical significance in modern clinical practice.
Keywords: Cardiovascular diseases; Acute myocardial infarction; Biomarkers; Troponin T; Troponin I; False-positive
| Introduction | ▴Top |
Cardiac troponins (troponin T (cTnT) and troponin I (cTnI)) are the main and most specific biomarkers for the early diagnosis of acute myocardial infarction (AMI) [1-4]. In accordance with the main guidance document (Fourth Universal Definition of Myocardial Infarction), the main criteria for AMI are the following: 1) myocardial damage detected using cTns; 2) symptoms of myocardial ischemia; 3) ischemic changes on an electrocardiogram and in particular the appearance of a pathological Q wave; 4) identification of areas of non-viable myocardium using imaging methods; and 5) detection of a blood clot in the coronary arteries using coronary angiography or autopsy [1].
Due to modern ultra-sensitive tests, medical practitioners got the opportunity to early diagnose AMI (within the first 2 h from admission of the patient) through the evaluation of dynamic changes of cardiac troponins. The changes (increase) of the concentration of cTn molecules within the first 2 h are very small (may amount to as little as several ng/L) and cannot be detected by moderately sensitive test systems. It should be noted that due to a number of multicenter studies, there have been validated algorithms of early diagnostics (0 → 1 h and 0 → 2 h) of non-ST-segment elevation AMI (NSTEMI) for ultra-sensitive test systems of various manufacturers (Tables 1 and 2) [5]. These diagnostic algorithms of AMI are recommended by the European Society of Cardiology (ESC) for clinical practice [6].
 Click to view | Table 1. Current Diagnostic Algorithms for Confirmation/Exclusion of NSTEMI Approved by the ESC: One-Hour NSTEMI Diagnostic Algorithm |
 Click to view | Table 2. Current Diagnostic Algorithms for Confirmation/Exclusion of NSTEMI Approved by the ESC: Two-Hour NSTEMI Diagnostic Algorithm |
In addition, cTns can be used to assess the prognosis of patients suffering from many non-cardiac pathologies that damage cardiac myocytes (Fig. 1) [7-15].
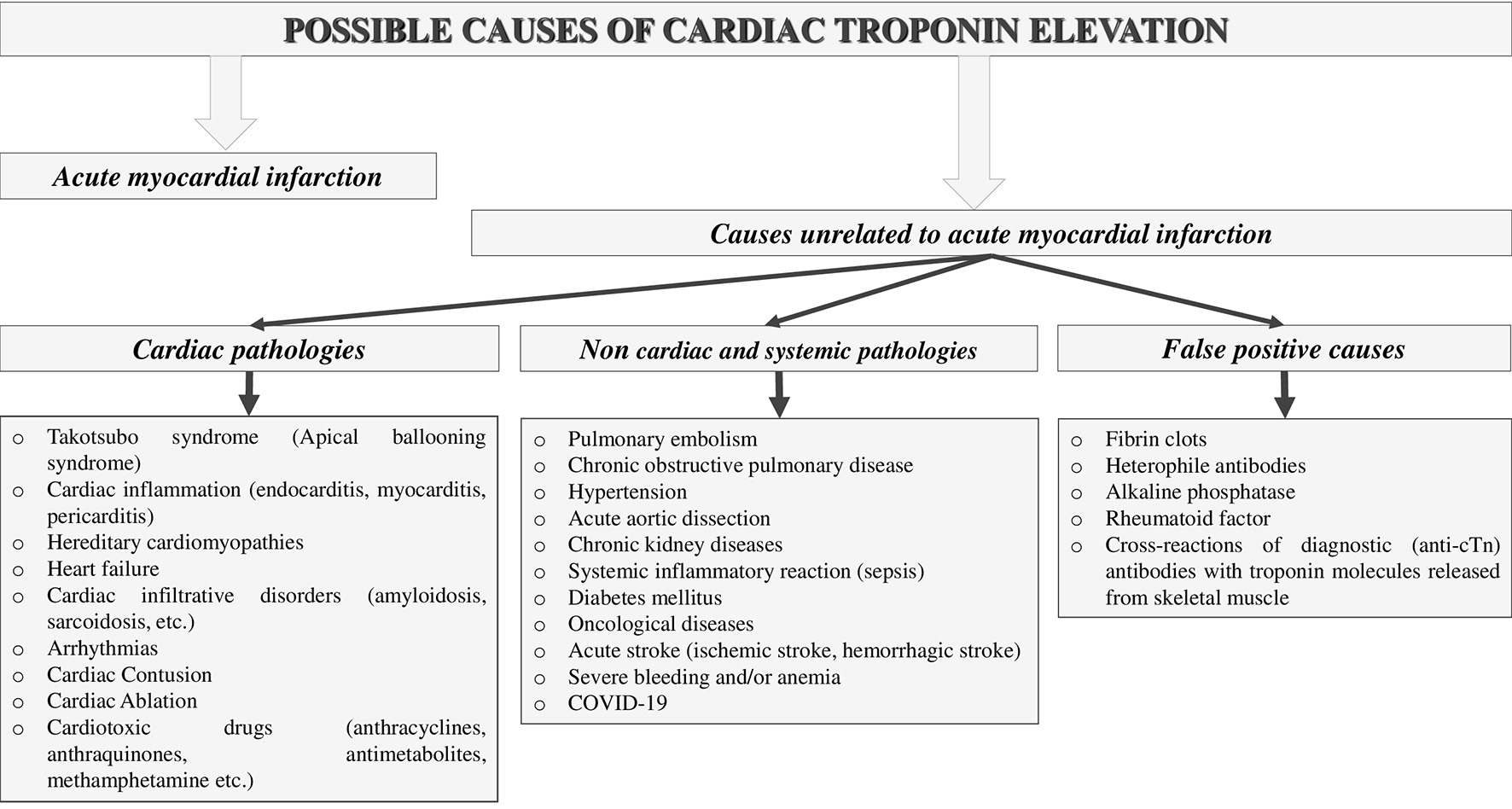 Click for large image | Figure 1. Possible causes of cardiac troponin elevation. |
However, in some cases, elevated (positive) troponin concentrations cannot be explained, even after careful clinical examination and exclusion of all possible pathologies that may cause cardiomyocyte damage. Such cases are called false-positive and are most often associated with the following reasons: fibrin clots, heterophile antibodies, alkaline phosphatase, rheumatoid factor, and cross-reactions of diagnostic (anti-cTn) antibodies with troponin molecules released from skeletal muscle [16-28]. Knowledge of the main causes and mechanisms of a false-positive increase in cTn concentrations is important in clinical practice, since many physicians may make incorrect diagnoses and prescribe unnecessary treatment based on laboratory results, which can be harmful to the patient and lead to unnecessary economic costs. The main causes and mechanisms of false-positive troponin elevations, as well as ways to combat these types of interference, are sequentially discussed below.
