| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 13, Number 10-11, November 2021, pages 502-509
Anagliptin Monotherapy for Six Months in Patients With Type 2 Diabetes Mellitus and Hyper-Low-Density Lipoprotein Cholesterolemia Reduces Plasma Levels of Fasting Low-Density Lipoprotein Cholesterol and Lathosterol: A Single-Arm Intervention Trial
Yuichi Ikegamia, d, Yasuhiro Takenakaa, b, d, Daigo Saitoa, Akira Shimadaa , Ikuo Inouea, c, e
aDepartment of Endocrinology and Diabetes, Saitama Medical University, Faculty of Medicine, 38 Morohongo, Moroyamacho, Irumagun, Saitama 350-0495, Japan
bDepartment of Physiology, Graduate School of Medicine, Nippon Medical School, 1-25-16 Nezu, Bunkyo, Tokyo 113-0031, Japan
cPreventive Medicine Research Center, Saitama Medical University, 38 Morohongo, Moroyamacho, Irumagun, Saitama 350-0495, Japan
dThese authors contributed equally to this work.
eCorresponding Author: Ikuo Inoue, Department of Endocrinology and Diabetes, Saitama Medical University, Faculty of Medicine, 38 Morohongo, Moroyamacho, Irumagun, Saitama 350-0495, Japan
Manuscript submitted October 19, 2021, accepted November 10, 2021, published online November 20, 2021
Short title: Reduced Lathosterol by Anagliptin Monotherapy
doi: https://doi.org/10.14740/jocmr4623
| Abstract | ▴Top |
Background: Anagliptin, a dipeptidyl peptidase-4 (DPP-4) inhibitor, has been shown to decrease plasma low-density lipoprotein cholesterol (LDL-C) levels. The objective of our study was to elucidate the mechanisms responsible for the anagliptin-mediated improvements in high LDL-C levels (hyper-LDL cholesterolemia).
Methods: We prospectively examined the effects of anagliptin monotherapy on fasting plasma lathosterol, sitosterol, and campesterol levels in patients with type 2 diabetes mellitus and hyper-LDL cholesterolemia for 6 months. We examined 14 patients who did not use hypoglycemic or lipid-lowering drugs for 4 months before initiating the study. Plasma variables related to glucose and lipid metabolism were measured before and after 6 months of treatment and pre- and postprandially using the cookie-loading test.
Results: After treatment, anagliptin monotherapy (n = 14) significantly decreased fasting LDL-C (175.6 to 148.5 mg/dL, mean values before and after the treatment, respectively) and plasma lathosterol levels (3.56 to 2.49 mg/dL), whereas it did not lower fasting sitosterol or campesterol levels. Furthermore, fasting plasma lathosterol levels were negatively correlated with preprandial glucagon-like peptide-1 (GLP-1) levels after anagliptin treatment.
Conclusions: Anagliptin monotherapy may have a beneficial effect on lipid metabolism, which could be mediated by the inhibition of hepatic cholesterol synthesis rather than the inhibition of intestinal lipid transport.
Keywords: Anagliptin; Dipeptidyl peptidase-4 inhibitor; Low-density lipoprotein cholesterol; Lathosterol; Glucagon-like peptide-1
| Introduction | ▴Top |
Dipeptidyl peptidase-4 (DPP-4) inhibitors increase the plasma levels of incretins, such as glucagon-like peptide-1 (GLP-1), by selectively inhibiting DPP-4 and thereby promoting insulin secretion, which then results in hypoglycemic effects in patients with type 2 diabetes mellitus (T2DM). The degree of insulin secreted by the pancreatic beta cells, following the stimulation of incretins, is dependent on blood glucose levels. Therefore, the risk of hypoglycemia by DPP-4 inhibitor treatments is lower than that by other diabetes drugs, such as sulfonylureas (SUs). DPP-4 inhibitors are currently used worldwide, and each DPP-4 inhibitor has different clinical features depending on the type of agent [1]. Similar to other DPP-4 inhibitors, anagliptin decreases glycosylated hemoglobin (HbA1c) and plasma glucose levels [2, 3]. It has been shown to achieve the strongest effect in increasing plasma GLP-1 levels by administering a dose of 100 - 200 mg twice daily to patients with T2DM [4]. A previous study has reported that anagliptin treatment decreases plasma low-density lipoprotein cholesterol (LDL-C) levels [5]. Thus, in the present study, we examined the effects of anagliptin on fasting plasma lathosterol, sitosterol, and campesterol levels in patients with T2DM and hyper-LDL cholesterolemia for 6 months to elucidate the mechanisms that lead to improvements in plasma LDL-C levels. Lathosterol is a whole-body cholesterol synthesis marker in the liver, while sitosterol and campesterol (both plant sterols) are sterol absorption markers in the intestines [6].
