| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 13, Number 7, July 2021, pages 377-386
Association Between Obesity and Cancer Mortality: An Internal Medicine Outpatient Clinic Perspective
Vede Ramdassa, b, d, Elizabeth Caskeya, Tammarah Sklarza, Saaniya Ajmeria, Vaishali Patela, Ayobamidele Baloguna, Victor Pomarya, Jillian Hallb, Omar Qarib, Rahul Tripathib, Krystal Hunterc, Satyajeet Roya, b
aDepartment of Medicine, Cooper University Health Care, Camden, NJ, USA
bCooper Medical School of Rowan University, Camden, NJ, USA
cCooper Research Institute, Cooper Medical School of Rowan University, Camden, NJ, USA
dCorresponding Author: Vede Ramdass, Department of Medicine, Cooper University Health Care, Cooper Medical School of Rowan University, 1103 North Kings Highway, Suite 203, Cherry Hill, NJ 08034, USA
Manuscript submitted June 17, 2021, accepted July 2, 2021, published online July 28, 2021
Short title: Obesity and Cancer Mortality
doi: https://doi.org/10.14740/jocmr4543
| Abstract | ▴Top |
Background: Obesity is one of the leading preventable causes of cancer that has a causal relationship with cancers of esophagus, breast and colon. Paradoxically, there are studies demonstrating that obesity is associated with improved survival in cancer patients. The aim of our study was to investigate the association of obesity and cancer mortality in adult patients.
Methods: Retrospective medical record review of 784 adult patients was performed who had a diagnosis of cancer and who were seen in our outpatient Internal Medicine Clinic between January 1, 2019 and December 31, 2019.
Results: Forty-three (5.2%) patients were cancer non-survivors and 741 (94.8%) were cancer survivors. The mean age of the cancer non-survivors group was significantly higher than that of the cancer survivors (78.7 vs. 68.0 years, respectively; P < 0.001). For every unit increase in age, there was 7.6% increased odds of cancer death (95% confidence interval (CI): 3-12%) (P = 0.001). Average body mass index (BMI) of the patients in the cancer non-survivors group was significantly lower than that of the cancer survivors group (25.0 vs. 28.1 kg/m2; P = 0.008). Non-obese patients had 4.9 times greater odds of cancer death (95% CI: 1.51 - 15.81) (P = 0.008). The mean glycosylated hemoglobin (HbA1c) was significantly higher in the cancer non-survivors group compared to the cancer survivors group (7.1% vs. 6.0%; P < 0.001), and for every unit increase in HbA1c there was 1.6 times greater odds of cancer death (95% CI: 1.14 - 2.23) (P = 0.006). Patients with peripheral artery disease (PAD) had 3.5 times greater odds of cancer death compared to those without PAD (95% CI: 1.18 - 10.19) (P = 0.023).
Conclusions: Non-obese patients with cancer had higher odds of cancer death. Rising HbA1c, increasing age, and presence of PAD were associated with increased cancer mortality.
Keywords: Obesity; Cancer mortality; Obesity paradox
| Introduction | ▴Top |
Obesity is an important public health concern that currently affects 42.4% of adults in the USA [1, 2]. The Center for Disease Control and Prevention (CDC) uses body mass index (BMI) as an indirect measure of body fatness given its correlation with other direct measurements, and defines overweight as a BMI of 25.0 - 29.9 kg/m2 and obesity as a BMI ≥ 30.0 kg/m2 [3]. The consequences of obesity are well known, which contribute to a wide spectrum of disease states that include diabetes mellitus, end-stage renal disease, coronary artery disease and even cancer [4]. With regard to cancer, obesity is becoming one of the leading preventable causes of cancer and it has been shown to have a causal relation in malignancies such as adenocarcinoma of the esophagus, breast cancer and colon cancer [5]. On the other hand, there has been a lack of consensus on the association between obesity and cancer mortality [4, 6, 7]. For instance, in a prospective study by Calle and associates, there was a significant association between BMI and rates of death, estimating that in the USA, obesity and overweight account for 14% and 20% of deaths, respectively among men and women who suffer from cancer [8]. Elevated BMI was specifically associated with death by cancer of the esophagus, pancreas and multiple myeloma [8, 9]. Schmitz and associates explained that there were no clear mechanisms for the association, but potential causes include cancer treatment toxicities that vary with obesity and growth factors that are expressed on malignant cells, and are believed to be increased in the obese subjects [8, 9].