| Fibrin Clots | ▴Top |
Fibrin clots are one of the most important factors causing interferences in laboratory studies of blood serum. Fibrin clots are formed due to incomplete blood clotting under the action of coagulants added to the test blood to obtain serum. Standard biochemical test tubes (vacuum tubes with a red lid) use dry clot activator (silica) applied to the inner surface of the test tube wall as a coagulant. The main reason for the formation of fibrin clots is incomplete clotting of blood prior to centrifugation. Most often this occurs in patients with coagulopathies or against the background of anticoagulant therapy [16, 29-31]. In addition, extra-laboratory errors (violation of blood collection technique) and intra-laboratory violations (reduction of recommended time from blood receipt to centrifugation) leading to formation of fibrin clots are also possible. The optimal time for the complete clotting of the blood sample is approximately 30 - 60 min from the time of blood collection. However, in some cases, laboratory staff, under pressure from clinicians, are forced to reduce the time allotted for clotting a blood sample. This increases the likelihood of fibrin clots and filaments formation in the tubes after centrifugation. Additional factors increasing the risk of fibrin clots are hypocoagulant states (e.g., in patients taking anticoagulant drugs). Cases of false-positive increases in cTn concentrations in sera with fibrin clots on Abbott AxSYM [17] and Dade Stratus II immunoanalyzers [18] can be found in the literature. A presumed mechanism underlying false-positive troponin tests is the competitive interaction of fibrin clots with diagnostic antibodies (anti-cTn). The prevalence of false-positive increases in cTn levels differs significantly according to different studies. Thus, Nosanchuck et al (1999) found false-positive results due to fibrin clots in all serum samples (n = 8) [17]. Roberts et al (1997) in their study identified 2.2% of false-positive results from > 900 patient specimens due to fibrin clots [18]. Ways to combat fibrin clots are: adherence to blood collection and sample preparation guidelines (paying particular attention to the clotting time guidelines), careful visual inspection of the blood sample after centrifugation, and switching to the routine use of whole blood or plasma as biomaterial instead of serum. The latter condition is the most optimal for laboratories involved in the diagnosis of acute conditions, including AMI.