| Materials and Methods | ▴Top |
Subjects
The inclusion criterion of the present study was as follows: patients with poorly controlled T2DM (HbA1c < 8.0% or 63 mmol/mol) and an LDL-C level of more than 140 mg/dL. Participating T2DM patients had inadequate glycemic and lipid control and were being treated with dietary and exercise interventions. The participants aged between 20 and 80 years of males and females. Key exclusion criteria were as follows: 1) patients using hypoglycemic agents (SUs, biguanides, alpha-glucosidase inhibitors, glinides, dipeptidyl peptidase-4 inhibitors, insulin, and GLP-1 receptor agonists); 2) hypersensitivity to anagliptin; 3) patients with severe diabetic ketoacidosis, diabetic coma/pre-coma, or type 1 diabetes; 4) patients with sepsis, those scheduled for surgery and who underwent surgery, and those with severe trauma; 5) patients with severe renal dysfunction (serum creatinine level > 2.4 mg/dL for male and > 2.0 mg/dL for female), and patients undergoing hemodialysis or peritoneal dialysis; 6) female patients who were pregnant or suspected to be pregnant; and 7) patients deemed by the investigator to be ineligible for participation in the study.
Study protocol
Anagliptin (100 mg) was administered in the morning and evening. The Institutional Review Board of Saitama Medical University Hospital approved this experimental protocol. This was a single-arm, non-randomized, open-label prospective study with blinded assessors. The protocol was registered at the University Hospital Medical Information Network (UMIN) on August 5, 2013, with the identification number UMIN000011256, and conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration. Patients who provided written informed consent were enrolled in the pre-drug intervention phase for 4 months with only lifestyle interventions; this was done to ensure that any observed effects with pharmacotherapy were attributed to anagliptin monotherapy. During the period of anagliptin monotherapy, patients were asked to follow a diet that provided a total energy of 25 kcal/kg of target body weight/day, consisting of 45-65% carbohydrate energy, 25-35% fat energy, and 15-25% protein energy per total energy. Patients were instructed to avoid alcohol consumption and smoking during the period of anagliptin monotherapy.
We assessed the following parameters at the start of the pre-drug period: both pre- and 1- and 2-h postprandial blood levels of glucose; lipid, insulin, C-peptide, and proinsulin levels; pre- and 1-h postprandial active GLP-1 levels using the cookie-loading test (CLT); and fasting plasma lathosterol, sitosterol, and campesterol levels. During this period, six patients dropped out because of the necessity for interventions other than anagliptin for dyslipidemia or hyperglycemia. After the pre-drug intervention phase, patients received anagliptin monotherapy for 6 months. During the last visit of this study, patients underwent CLT again. To assess the effects of anagliptin monotherapy on lipid metabolism, patients receiving drugs other than anagliptin were excluded from the main analysis.
Measurements
The metabolic parameters described above, including glucose and lipid levels, were measured at SRL, Inc. (Tokyo, Japan). These data were obtained to investigate the effects of 6-month anagliptin monotherapy on both pre- and 1- and 2-h postprandial levels of blood glucose, lipid, insulin using Lumipulse® PrestoInsulin (Fujirebio Inc., Tokyo, Japan), C-peptide using Lumipulse® PrestoC-peptide (Fujirebio Inc., Tokyo, Japan), proinsulin using Human Proinsulin RIA KIT (Millipore Cor., MA, USA), and pre- and 1-h postprandial active GLP-1 levels using Glucagon-Like Peptide-1 (Active) enzyme-linked immunosorbent assay (ELISA) KIT (Millipore Corp., MA, USA) in participating patients after 6 months. Moreover, markers for cholesterol synthesis (lathosterol) and absorption (sitosterol and campesterol) were determined using gas chromatography, GC-2010 (Shimadzu Co., Ltd, Kyoto, Japan). Serum apoB-48 levels were measured using Apo B-48 chemiluminescent enzyme immunoassay KIT (Fujirebio Inc., Tokyo, Japan); these levels reflect exogenous lipoproteins, such as chylomicrons, and their remnants. LDL-C levels were calculated using the Friedewald equation because all patients had triglyceride (TG) levels less than 400 mg/dL.
CLT
CLT was performed after overnight fasting using previously described methods at baseline and at 6 months after starting anagliptin treatment. Patients were instructed to ingest a cookie with water within 30 min: half of the cookie was to be eaten within 10 min and the remainder within the last 20 min. Anagliptin was administered immediately after CLT. Time measurements were started when half the cookie was ingested. Blood samples were obtained at 0, 1, and 2 h after ingesting the cookie, which consisted of 75 g of carbohydrates, 25 g of fat, and 8 g of protein, and 592 kcal of energy (Saraya Co., Ltd, Osaka, Japan).