Interestingly, there are emerging studies demonstrating that obesity is associated with improved survival in cancer patients, and this finding is termed as “obesity paradox” [7, 10]. For instance, the COMPARZ trial found that obese patients with clear cell renal cell carcinoma had a lower mortality than patients who were of normal weight [11]. This relationship has also been appreciated in other malignancies such as lung cancer and melanoma [10, 12]. In a review by Trestini and associates, some of the proposed mechanisms for this effect are proposed as obesity can be associated with less aggressive tumor subtypes and lower stage disease [13]. Given the complex interaction between obesity and cancer, the American Clinical Society on Oncology (ASCO) is advocating for the increase in education and research on overweight-obesity and cancer [4]. Overall, there is lack of consensus regarding the effect of overweight and obesity on cancer mortality. The aim of our study was to investigate the association of obesity and cancer mortality in adult patients.
| Materials and Methods | ▴Top |
Study selection
This study was a retrospective study of patients with a diagnosis of cancer who were seen in our outpatient clinic between January 1, 2019 and December 31, 2019. The study was reviewed and approved by the Institutional Review Board of the Cooper University Health Care, Camden, New Jersey, USA. This study was fully compliant with the ethical standards set forth by the CUHC institutional review board. The inclusion criteria were patients aged 18 years or older who had a diagnosis of cancer.
Data collection
The data were collected by reviewing the existing electronic medical records of our patients who fulfilled the selection criteria. The following data were collected for each patient: age, gender, BMI, type of cancer (breast, colon, prostate, gynecological and other types), race (non-Hispanic, Caucasian, African American, Alaskan native, American Indian, Asian, Hispanic, Pacific islander, and other), cigarette smoking history (current or former use), alcohol use, recreational drugs use, family history (obesity, cancer and type of cancer), associated medical conditions (hypertension, diabetes mellitus (DM), hyperlipidemia, hypothyroidism, coronary artery disease (CAD), cerebral vascular disease, peripheral vascular disease, carotid artery stenosis, congestive heart failure (CHF), arthritis and other rheumatologic disorder), psychiatric conditions, glycosylated hemoglobin (HbA1c), and living status (survivor or non-survivor).
The majority of our patients received their cancer care outside our healthcare system. The details of their cancer staging and management were not linked with our electronic medical records, hence the data were not available to us. We had to rely on the scanned reports of the consultant oncologists from various healthcare systems which varied widely based on the patient-specific cancer care needs, and lacked many details, such as consistent staging, symptoms of loss of appetite, treatments other than chemotherapy, radiotherapy or surgery, as well as follow-up course. In order to mitigate the influence of such factors on the BMI, for each patient we collected the average BMI out of the preceding 5-year BMIs documented in our electronic medical record.
Statistical analysis
All of the collected data were entered into a Microsoft Excel (2016, Remond, Washington, USA) spreadsheet. Statistical analysis was done by using SPSS (Statistical Package for the Social Sciences, version 15.01, IBM, Armonk, New York, USA). We calculated an approximate sample size of 784 patients based on the calculation of the approximate number of patients with a diagnosis of cancer who visited our outpatient office during the previous 12 months.
The patients were divided into two groups: first group represented the patients with cancer who were cancer non-survivors, and the second group represented patients who were cancer survivors. Unifactorial analysis was conducted by applying independent t-tests to compare the means of continuous variables between the study groups. Chi-square tests were used to compare the categorical (dichotomous) variables between the study groups. For multifactorial analysis, we used logistic regression. The model was used to examine the relationship between the variable of interest (obesity) and the dependent variable (cancer mortality) after adjusting (or controlling) for other independent variables that may also be related to the outcome (confounding factors or covariates). In this study, significance was defined as a P < 0.05.
| Results | ▴Top |
A total of 784 patients were included in the study. These comprised of 43 patients (5.2%) who were deceased at the time of chart review hence grouped as cancer non-survivors and 741 patients (94.8%) who were cancer survivors. The age range for all patients was between 21 and 99 years. The mean age of the cancer survivors group was 68.0 years and the mean age of the cancer non-survivors patient group was 78.7 years (Table 1). The difference between both groups of patients was statistically significant (P < 0.001). Multivariate analysis revealed that for every unit increase in age, there was 7.6% increased odds of cancer death (95% confidence interval (CI): 3-12%) (P = 0.001) (Table 2).