| Heterophile Antibodies | ▴Top |
Heterophile antibodies are immunoglobulins (antibodies) formed by B lymphocytes against poorly recognized antigens (such as foreign animal proteins). Heterophilic antibodies have a weak but polyvalent activity (avidity) to antigens. The main reasons for the formation of heterophile antibodies in humans are: the use of mouse monoclonal sera (antibodies) or incompletely humanized (human) antibodies for the treatment of a number of diseases (e.g., systemic connective tissue diseases or oncopathology); frequent contact with microbial antigens, animal antigens (e.g., when keeping pets), foreign proteins (e.g., in food workers, veterinarians, farmers); vaccination; blood transfusion and long-term persistence of viral agents in the body [19, 20, 32-35]. According to various estimates, the prevalence of heterophile antibodies in the population ranges from < 1% to 80%. However, not all patients with heterophile antibodies in the blood have false-positive reactions [19, 20]. Unlike a number of pre-laboratory factors (hemolysis, lipemia, and fibrin clots), heterophile antibodies cannot be detected by visual inspection of the specimen under examination.
The mechanism of false-positive elevation of cTn concentrations lies in the cross-interaction of heterophile antibodies with anti-cTn included in the diagnostic test system. Lum et al (2006) described an interesting clinical case of a false-positive increase in cTnI concentration in a patient without myocardial infarction. A 57-year-old patient admitted to the emergency department had complaints and symptoms similar to AMI. The cTnI concentration measured on admission with the Beckman Coulter immunoassay was 41.0 ng/mL, significantly higher than normal (0 - 0.5 ng/mL). However, the levels of total creatine kinase and creatine kinase-MB (CK-MB) isoform were within the normal range and the electrocardiogram data also did not indicate AMI. After careful examination, other possible causes of elevated troponin I concentration were also excluded. Based on these data, cardiologists suggested the presence of a false-positive result. When troponin I concentrations were repeatedly tested with diagnostic test systems from different manufacturers (Beckman Coulter, Abbot, Bayer, Roche), troponin I levels were positive only with the Beckman Coulter immunoassay, whereas all other immunoassays were negative. Serial dilution of the patient’s plasma samples with control plasma (with normal troponin I levels) revealed nonlinear results and led to the assumption of heterophilic antibody interference. To finally confirm this assumption, blood plasma samples were transferred to the research laboratory of Beckman Coulter, where after adding heterophilic antibody blockers to the patient’s original blood sample, the troponin I concentration decreased from 41.0 to 1.04 ng/mL [19].
Researchers Zaidi et al (2010) described a clinical case of a false-positive increase in cTnI concentration in a 53-year-old female patient admitted to the emergency department with complaints of chest pain. The medical history revealed that the patient had been admitted with similar symptoms three times during the current year. The cTnI concentration at the time of admission (0.37 ng/mL) was five times the upper reference limit (0.00 - 0.069 ng/mL). However, electrocardiography (ECG) and coronarography data did not reveal signs of ischemia and obstruction of the coronary arteries, so physicians suspected a false-positive cTnI increase. The blood sample was sent to another laboratory, where troponin T was measured and was negative. Further analysis revealed the presence of heterophile antibodies in the patient’s blood, which led to a false-positive increase in cTnI [36].
The largest systematic literature review by Lippi et al (2012) summarized 16 studies and clinical cases demonstrating the effect of heterophile antibodies on cTn concentrations. On average, the rate of false-positive increases in cTn levels ranged from 0.1% to 3.0%, and in some studies, it was significantly higher, up to 50%. The effect of heterophile antibodies is an unpredictable phenomenon and can affect both cTnI and cTnT test systems of any manufacturer. According to a systematic literature review, the best way to detect false-positive troponin levels caused by heterophilic antibodies is to pretreat the blood sample with heterophilic antibody blockers. According to most studies, the addition of a blocking reagent led to a dramatic decrease in cTn concentrations in patients’ blood [20]. Some researchers believe that the prevalence of false-positive results due to the influence of heterophile antibodies may increase significantly in the future due to the widespread use of immunotherapy for the treatment of many diseases, as well as the use of antibodies in diagnostic immunoscintigraphic studies [37].