Data analysis
The primary outcome was the change in LDL-C levels after 6 months. The secondary outcome was changes in plasma lathosterol, sitosterol, and campesterol levels. Data are presented as the mean ± standard deviation (SD) of the parametric variables. Differences before and after anagliptin treatment were examined using the Wilcoxon matched-pairs signed-rank test, and the significance was set at a P value of less than 0.05. A subanalysis of the correlation between lathosterol and active GLP-1 levels was performed by Spearman’s correlation test using samples from the remaining 13 participating patients at the end of the study. All analyses were performed using the GraphPad Prism 5.0 software (GraphPad Software, San Diego, CA, USA, www.graphpad.com).
| Results | ▴Top |
Twenty-three patients with T2DM and hyper-LDL cholesterolemia were included in the study. Pre- and postprandial (1 and 2 h) levels of blood glucose, lipids, insulin, C-peptide, and proinsulin; pre- and postprandial (1 h) levels of active GLP-1, fasting plasma lathosterol, sitosterol, and campesterol were measured. Six patients dropped out because of worsening hyperlipidemia and/or hyperglycemia before the initiation of the anagliptin treatment. Among the 17 patients who were followed until the end of the study, one patient received additional treatment with 10-mg ezetimibe daily and two patients received 5-mg pravastatin daily to manage hyperlipidemia. We mainly analyzed 14 patients for whom therapeutic agents were not changed during the test period (Fig. 1). These 14 patients did not use hypoglycemic agents, such as SUs, biguanides, alpha-glucosidase inhibitors, glinides, thiazolidinedione, other DPP-4 inhibitors, sodium-glucose co-transporter 2 inhibitors, insulin, and GLP-1 receptor agonists, and did not use oral drugs for hyperlipidemia including statins, fibrates, and ezetimibe.
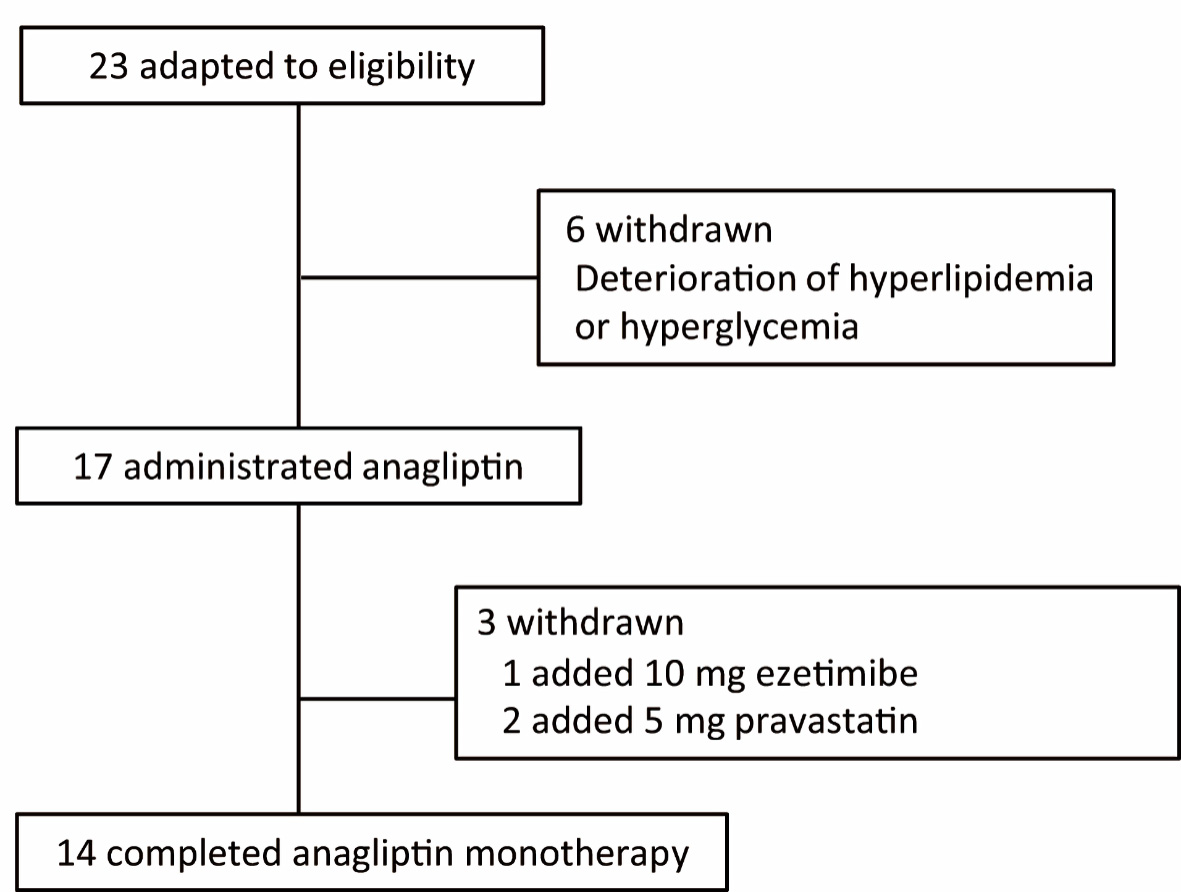 Click for large image | Figure 1. Flowchart of participant selection in this study. |
The baseline biochemical characteristics of these 14 patients are summarized in Table 1; and their metabolic profiles before and after anagliptin monotherapy are shown in Table 2. As the baseline characteristics, patients A, K, O, and Q were mild drinker (0 - 20 g/week) and other 10 patients were nondrinkers (28.6% of the participants). All patients were nonsmokers. After 6 months of anagliptin monotherapy, HbA1c (6.51% to 6.14 %, mean values before and after treatment, Fig. 2a) and LDL-C (175.6 to 148.5 mg/dL, Fig. 2b) levels significantly decreased in these 14 patients. Furthermore, the monotherapy for 6 months significantly decreased fasting lathosterol (3.56 to 2.49 µg/mL, Fig. 3a), total cholesterol (257.8 to 233.1 mg/dL), and triglyceride (135.4 to 102.3 mg/dL) levels, whereas no such significant changes were noted in fasting campesterol (6.01 to 6.74 µg/mL, Fig. 3b), sitosterol (3.22 to 3.53 µg/mL, Fig. 3c), and high-density lipoprotein cholesterol (58.6 to 60.8 mg/dL) levels as well as body mass index (BMI, 24.7 to 25.0 kg/m2).