 Click to view | Table 1. Baseline Characteristics |
 Click to view | Table 2. Influence of Risk Factors on Cancer Mortality |
In gender analysis, the difference between both cancer non-survivors and cancer survivors groups was significant (P = 0.019), with the cancer non-survivors group having more males than females (53.5% vs. 46.5%), whereas the cancer survivors group had more females than males (64.2% vs. 35.8%). However, gender did not have significant influence on mortality in our patient population (Table 2). The breakdown of races was similar between the cancer non-survivors group and the survivors group, with White race being the majority (76.7% vs. 68.7%), followed by other races (11.6% vs. 18.9%), Black race (9.3% vs. 8.5%) and Hispanic race (2.3% vs. 3.9%) (Table 1). There was no significant difference between both groups with regard to race (P = 0.606). About half of the patients in both the groups had history of cigarette smoking or alcohol use, but the differences were not statistically different (Table 1).
The breakdown of cancer types between both groups was also similar (Fig. 1). In the cancer non-survivors group, frequency of breast cancer was 11.6%, followed by colorectal cancer 9.3%, prostate cancer 14%, gynecological cancer 2.3% and other cancers 62.8%. In the survivors group, frequency of breast cancer was 24.4%, followed by colorectal cancer 5.7%, prostate 13.2%, gynecological cancer 7.4% and other cancers 49.3%. Although the frequency of breast cancer was higher in the cancer survivors group compared to the cancer non-survivors group, the differences in the frequencies of various cancers between the two groups were not statistically significant (Table 1).
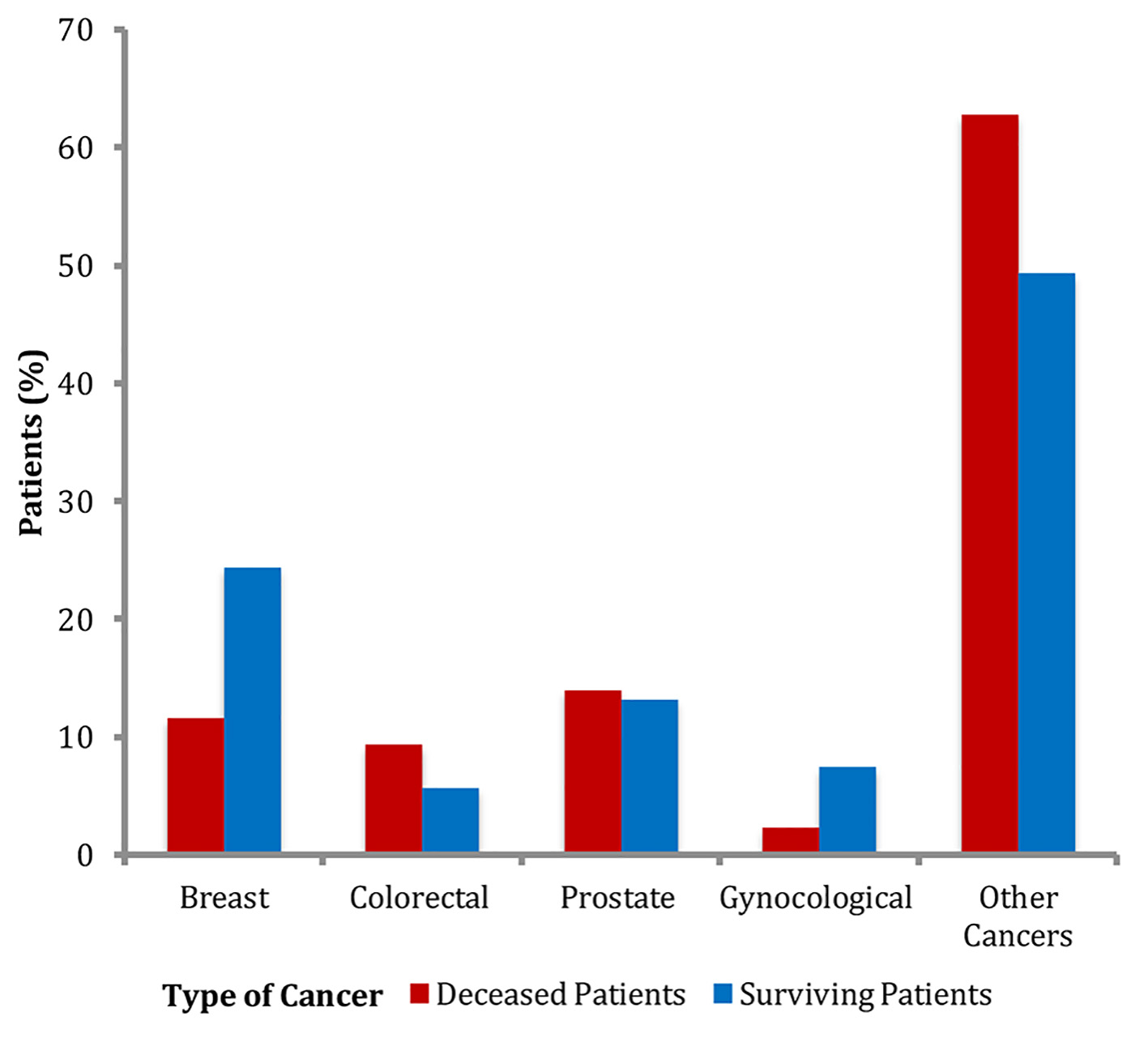 Click for large image | Figure 1. Frequencies of types of cancers. |
We found that the frequencies of arthritis, hypertension, hyperlipidemia, other rheumatologic disorders, hypothyroidism, depression and anxiety were greater in the cancer non-survivors group compared to the cancer survivors group, but the differences were not statistically significant (Table 1). However, for other comorbid conditions, there were statistically significant differences between the groups, but the comorbid condition did not have any effect on the cancer mortality. For instance, there was a higher frequency of cerebral vascular accidents (CVAs) in the cancer non-survivors group