Fast detection of false-positive elevation of cTn levels is important in the emergency diagnosis of AMI. It is possible only with the coordinated interaction of clinicians and laboratory diagnostics specialists. This is due to the fact that laboratory diagnosticians only have access to laboratory results and therefore cannot compare troponin levels with data from other test methods. Clinical laboratory diagnosticians may suspect incorrect (false-positive) results if, in addition to cTns, a patient has been prescribed study of other myocardial damage biomarkers (total creatine kinase and its MB isoform, aspartate aminotransferase, myoglobin, lactate dehydrogenase and others). Thus, normal levels of these biomarkers with sharply elevated levels of cTns should alert laboratory diagnosticians. An equally important role in identifying a possible false-positive result of troponin immunoassay is played by clinicians, who have maximum access to the results of all diagnostic methods used in relation to a particular patient. If the laboratory results are inconsistent with the clinical and instrumental data, clinicians should notify the diagnostic laboratory and initiate further investigation. Possible ways to detect false-positive troponin immunoassay results in the laboratory are: 1) testing the sample on another analyzer (if available), or measuring another cardiac marker (another cardiac isoform of troponin, CK-MB, myoglobin, and others); 2) serial dilution of biomaterial with control samples or saline several times and assessment of linearity of the values obtained; and 3) pretreating samples with special reagents that block heterophile antibodies (if available) or sending samples to specialized laboratories for these manipulations.
| Alkaline Phosphatase | ▴Top |
Alkaline phosphatase is a hydrolase enzyme which is widely used to diagnose liver and biliary tract diseases. In addition to its diagnostic value, this enzyme is also used in some immunoassays, including troponin immunoassays, for signal amplification. Some immunoassays using alkaline phosphatase as a component of the immunochemical reaction have been reported to be affected by endogenous (serum) alkaline phosphatase interference [21, 22, 38, 39]. Butch et al (1989) first established that alkaline phosphatase can have a significant effect on the concentration of a cardiac-specific enzyme CK-MB, measured on a Stratus immunochemical analyzer. The researchers found that in 12 of 23 patients with elevated serum alkaline phosphatase activity, CK-MB levels were falsely elevated [37]. Subsequently, Dasgupta et al (2001) reported the effect of alkaline phosphatase on cTnI concentration. With alkaline phosphatase activity of 46 U/L, the serum troponin I concentration in sample was 0.5 ng/mL. Researchers then added alkaline phosphatase solutions to this serum to increase the activity of this enzyme and evaluate its effect on the troponin concentration. With alkaline phosphatase activity of 129 U/L, the troponin I concentration increased to 4.3 ng/mL. A further increase in alkaline phosphatase activity to 222 and 913 U/L also proportionally increased the troponin I concentration to 9.4 and 40.1 ng/mL, respectively. Other test systems not using alkaline phosphatase as an immunochemical reaction component are unresponsive to such influence [22].
In a recent research, Marinheiro et al (2018) also proved that alkaline phosphatase was the cause of the false-positive troponin I result in a patient [38]. According to some authors, immunoassays that do not use this enzyme should be used for serum testing in patients with increased alkaline phosphatase activity [39]. In the absence of such a possibility, the results of patients who have elevated serum/plasma alkaline phosphatase activity should be interpreted with care.
| Rheumatoid Factor | ▴Top |
Rheumatoid factors are autoantibodies (immunoglobulins) that are directed against their own IgG. Elevated levels of rheumatoid factor are not only of diagnostic value, but can also have a significant impact on the results of laboratory tests performed on immunochemical analyzers [23, 40-45]. In patients with autoimmune diseases (rheumatoid arthritis, systemic lupus erythematosus, etc.), the main cause of falsely elevated troponins is rheumatoid factor [42-44]. According to Al-Awadhi et al (2007), five of 50 patients with seropositive rheumatoid arthritis had troponin I concentrations > 0.1 ng/mL (diagnostic threshold for AMI), while none of patients with seronegative rheumatoid arthritis had troponin I concentrations above the reference limit. One-factor regression analysis showed a positive correlation between troponin I and rheumatoid factor concentrations (r = 0.35; P < 0.02) [44]. Dasgupta et al (1999) in their research found false-positive troponin I concentrations in four of 12 patients with elevated rheumatoid factor levels. To eliminate the interference, the researchers used polyclonal antisera against rheumatoid factor, which resulted in a normalization of cTnI levels [42].
A large multi-center study of analytical interferences on laboratory results concentration examined the prevalence of false-positives. This study included patients with autoimmune diseases associated with elevated rheumatoid factor concentrations. In general, about 8.7% of the 3,445 results were false-positive. However, only a small fraction (21% of all false-positives) of the results were corrected with a blocking reagent, whereas 49% of the false-positives were not corrected with blocking reagents and would potentially mislead clinicians in making the diagnosis [45]. Thus, clinicians should be very careful when interpreting laboratory immunochemical studies in patients with autoimmune diseases and elevated serum rheumatoid factor levels.