 Click to view | Table 1. Characteristics of Glucose Metabolism of 14 Patients Before and After Treatment With Anagliptin Monotherapy |
 Click to view | Table 2. Comparison of Lipid Profiles of 14 Patients Before and After Treatment With Anagliptin Monotherapy |
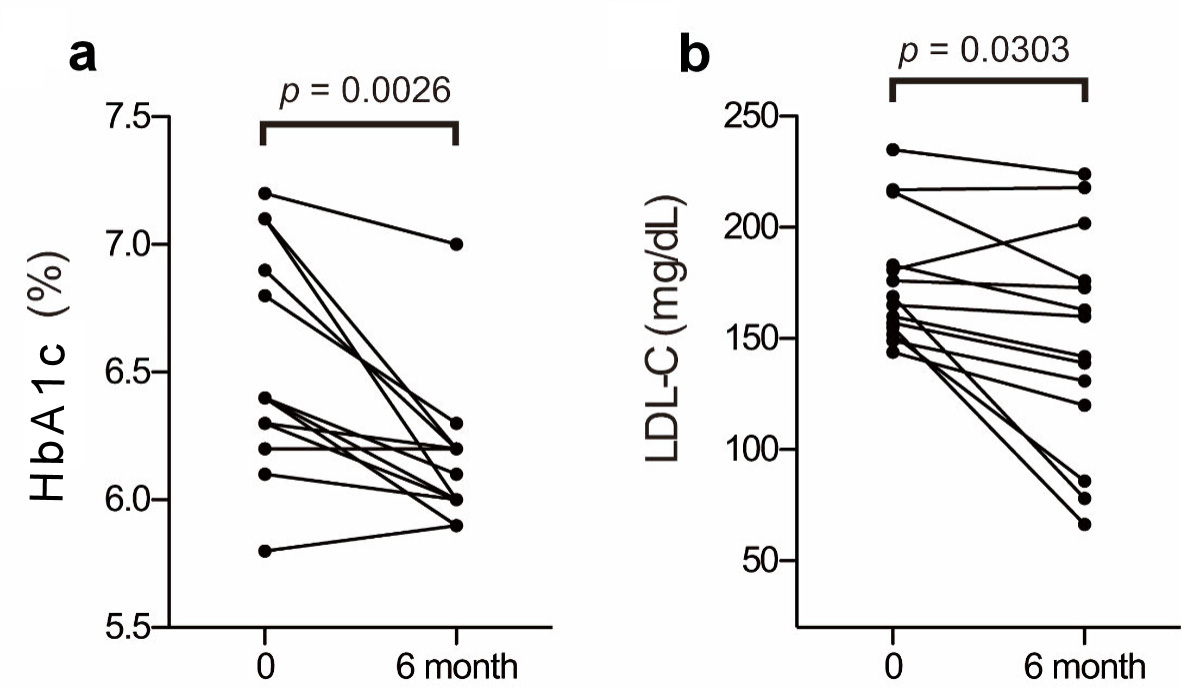 Click for large image | Figure 2. Changes in HbA1c and LDL-C before and after anagliptin monotherapy: plasma Hb1Ac (a) and LDL-C (b) levels. The two groups were compared using the Wilcoxon matched-pairs signed-rank test. HbA1c: glycosylated hemoglobin; LDL-C: low-density lipoprotein cholesterol. |
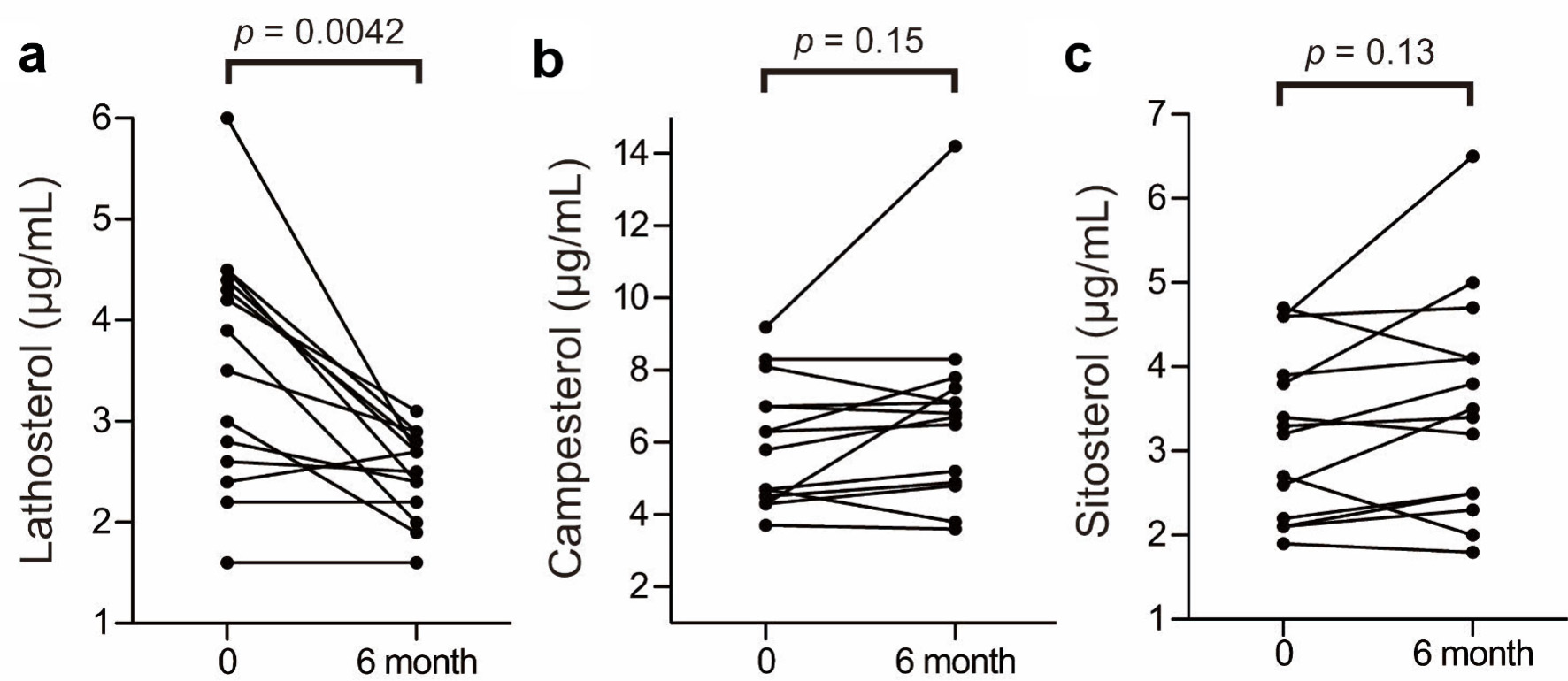 Click for large image | Figure 3. Changes in lathosterol, campesterol, and sitosterol levels before and after anagliptin monotherapy: plasma lathosterol (a), campesterol (b) and sitosterol (c) levels. The two groups were compared using the Wilcoxon matched-pairs signed-rank test. |
We examined the relationship between active GLP-1 and lathosterol, campesterol, and sitosterol levels in a subanalysis. Figure 4a shows a scatter plot of the relationship between fasting active GLP-1 levels and fasting plasma lathosterol levels in 13 patients at the end of the study. Similarly, Figure 4b, c shows scatter plots corresponding to the relationship between fasting active GLP-1 levels and fasting plasma campesterol and sitosterol levels, respectively. Since GLP-1 levels of 2.0 pmol/L or lower were not detectable, plots corresponding to these levels in one patient were omitted from the figure. A negative correlation was observed between fasting active GLP-1 levels and fasting plasma lathosterol levels but not between active GLP-1 levels and fasting plasma campesterol or sitosterol levels. No correlation was noted between postprandial active GLP-1 levels and fasting plasma lathosterol, campesterol, or sitosterol levels based on the CLT (data not shown).