compared to the cancer survivors group (14% vs. 4.9%; P = 0.023) (Table 1); however, multivariate analysis revealed that CVA did not have a significant effect on cancer mortality (Table 2). Similarly, the frequencies of association of DM, CAD and CHF were significantly higher in the cancer non-survivors group compared to the cancer survivors group (41.9% vs. 20.6%, P = 0.015; 27.9% vs. 14.3%, P = 0.015; and 20.9% vs. 5.5%, P = 0.001), but these comorbidities had no effect on cancer mortality (Table 2). Interestingly, we found that the frequency of peripheral artery disease (PAD) was significantly higher in the cancer non-survivors group (23.3%) compared to the cancer survivors group (4.2%) (P < 0.001) (Table 1). Multivariate analysis showed that the patients with PAD had 3.5 times greater odds of cancer death (95% CI: 1.18 - 10.19) compared to those without PAD (P = 0.023) (Table 2).
Analysis of family history of cancers revealed that the frequencies of breast cancer, prostate cancer, gynecological cancer and other cancers were not significantly different between the two groups. For family history of colorectal cancer, we found that the cancer survivors group had a significantly higher frequency of association compared to the cancer non-survivors group (17.5% vs. 4.7%) (P = 0.028) (Table 1); however, multivariate analysis showed that it did not have an effect on cancer mortality (Table 2).
As mentioned earlier, we found a statistically significant difference in frequency of association between DM in the cancer non-survivors group compared to the cancer survivors group. Although DM by itself had no effect on the cancer mortality (Table 2), the mean HbA1c was significantly higher in the cancer non-survivors group compared to the cancer survivors group (7.1% vs. 6.0%, P < 0.001) (Table 1) and for every unit increase in HbA1c, there was 1.6 times greater odds of cancer death (95% CI: 1.14 - 2.23) (P = 0.006) (Table 2).
In the assessment of overweight and obesity, we found that the average BMI of the patients in the cancer non-survivors group was significantly lower than that in the cancer survivors group (25.0 vs. 28.1 kg/m2) (P = 0.008) (Table 1). The frequencies of patients in each BMI category also differed significantly (Fig. 2). Multivariate analysis showed that non-obese patients had 4.9 times greater odds of cancer death (95% CI: 1.51 - 15.81) (P = 0.008) (Table 2).