| Cross-Reactions of Diagnostic (Anti-cTn) Antibodies With Troponin Molecules Released From Skeletal Muscle | ▴Top |
Damage to striated skeletal muscle in congenital and acquired diseases (myopathies, rhabdomyolysis) can lead to a false-positive increase in cTn levels due to the cross-reactions of anti-cTnI and anti-cTnT antibodies with skeletal troponin molecules. Most often, such false-positive reactions occurred with first and second generation troponin immunoassays with weakly specific antibodies that could interact with skeletal troponin molecules [46-49]. However, subsequently, a considerable number of cases of false-positive increases in cTns were registered when using more specific third and fourth generation troponin immunoassays [24-27]. The specific cause and mechanism of increase in cTns in patients with skeletal myopathies has not been unraveled yet, and cases of false-positive increases in cTns have been described even with the use of modern highly sensitive immunoassays [49]. There are two possible mechanisms for increase in the levels of cTns in diseases and injuries of skeletal muscles: 1) re-expression of cTn molecules in skeletal muscles after injury and the release of these molecules into the bloodstream from skeletal muscle fibers [50-52]; and 2) cross-reactions of diagnostic antibodies (anti-cTnI and anti-cTnT) with skeletal troponin molecules released into the bloodstream during skeletal muscle injury [47-49, 53]. Discussions about these mechanisms are still ongoing [54, 55].
A number of studies have reported elevated serum cTn levels in many patients with skeletal myopathies even in the absence of ischemia and myocardial injury. Punukollu et al (2004) reported elevated serum cTnT concentrations in 19 of 91 patients with rhabdomyolysis with no signs of coronary artery damage [25]. Egholm et al (2015) described a clinical case of a significant increase in high-sensitivity troponin T (hs-TnT) (471 ng/L, 99th percentile < 14 ng/L) in a 48-year-old patient with drug-induced rhabdomyolysis. The concentrations of myoglobin (29,120 µg/L), total creatine kinase (30,750 U/L) and its MB isoform (162 µg/L) were also significantly increased [26].
Rheumatologists revealed elevated cTnT concentrations in many patients with idiopathic inflammatory myopathies (polymyositis, dermatomyositis, and myositis associated with systemic connective tissue disease). Eighteen of 23 patients with myopathies had elevated levels of creatine kinase and cTnT, while the remaining five patients had normal creatine kinase and cTnT levels. Only one patient with myopathy had elevated cTnI level. Researchers also noted that creatine kinase levels correlated closely with cTnT levels (r = 0.62; P = 0.001) [46]. The most likely mechanism for the elevation of troponin T in this study is the cross-reaction of anti-cTnT with skeletal troponin T molecules. This is evidenced by the close correlation of cTnT with another skeletal muscle damage marker (creatine kinase) and the absence of a significant increase in cTnI. Thus, cTnT and cTnI have almost the same diagnostic value, and in case of cardiomyocyte damage, the concentration of cTnT and cTnI in serum would increase proportionally. A significant increase in only one cardiac troponin isoform (cTnT or cTnI), however, would be more indicative of analytical problems, particularly, cross-reactivity of the diagnostic antibodies included in the corresponding troponin immunoassay.
In another study, cTnT or cTnI levels were measured in 78 patients with skeletal myopathies including muscular dystrophies, myotonic dystrophies, inflammatory myopathies, myotonia, and neurogenic muscle pathologies. The cTnT was increased in 56 patients (72.8%) and cTnI in only two (2.6%). When grouping patients with elevated troponin T levels by nosology, it turned out that cTnT was elevated in all patients (100%) with neurogenic muscle pathologies, in 87% of patients with muscular dystrophy, in 75% of patients with inflammatory myopathies, in 72% of patients with myotonic dystrophy and in none of the patients with myotonia (0%). Studies of skeletal muscle biopsy specimen using western blotting and mass spectrometry revealed no cardiac troponins [53], which indicates the absence of cTns expression in skeletal muscle. Based on these results, the most likely mechanism for the troponins elevation in this study is the cross-reactions (false-positive) of diagnostic antibodies with skeletal troponin molecules that are released from damaged muscle fibers.