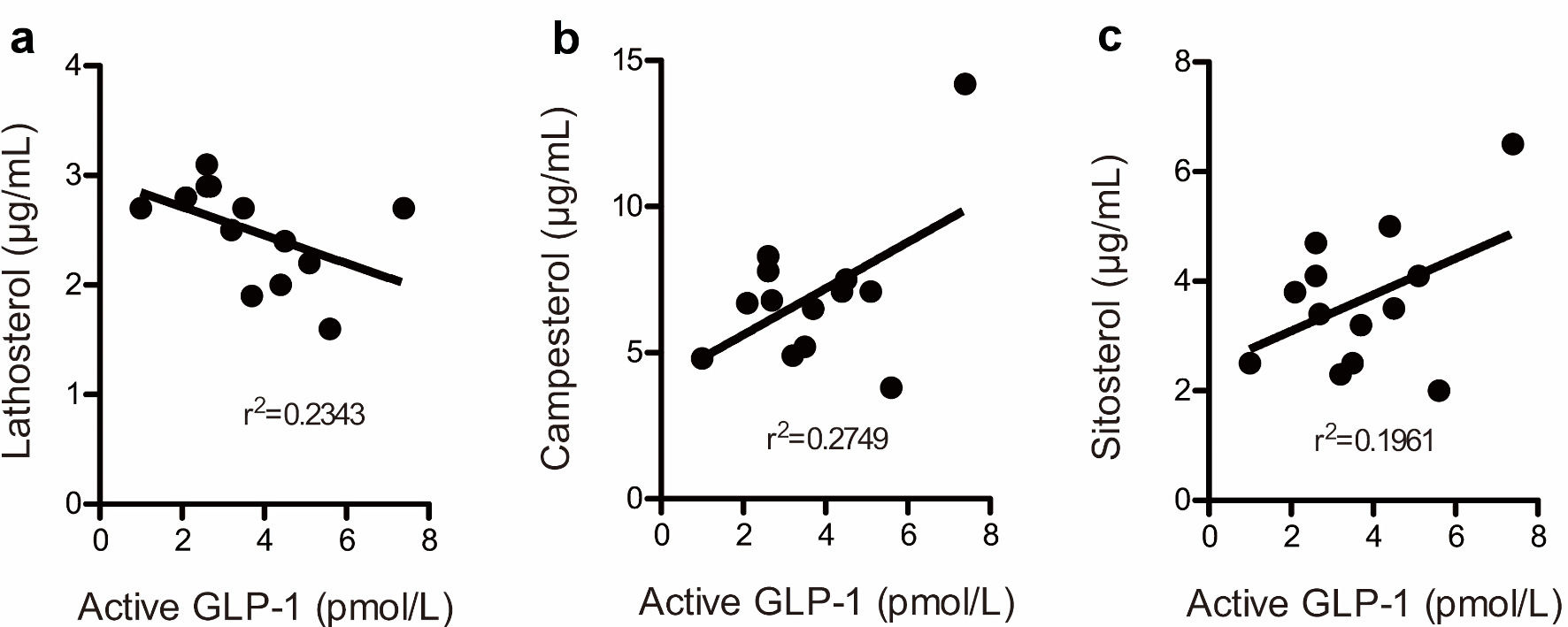 Click for large image | Figure 4. The correlation between fasting active glucagon-like peptide-1 levels and lathosterol (a), campesterol (b), and sitosterol (c) levels at the end of this study. Plots below the detection limit of (2.0 pmol/L) plasma GLP-1 were omitted. The figure presents 13 plots. GLP-1: glucagon-like peptide-1. |
Plasma glucose, insulin, and proinsulin levels obtained before and after anagliptin monotherapy using the CLT are shown in Figure 5. No significant changes were noted in fasting plasma glucose (Fig. 5a) or insulin levels (Fig. 5b) after 6 months of anagliptin monotherapy, whereas fasting plasma proinsulin levels significantly decreased (Fig. 5c). After 6 months of anagliptin monotherapy, glucose levels at 1 h (data not shown) and 2 h (Fig. 5d) after the CLT were significantly reduced, although plasma insulin levels before and after 6 months of monotherapy at 1 h (data not shown) and 2 h (Fig. 5e) after the CLT did not significantly increase. After 6 months of anagliptin monotherapy, proinsulin levels were significantly decreased at 1 h (data not shown) and 2 h (Fig. 5f) after the CLT.
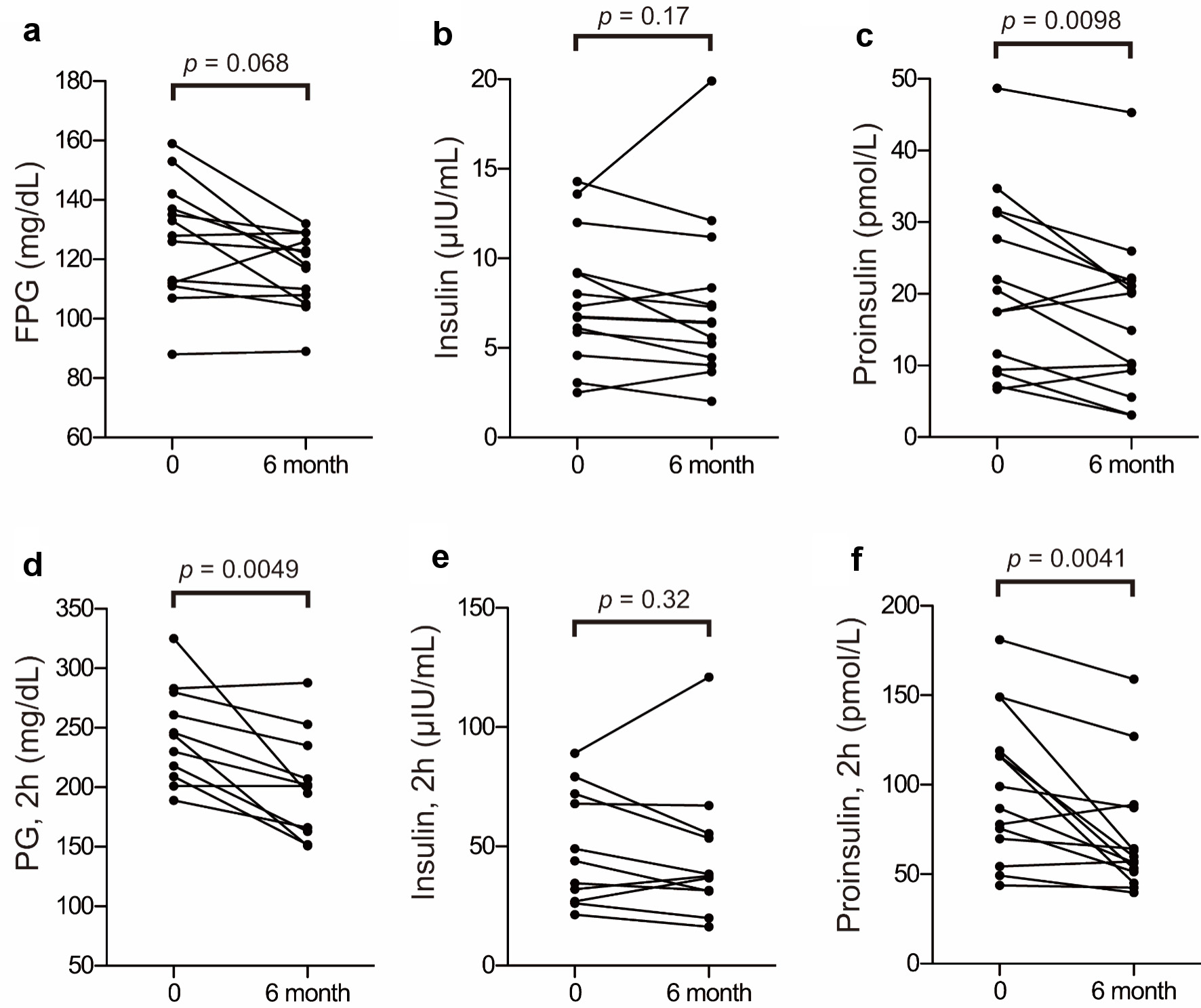 Click for large image | Figure 5. Changes in proinsulin, plasma glucose, and insulin before and after anagliptin monotherapy: fasting plasma glucose (FPG) (a), insulin (b), and proinsulin (c) levels before and after anagliptin monotherapy; and plasma glucose (PG) (d), insulin (e), and proinsulin (f) levels at 2 h after the CLT before and after anagliptin monotherapy. The two groups were compared using the Wilcoxon matched-pairs signed-rank test. |
| Discussion | ▴Top |
The results of the present study are consistent with those of previous studies, showing that DPP-4 inhibitors decrease LDL-C levels [7, 8]. Moreover, our results showed that anagliptin significantly decreased fasting plasma lathosterol levels (Fig. 3a), whereas it did not significantly alter fasting sitosterol or campesterol levels (Fig. 3b, c). Thus, anagliptin monotherapy may have reduced cholesterol synthesis in the liver, but it did not appear to have reduced cholesterol absorption in the intestines. Our results suggest that higher fasting active GLP-1 levels are associated with lower fasting plasma lathosterol levels (Fig. 4a). However, a previous study by Juntti-Berggren et al [9] showed that continuous administration of GLP-1 to patients