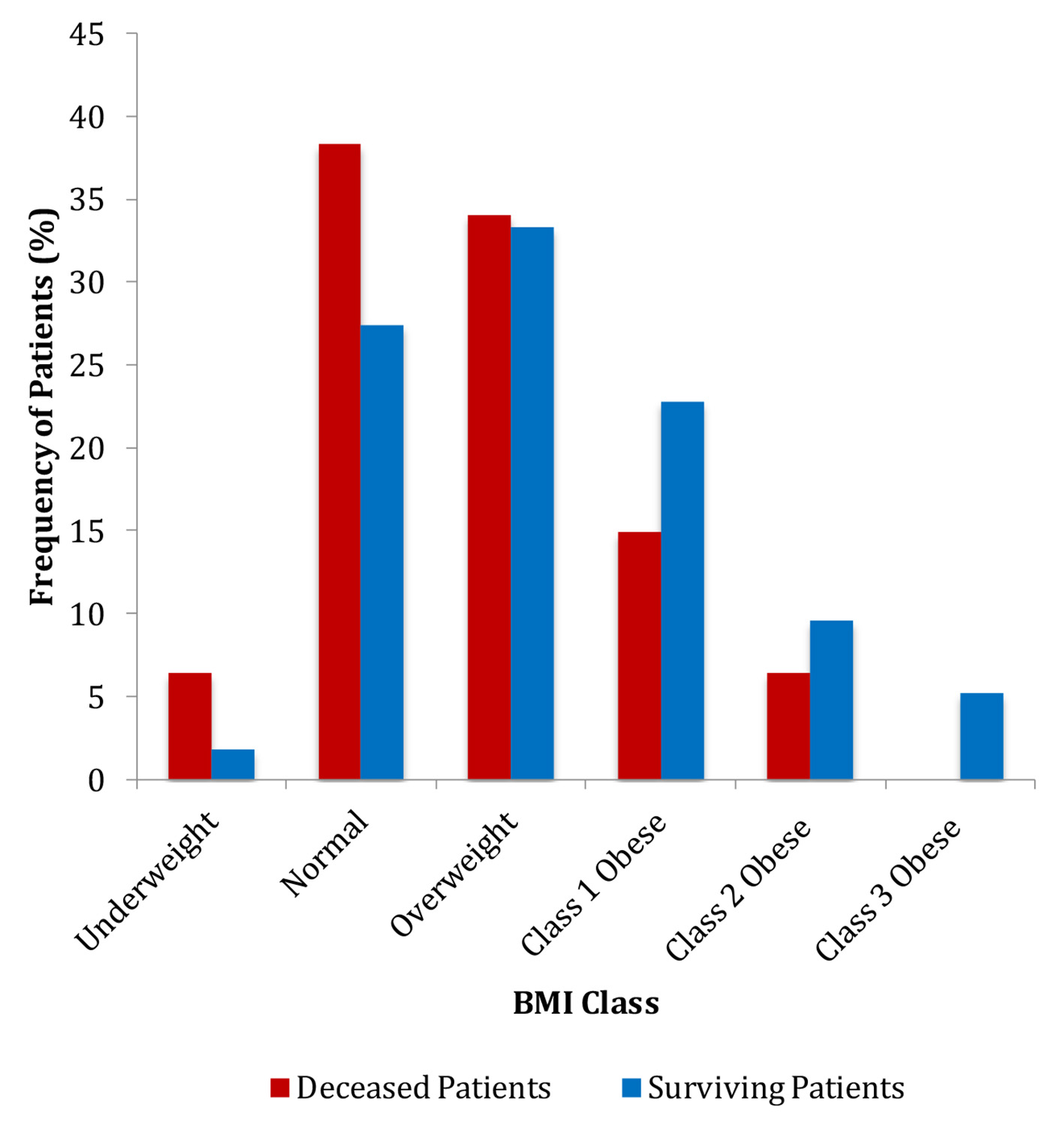 Click for large image | Figure 2. Frequencies of patients in each BMI category. BMI: body mass index. |
| Discussion | ▴Top |
The major finding of our study was that the non-obese patients with cancer had higher odds of cancer death. Our findings are supported by the findings of many studies [14-20] that have documented that among patients with cancer, elevated BMI is associated with improved survival compared to the patients who had normal BMI. Hines and associates found that in patients with colon cancer, being underweight increased the risk of death; however, being overweight and obese was protective [14]. Navarro and associates studied the role of high-dose therapy with autologous hematopoietic cell transplantation in patients with lymphoma. They reported that the overall mortality was higher in the underweight group, and lower in the overweight and obese groups compared with the normal BMI group [15]. Parker and associates found that the overweight patients with renal cell carcinoma were at a reduced risk of death compared with patients with BMI in the normal range [16]. A meta-analysis of colorectal cancer survivors conducted by Schlesinger and associates concluded that patients with BMI in the underweight category were at an increased risk for all-cause mortality, whereas patients who were overweight had a lower risk, compared to the patients who were in the normal BMI range [17]. Similarly, Tsang and associates studied the association between BMI and overall survival in patients with distant metastases and who had favorable performance status. They found that the median overall survival time was 3.23 months for underweight patients, 6.08 months for normal weight patients, 7.99 months for overweight patients, and 12.49 months for obese patients. In their study they also found that compared with normal weight patients, both obese and overweight patients had a reduced risk of all-cause mortality [20].