Schmid et al (2018) used highly sensitive assays to measure troponin I and troponin T during the examination of 74 patients with hereditary and acquired skeletal myopathies. Hs-TnT levels were elevated in a much larger number of patients (> 14 ng/L; 68.9%) compared with hs-cTnI levels (> 26 ng/L; 4.1%). There was a close correlation of hs-cTnT with creatine kinase (r = 0.679) and even closer one with myoglobin (r = 0.786). Serial measurements of hs-TnT concentrations revealed a chronic elevation of hs-cTnT in most patients. The study of skeletal muscle biopsy specimens showed no expression of cTn isoforms in them, leading the researchers to a conclusion that there is no re-expression of cTn molecules in skeletal muscle. According to the researchers, the most likely reason for the increase in serum hs-cTnT and hs-cTnI levels was the false-positive (cross) reactions of anti-hs-cTnT and anti-hs-cTnI with skeletal troponin isoforms [49, 56, 57]. However, several other studies have revealed the expression of cTn molecules in skeletal muscle in skeletal myopathies, which may indicate the possibility of increasing the serum cTns concentration through the release of cTn molecules from skeletal muscle fibers into the bloodstream [50, 51]. A recent cohort study has also detected messenger RNA and peptide fragments of cTnT using mass spectrometry in patients with Pompe disease, an inherited glycogen storage disease predominantly damaging nerve and muscle cells throughout the body [52]. Thus, the data regarding the source and mechanism of positive troponin tests results in skeletal myopathies are inconsistent and need further clarification.
False-positive causes of increased cTn levels are summarized in Table 3 [3, 17-22, 27, 30-45, 47-49, 53].
 Click to view | Table 3. False-Positive Causes in Serum Cardiac Troponin Levels |
| Conclusions | ▴Top |
Physicians and researchers should also keep in mind that there are a significant number of factors (fibrin clots, heterophilic antibodies, rheumatoid factor, alkaline phosphatase, and cross-reactions of diagnostic antibodies (anti-cTn) with skeletal troponin molecules) that cause false-positive elevations in cTns, as well as ways to detect false-positive results and counteract them. Understanding these causes and mechanisms of a false-positive increase in cTns in blood serum will help practitioners and researchers improve the diagnosis of cardiovascular diseases, in particular myocardial infarction, and reduce the risk of misdiagnoses.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Data Availability
The author declares that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, White HD, et al. Fourth universal definition of myocardial infarction (2018). Circulation. 2018;138(20):e618-e651.
doi pubmed - Chaulin AM, Karslyan LS, Bazyuk EV, Nurbaltaeva DA, Duplyakov DV. [Clinical and diagnostic value of cardiac markers in human biological fluids]. Kardiologiia. 2019;59(11):66-75.
doi pubmed - Chaulin A. Cardiac troponins: contemporary biological data and new methods of determination. Vasc Health Risk Manag. 2021;17:299-316.
doi pubmed - Chaulin AM. Cardiac troponins metabolism: from biochemical mechanisms to clinical practice (Literature Review). Int J Mol Sci. 2021;22(20):10928.
doi pubmed - Collet JP, Thiele H, Barbato E, Barthelemy O, Bauersachs J, Bhatt DL, Dendale P, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2021;42(14):1289-1367.
doi pubmed - URL: https://www.escardio.org/Sub-specialty-communities/Association-for-Acute-CardioVascular-Care-(ACVC)/Education/rapid-rule-in-and-rule-out-algorithms. Access date: July 2, 2021.
- Chaulin AM, Duplyakov DV. MicroRNAs in atrial fibrillation: Pathophysiological aspects and potential biomarkers. Int J Biomed. 2020;10:198-205.
doi - Chaulin AM, Duplyakov DV. On the potential effect of circadian rhythms of cardiac troponins on the diagnosis of acute myocardial infarction. Signa Vitae. 2021;17(3):79-84.
doi - Chaulin AM, Abashina OE, Duplyakov DV. High-sensitivity cardiac troponins: detection and central analytical characteristics. Cardiovascular Therapy and Prevention. 2021;20(2):2590 (In Russ).
doi - Giannitsis E, Katus HA. Cardiac troponin level elevations not related to acute coronary syndromes. Nat Rev Cardiol. 2013;10(11):623-634.
doi pubmed - Chaulin AM, Abashina OE, Duplyakov DV. Pathophysiological mechanisms of cardiotoxicity in chemotherapeutic agents. Russian Open Medical Journal. 2020;9:e0305.
doi - Chaulin AM, Duplyakov DV. Arrhythmogenic effects of doxorubicin. Complex Issues of Cardiovascular Diseases. 2020;9(3):69-80 (In Russ).
doi - Park KC, Gaze DC, Collinson PO, Marber MS. Cardiac troponins: from myocardial infarction to chronic disease. Cardiovasc Res. 2017;113(14):1708-1718.
doi pubmed - Chaulin AM, Duplyakov DV. Comorbidity in chronic obstructive pulmonary disease and cardiovascular disease. Cardiovascular Therapy and Prevention. 2021;20(3):2539 (In Russ).
doi - Chaulin AM. Elevation mechanisms and diagnostic consideration of cardiac troponins under conditions not associated with myocardial infarction. Part 1. Life (Basel). 2021;11(9):914.