with T2DM did not change LDL-C levels. GLP-1 analogs, when injected subcutaneously, directly flow into the systemic circulation. In contrast, active GLP-1, which is elevated by DPP-4 inhibitor, is secreted from L-cells in the ileum and enters the liver via the portal vein before entering the systemic circulation. Therefore, endogenous GLP-1 may exert different effects on cholesterol metabolism, whereas GLP-1 analogs may not. A previous study has reported that administration of anagliptin twice a day increases active GLP-1 levels after dinner more than administration of sitagliptin once a day [4]. Administration of DPP-4 inhibitors twice a day may be more advantageous for lowering LDL-C levels than administering them once a day. Although vildagliptin is also administered twice a day, its half-life is markedly shorter than that of anagliptin, which might lead to different LDL-C lowering effects. Therefore, further studies are warranted. The effect of GLP-1 on 3-hydroxy-3-methyl-glutaryl-CoA (HMG-CoA) activity in the liver is still unclear. In contrast, the administration of anagliptin has been shown to suppress the expression of SREBP-2, a transcription factor for cholesterol synthesis, and decrease LDL-C levels in LDL receptor-knockout mice, a model for studying hyperlipidemia, using that mechanism of action. Thus, the suppression of SREBP-2 by anagliptin appears to be one of the mechanisms underlying the drug’s LDL-C lowering effect [10].
Onoue et al recently observed that anagliptin, in combination with other hypoglycemic drugs, reduces fasting apoB-48 levels; hence, they proposed that anagliptin is beneficial for lipid metabolism by acting via the inhibition of intestinal lipid transport [11]. This effect was particularly observed in previous studies that included the concomitant use of SUs, which have significant hypolipidemic effects and hypoglycemic activity. It is, therefore, possible that SUs masked the LDL-C-lowering effects of anagliptin, especially in long-term treatments. Another study has showed that anagliptin therapy for 4 weeks decreases the level of the cholesterol synthesis marker lathosterol without affecting the levels of cholesterol absorption markers [12]. The present study differed from these studies with regard to the following: 1) monotherapy was performed for long period of 6 months; and 2) the relationship between lathosterol and active GLP-1 levels was examined (Fig. 4a).