There have been several postulations to explain this phenomenon. One such explanation is that some tumors are less aggressive in obese patients such as renal cell carcinoma, where obesity is associated with more indolent forms of tumor characteristics [21]. Another potential explanation is that obese patients have altered pharmacokinetics to treatment regimens and they respond differently to certain surgeries, both resulting in improved outcomes [22, 23]. It is also possible that obesity plays a role in having a “nutrient reserve” and patients experience a mortality benefit [7]. Although BMI has been implicated as a poor proxy for estimation of body adipose tissue and composition [24], it correlates positively with waist circumference, and negatively with mortality [25]. BMI also has an inverse association with the expression of fatty acid synthase (FASN) [20], an oncogene that is involved in the fatty acid synthesis, and found to be overexpressed in several malignancies. It has been reported that FASN is significantly downregulated in obese patients with renal cell carcinoma offering favorable effects on cancer-specific survival [21]. Additionally, muscle wasting negatively impacts BMI. It is a common feature of aging. In our study, we did find that with every unit increase in age, there was 7.6% increased odds of cancer death in our patients. Muscle wasting is also associated with distant metastasis, treatment toxicities, and mortality [26-29]. Although the exact mechanism remains unclear but increased levels of pro-inflammatory interleukin 6 (IL-6) have been associated with muscle wasting and low BMI [30, 31]. Studies have shown that increased levels of IL-6 activate pro-inflammatory and angiogenic factors that drive and promote tumorigenesis [32]. Increased levels of IL-6 also trigger epithelial mesenchymal transformation in breast cancer cells, which promote distant metastasis [33]. To summarize, several studies have reported the “obesity paradox” where the mortality benefit had been seen in patients who were overweight or obese [7, 10] which further support our findings of the higher odds of cancer deaths in non-obese patients.
Our findings contrast with the findings of several studies that have suggested that a higher BMI increases the incidence of cancers [34-36], particular in the highest weight category (class III: BMI ≥ 40 kg/m2) [8]. Calle and associates reported an increase in mortality associated with obesity in patients with all cancers, and especially for the esophageal cancer, colorectal cancer and breast cancer [8]. It is possible that relationships between obesity and cancer mortality differ with tumor site. In our study, in the cancer non-survivors group, approximately 20% of the patients had breast cancer while the majority of patients had other cancers that included malignancies, such as lung cancer, basal cell carcinoma, melanoma, etc. Interestingly, Taghizadeh and associates found no significant association between long-term annual change in BMI and cancer mortality risk, while both short-term annual increase and decrease in BMI were associated with a lower mortality risk from any cancer [37]. They also reported a lower risk of mortality from lung cancer among overweight subjects, especially males. There are several studies that have consistently found an inverse association between BMI and cancer mortality and suggested that this association is independent of smoking and weight loss because of preclinical disease [38-41].
In our study, with each year increase in age, there was a 7.6% increase in odds of cancer death. Several studies have investigated the effect of age on specific malignancies, and the data are consistent with the fact that increasing age confers a worse prognosis. For instance, when considering breast cancer, a prospective study showed that increasing age was associated with higher cancer mortality [42]. In other studies, the same relationship was seen in liver cancer and cervical cancer [43, 44]. Furthermore, the CDC performed a study in which information was collected by the CDC’s National Center for Health Statistics which used death certificates from all 50 states in the USA [17]. The study found an upward trend in death rate (defined as deaths per 100,000 population) with the increase in the age group [45]. Our findings correlate with the findings from the CDC and by other studies. In a study by Pal and associates, there were some proposed mechanisms for these findings, such as decreased physiological reserve that include changes to renal and gastric function in elderly patients. Also, in patients who developed a malignancy in older age, the cancer characteristics could have been associated with worse outcomes [46].