doi pubmed - Chaulin AM. Main analytical characteristics of laboratory methods for the determination of cardiac troponins: a review from the historical and modern points of view. Orv Hetil. 2022;163(1):12-20.
doi pubmed - Nosanchuk JS, Combs B, Abbott G. False increases of troponin I attributable to incomplete separation of serum. Clin Chem. 1999;45(5):714.
doi pubmed - Roberts WL, Calcote CB, De BK, Holmstrom V, Narlock C, Apple FS. Prevention of analytical false-positive increases of cardiac troponin I on the Stratus II analyzer. Clin Chem. 1997;43(5):860-861.
doi pubmed - Lum G, Solarz DE, Farney L. False positive cardiac troponin results in patients without acute myocardial infarction. Laboratory Medicine. 2006;37(9):546-550.
doi - Lippi G, Aloe R, Meschi T, Borghi L, Cervellin G. Interference from heterophilic antibodies in troponin testing. Case report and systematic review of the literature. Clin Chim Acta. 2013;426:79-84.
doi pubmed - Butch AW, Goodnow TT, Brown WS, McClellan A, Kessler G, Scott MG. Stratus automated creatine kinase-MB assay evaluated: identification and elimination of falsely increased results associated with a high-molecular-mass form of alkaline phosphatase. Clin Chem. 1989;35(10):2048-2053.
doi pubmed - Dasgupta A, Chow L, Wells A, Datta P. Effect of elevated concentration of alkaline phosphatase on cardiac troponin I assays. J Clin Lab Anal. 2001;15(4):175-177.
doi pubmed - Katwa G, Komatireddy G, Walker SE. False positive elevation of cardiac troponin I in seropositive rheumatoid arthritis. J Rheumatol. 2001;28(12):2750-2751.
- Li SF, Zapata J, Tillem E. The prevalence of false-positive cardiac troponin I in ED patients with rhabdomyolysis. Am J Emerg Med. 2005;23(7):860-863.
doi pubmed - Punukollu G, Gowda RM, Khan IA, Mehta NJ, Navarro V, Vasavada BC, Sacchi TJ. Elevated serum cardiac troponin I in rhabdomyolysis. Int J Cardiol. 2004;96(1):35-40.
doi pubmed - Egholm G, Pareek M. Drug-induced rhabdomyolysis with elevated cardiac troponin T. Case Rep Med. 2015;2015:270204.
doi pubmed - Chaulin AM, Duplyakova PD, Duplyakov DV. Circadian rhythms of cardiac troponins: mechanisms and clinical significance. Russian Journal of Cardiology. 2020;25(3S):4061.
doi - Chaulin AM, Duplyakova PD, Bikbaeva GR, et al. Concentration of high-sensitivity cardiac troponin I in the oral fluid in patients with acute myocardial infarction: a pilot study. Russian Journal of Cardiology. 2020;25(12):3814.
doi - Hyytia H, Heikkila T, Hedberg P, Puolakanaho T, Pettersson K. Skeletal troponin I cross-reactivity in different cardiac troponin I assay versions. Clin Biochem. 2015;48(4-5):313-317.
doi pubmed - Reichstein E. The Importance of Preanalytical Factors in Immunodiagnostic Testing. EJIFCC. 2003;14(3):124-127.
- Beyne P, Vigier JP, Bourgoin P, Vidaud M. Comparison of single and repeat centrifugation of blood specimens collected in BD evacuated blood collection tubes containing a clot activator for cardiac troponin I assay on the ACCESS analyzer. Clin Chem. 2000;46(11):1869-1870.
doi pubmed - Kaplan IV, Levinson SS. When is a heterophile antibody not a heterophile antibody? When it is an antibody against a specific immunogen. Clin Chem. 1999;45(5):616-618.
doi - Kricka LJ. Human anti-animal antibody interferences in immunological assays. Clin Chem. 1999;45(7):942-956.
doi pubmed - Lahat Y, Shiloah E, Rapoport M. [Recurrent hospitalizations due to false positive troponin in a patient presenting with chest pain]. Harefuah. 2009;148(6):359-361, 413.