In conclusion, anagliptin monotherapy for 6 months significantly decreased the levels of LDL-C and the cholesterol synthesis marker lathosterol without affecting the levels of the cholesterol absorption markers. Lathosterol levels were negatively correlated with fasting active GLP-1 levels. Thus, the beneficial effect of anagliptin monotherapy on lipid metabolism could be mediated by the inhibition of hepatic cholesterol synthesis rather than by the inhibition of intestinal lipid transport.
Acknowledgments
The authors thank Ms. Sawako Sato for her technical and secretarial assistance.
Financial Disclosure
This work was supported by an annual budget of Department of Endocrinology and Diabetes, Saitama Medical University.
Conflict of Interest
There is no conflict of interest associated with this study.
Informed Consent
Informed consent was obtained.
Author Contributions
II: study concept and design; YI and II: acquisition of data; YI, YT and II: analysis and interpretation of data; YT: statistical support; YI, YT and II: drafting of the manuscript; YT, II, DS and AS: critical revision of the manuscript.
Abbreviations
BMI: body mass index; CLT: cookie-loading test; DPP-4: dipeptidyl peptidase-4; GLP-1: glucagon-like peptide-1; HbA1c: glycosylated hemoglobin; HDL-C: high-density lipoprotein cholesterol; HMG-CoA: 3-hydroxy-3-methyl-glutaryl-CoA; LDL-C: low-density lipoprotein cholesterol; SD: standard deviation; SUs: sulfonylureas; T2DM: type 2 diabetes mellitus; TC: total cholesterol; TG: triglyceride
| References | ▴Top |
- Gerich J. DPP-4 inhibitors: what may be the clinical differentiators? Diabetes Res Clin Pract. 2010;90(2):131-140.
doi pubmed - Kaku K. Efficacy and safety of anagliptin add-on therapy in Japanese patients with type 2 diabetes - Placebo-controlled, double-blind, parallel-group study with an open-label, long-term extension. Jpn Pharmacol Ther. 2012;40(9):745-770.
- Kaku K. Efficacy and safety of long-term monotherapy with Anagliptin in Japanese patients with type 2 diabetes - A multi-centre, randomized, open-label, parallel-group study (administered before meals vs. after meals). Jpn Pharmacol Ther. 2012;40(9):733-744.
- Uchino H, Kaku K. A novel dipeptidyl peptidase-4 inhibitor, anagliptin, improved the daily blood glucose profile. Jpn Pharmacol Ther. 2012;40(10):859-869.
- Kaku K. Effects of anagliptin on serum lipids in Japanese patients with type 2 diabetes - A pooled analysis of long-term therapy with anagliptin. Jpn Pharmacol Ther. 2012;40(9):771-784.
- Miettinen TA, Gylling H. Synthesis and absorption markers of cholesterol in serum and lipoproteins during a large dose of statin treatment. Eur J Clin Invest. 2003;33(11):976-982.
doi pubmed - Cha SA, Park YM, Yun JS, Lim TS, Song KH, Yoo KD, Ahn YB, et al. A comparison of effects of DPP-4 inhibitor and SGLT2 inhibitor on lipid profile in patients with type 2 diabetes. Lipids Health Dis. 2017;16(1):58.
doi pubmed - Monami M, Lamanna C, Desideri CM, Mannucci E. DPP-4 inhibitors and lipids: systematic review and meta-analysis. Adv Ther. 2012;29(1):14-25.
doi pubmed - Juntti-Berggren L, Pigon J, Karpe F, Hamsten A, Gutniak M, Vignati L, Efendic S. The antidiabetogenic effect of GLP-1 is maintained during a 7-day treatment period and improves diabetic dyslipoproteinemia in NIDDM patients. Diabetes Care. 1996;19(11):1200-1206.
doi pubmed - Yano W, Inoue N, Ito S, Itou T, Yasumura M, Yoshinaka Y, Hagita S, et al. Mechanism of lipid-lowering action of the dipeptidyl peptidase-4 inhibitor, anagliptin, in low-density lipoprotein receptor-deficient mice. J Diabetes Investig. 2017;8(2):155-160.
doi pubmed - Onoue T, Goto M, Wada E, Furukawa M, Okuji T, Okada N, Kobayashi T, et al. Dipeptidyl peptidase-4 inhibitor anagliptin reduces fasting apolipoprotein B-48 levels in patients with type 2 diabetes: A randomized controlled trial. PLoS One. 2020;15(1):e0228004.
doi pubmed - Aoki K, Ijima T, Kamiyama H, Kamiko K, Terauchi Y. Anagliptin decreases serum lathosterol level in patients with type 2 diabetes: a pilot study. Expert Opin Pharmacother. 2015;16(12):1749-1754.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