In our patient group, the diagnosis of diabetes was significantly higher in the cancer non-survivors group compared to the cancer survivors group. Similarly, the mean HbA1c level was significantly higher in the cancer non-survivors group compared to the cancer survivors group, and for each unit increase in HbA1c, there was 1.6 times greater odds of cancer death (95% CI: 1.14 - 2.23). Our findings correspond with the study conducted by Harding and colleagues, in which they showed that cancer patients with diabetes had a 30% higher rate of cancer mortality than non-diabetic cancer patients [47]. In another study, Currie and colleagues found that cancer mortality was increased in those with diabetes, compared with those without (hazard ratio (HR): 1.09 (95% CI: 1.06 - 1.13)). They also found that the mortality was particularly increased in patients with breast cancer (1.32 (1.17 - 1.49)) and prostate cancer (1.19 (1.08 - 1.31)). After controlling for the confounding factors, they reported that diabetes was associated with approximately 10% increase in the mortality for all cancers compared to those without diabetes. Their prognosis estimation in patients with diabetes revealed that bladder, breast, and prostate cancers were associated with significantly diminished survival [48]. Interestingly, the management of diabetes in patients with cancer can have an impact on the type of cancer and survival, as well. It has been reported that patients managed with insulin, or insulin secretagogues, were found to be more likely to develop solid cancers than those managed with metformin alone [49]. Xiu and colleagues investigated the impact of diabetes on the progression-free survival and overall survival of extensive-stage small-cell lung cancer. They reported that the mean progression-free survival of patients without diabetes was 9 months, while it was significantly reduced for patients with diabetes (5 months (P < 0.0001)). They also reported that the overall survival of patients with diabetes was significantly shorter than that of patients without diabetes (HR: 1.455; 95% CI: 1.134 - 1.868; P = 0.003) [50]. Kelkar and colleagues investigated the association between diabetes and prostate cancer-specific mortality. They found that diabetes was associated with increased risks of prostate cancer progression and mortality among obese men [51]. Similarly, Wu and colleagues reported that long-term plasma glucose fluctuation was significantly associated with the risk of cancer mortality (HR: 1.41 (95% CI: 1.04 - 1.92)), especially in the highest quartile of coefficient of variation of plasma glucose [52]. This finding aligns with our observation that with each unit increase in HbA1c there was 1.6 times greater odds of cancer death (95% CI: 1.14 - 2.23). Additional studies have also reported that the increased mortality associated with poorly managed diabetes has been particularly appreciated in specific malignancies, such as colorectal cancer and breast cancer [53, 54]. The exact relationship between diabetes and cancer mortality remains to be fully understood, but uncontrolled diabetes may have a direct effect on tumorigenesis, and indirect effects, such as influence of diabetes in medical decision making [53, 54]. Other possible mechanisms include less aggressive treatment in diabetic patients, poor response to treatment, and an increased risk of adverse events due to the treatment [55, 56].
We also found that PAD was associated with an increase in cancer mortality, with the odds of cancer death being 3.5 times higher than patients without peripheral vascular disease. A similar report was published by Fiotti and associates, in which they found that the patients with PAD had a higher long-term mortality rate, and mortality rate from cancer exceeded that of cardiovascular diseases [57]. According to Yannoutsos and colleagues, and many other studies, the epidemiological evidence confirms that PAD is a marker for the development of lung cancer, independent of age [58-63]. Similarly, Kaschwich and colleagues found that patients suffering from symptomatic PAD had a markedly higher risk for incident cancer in the long-term follow-up, especially for certain types of cancers, such as cancer of the lung, bladder, pancreas, and colon [64]. Sundboll and colleagues examined cancer risk and prognosis of cancer in patients with lower limb PAD and arterial thrombosis. They reported that the risk of any cancer was 2.5% after 6 months of follow-up, which increased to 17.9% after 20 years [65]. Since cigarette smoking happens to be the common risk factor for many cancers, such as cancer of lung, head and neck, esophagus, stomach, liver, colon, pancreas, kidney, bladder, ovary, uterine cervix and myeloid leukemia, the association of PAD can be an indirect evidence of presence of cancer and overall influence on mortality. In our study, we found no significant difference in cigarette smoking between the cancer survivors and cancer non-survivors; hence we believe that the association of PAD in increased cancer mortality is a unique finding of our study.
The major strength of our study is a relatively large sample size of patients with cancer from one office location who were followed with the same small group of healthcare providers, which allowed proper documentation of cancers, comorbid conditions, mortality and other variables in the electronic medical record. The major limitation of our study was a small sample size of the cancer non-survivors which limited further analysis of BMI class on obesity. As seen in Figure 1, there were no patients in the cancer non-survivors group in the highest BMI class, as a result, we could not study the effect of increasing BMI on cancer mortality. Additionally, our patient selection limited to a suburban outpatient population limits generalization.
Conclusion
We found that non-obese patients with cancer had higher odds of cancer death. We also found that rising HbA1c, increasing age, and presence of PAD were associated with increased cancer mortality.
Acknowledgments
The authors thank Christine Rickette, RN (study coordinator) for her contribution to this study.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable. Being a retrospective chart review study the Institutional Review Board waived the need for informed consent.
Author Contributions
VR and SR made substantial contributions to the study design, drafting, data acquisition and analysis, and manuscript writing. All authors contributed in data collection and manuscript writing. KH analyzed the data. SR contributed in revising the manuscript critically for improved intellectual content, and final approval for the version to be published.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017-2018. NCHS Data Brief. 2020;360:1-8.
- National Institute of Health. Managing overweight and obesity in adults: systematic evidence review from the obesity expert panel. Accessed June 13, 2021, at: https://www.nhlbi.nih.gov/health-topics/managing-overweight-obesity-in-adults.
- CDC. About Adult BMI. 2017. Accessed June 13, 2021, at: https://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/ - Definition.