- Manjunath L, Yeluru A, Rodriguez F. 27-year-old man with a positive troponin: a case report. Cardiol Ther. 2018;7(2):197-204.
doi pubmed - Zaidi A, Cowell R. False positive cardiac troponin elevation due to heterophile antibodies: more common than we recognise? BMJ Case Rep. 2010;2010:bcr1120092477.
doi pubmed - Nguyen J, Thachil R, Vyas N, Marino T. Falsely elevated troponin: rare occurrence or future problem. J Community Hosp Intern Med Perspect. 2016;6(6):32952.
doi pubmed - Marinheiro R, Amador P, Parreira L, Rato Q, Caria R. False positive troponin I rendering two admissions for "Recurrent Acute Myopericarditis". Open Cardiovasc Med J. 2018;12:55-58.
doi pubmed - Herman DS, Ranjitkar P, Yamaguchi D, Grenache DG, Greene DN. Endogenous alkaline phosphatase interference in cardiac troponin I and other sensitive chemiluminescence immunoassays that use alkaline phosphatase activity for signal amplification. Clin Biochem. 2016;49(15):1118-1121.
doi pubmed - Ugolini A, Nuti M. Rheumatoid factor: a novel determiner in cancer history. Cancers (Basel). 2021;13(4):591.
doi pubmed - Bas S, Genevay S, Mensi N. False positive elevation of cardiac troponin I in seropositive rheumatoid arthritis. J Rheumatol. 2002;29(12):2665.
- Dasgupta A, Banerjee SK, Datta P. False-positive troponin I in the MEIA due to the presence of rheumatoid factors in serum. Elimination of this interference by using a polyclonal antisera against rheumatoid factors. Am J Clin Pathol. 1999;112(6):753-756.
doi pubmed - Kenny PR, Finger DR. Falsely elevated cardiac troponin-I in patients with seropositive rheumatoid arthritis. J Rheumatol. 2005;32(7):1258-1261.
- Al-Awadhi AM, Olusi S, Hasan EA, Abdullah A. Serum concentrations of cardiac troponin-I in patients with rheumatoid arthritis, systemic lupus erythematosus, primary Sjogren's syndrome and Graves' disease. Singapore Med J. 2007;48(9):847-849.
- Marks V. False-positive immunoassay results: a multicenter survey of erroneous immunoassay results from assays of 74 analytes in 10 donors from 66 laboratories in seven countries. Clin Chem. 2002;48(11):2008-2016.
doi pubmed - Lavoinne A, Hue G. Serum cardiac troponins I and T in early posttraumatic rhabdomyolysis. Clin Chem. 1998;44(3):667-668.
doi pubmed - Aggarwal R, Lebiedz-Odrobina D, Sinha A, Manadan A, Case JP. Serum cardiac troponin T, but not troponin I, is elevated in idiopathic inflammatory myopathies. J Rheumatol. 2009;36(12):2711-2714.
doi pubmed - Chaulin AM. Diagnostic considerations and analytical characteristics of methods for the determination of cardiac troponins: traditional review. Turkiye Klinikleri J Cardiovasc Sci. 2021;33(3):149-160.
doi - Schmid J, Liesinger L, Birner-Gruenberger R, Stojakovic T, Scharnagl H, Dieplinger B, Asslaber M, et al. Elevated cardiac troponin T in patients with skeletal myopathies. J Am Coll Cardiol. 2018;71(14):1540-1549.
doi pubmed - Messner B, Baum H, Fischer P, Quasthoff S, Neumeier D. Expression of messenger RNA of the cardiac isoforms of troponin T and I in myopathic skeletal muscle. Am J Clin Pathol. 2000;114(4):544-549.
doi pubmed - Ricchiuti V, Apple FS. RNA expression of cardiac troponin T isoforms in diseased human skeletal muscle. Clin Chem. 1999;45(12):2129-2135.
doi pubmed - Wens SC, Schaaf GJ, Michels M, Kruijshaar ME, van Gestel TJ, In 't Groen S, Pijnenburg J, et al. Elevated plasma cardiac troponin T levels caused by skeletal muscle damage in Pompe disease. Circ Cardiovasc Genet. 2016;9(1):6-13.
doi pubmed - Schmid J, Birner-Gruenberger R, Liesinger L, et al. Elevated cardiac troponin T but not troponin I in patients with skeletal muscle disease. Eur Heart J. 2017;38(1):ehx502.P2612.
doi - Chaulin AM. Cardiac troponins: current information on the main analytical characteristics of determination methods and new diagnostic possibilities. Medwave. 2021;21(11):e8498.
doi pubmed - Chaulin A. Clinical and diagnostic value of highly sensitive cardiac troponins in arterial hypertension. Vasc Health Risk Manag. 2021;17:431-443.
doi pubmed - Chaulin AM. Elevation Mechanisms and Diagnostic Consideration of Cardiac Troponins under Conditions Not Associated with Myocardial Infarction. Part 2. Life (Basel). 2021;11(11):1175.
doi pubmed - Chaulin AM. Updated information about methods of identification and diagnostic opportunities of cardiac troponins. Riv Ital Med Lab. 2021;17(3):154-164.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