- Ligibel JA, Alfano CM, Courneya KS, Demark-Wahnefried W, Burger RA, Chlebowski RT, Fabian CJ, et al. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol. 2014;32(31):3568-3574.
doi pubmed - Steele CB, Thomas CC, Henley SJ, Massetti GM, Galuska DA, Agurs-Collins T, Puckett M, et al. Vital Signs: Trends in Incidence of Cancers Associated with Overweight and Obesity - United States, 2005-2014. MMWR Morb Mortal Wkly Rep. 2017;66(39):1052-1058.
doi pubmed - Colditz GA, Peterson LL. Obesity and Cancer: Evidence, Impact, and Future Directions. Clin Chem. 2018;64(1):154-162.
doi pubmed - Lennon H, Sperrin M, Badrick E, Renehan AG. The Obesity paradox in cancer: a review. Curr Oncol Rep. 2016;18(9):56.
doi pubmed - Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625-1638.
doi pubmed - Schmitz KH, Neuhouser ML, Agurs-Collins T, Zanetti KA, Cadmus-Bertram L, Dean LT, Drake BF. Impact of obesity on cancer survivorship and the potential relevance of race and ethnicity. J Natl Cancer Inst. 2013;105(18):1344-1354.
doi pubmed - Zhang X, Liu Y, Shao H, Zheng X. Obesity Paradox in Lung Cancer Prognosis: Evolving Biological Insights and Clinical Implications. J Thorac Oncol. 2017;12(10):1478-1488.
doi pubmed - Sanchez A, Furberg H, Kuo F, Vuong L, Ged Y, Patil S, Ostrovnaya I, et al. Transcriptomic signatures related to the obesity paradox in patients with clear cell renal cell carcinoma: a cohort study. Lancet Oncol. 2020;21(2):283-293.
doi - Naik GS, Waikar SS, Johnson AEW, Buchbinder EI, Haq R, Hodi FS, Schoenfeld JD, et al. Complex inter-relationship of body mass index, gender and serum creatinine on survival: exploring the obesity paradox in melanoma patients treated with checkpoint inhibition. J Immunother Cancer. 2019;7(1):89.
doi pubmed - Trestini I, Carbognin L, Bonaiuto C, Tortora G, Bria E. The obesity paradox in cancer: clinical insights and perspectives. Eat Weight Disord. 2018;23(2):185-193.
doi pubmed - Hines RB, Shanmugam C, Waterbor JW, McGwin G, Jr., Funkhouser E, Coffey CS, Posey J, et al. Effect of comorbidity and body mass index on the survival of African-American and Caucasian patients with colon cancer. Cancer. 2009;115(24):5798-5806.
doi pubmed - Navarro WH, Loberiza FR, Jr., Bajorunaite R, van Besien K, Vose JM, Lazarus HM, Rizzo JD. Effect of body mass index on mortality of patients with lymphoma undergoing autologous hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2006;12(5):541-551.
doi pubmed - Parker AS, Lohse CM, Cheville JC, Thiel DD, Leibovich BC, Blute ML. Greater body mass index is associated with better pathologic features and improved outcome among patients treated surgically for clear cell renal cell carcinoma. Urology. 2006;68(4):741-746.
doi pubmed - Schlesinger S, Siegert S, Koch M, Walter J, Heits N, Hinz S, Jacobs G, et al. Postdiagnosis body mass index and risk of mortality in colorectal cancer survivors: a prospective study and meta-analysis. Cancer Causes Control. 2014;25(10):1407-1418.
doi pubmed - Amptoulach S, Gross G, Kalaitzakis E. Differential impact of obesity and diabetes mellitus on survival after liver resection for colorectal cancer metastases. J Surg Res. 2015;199(2):378-385.
doi pubmed - Brunner AM, Sadrzadeh H, Feng Y, Drapkin BJ, Ballen KK, Attar EC, Amrein PC, et al. Association between baseline body mass index and overall survival among patients over age 60 with acute myeloid leukemia. Am J Hematol. 2013;88(8):642-646.
doi pubmed - Tsang NM, Pai PC, Chuang CC, Chuang WC, Tseng CK, Chang KP, Yen TC, et al. Overweight and obesity predict better overall survival rates in cancer patients with distant metastases. Cancer Med. 2016;5(4):665-675.
doi pubmed - Hakimi AA, Furberg H, Zabor EC, Jacobsen A, Schultz N, Ciriello G, Mikklineni N, et al. An epidemiologic and genomic investigation into the obesity paradox in renal cell carcinoma. J Natl Cancer Inst. 2013;105(24):1862-1870.
doi pubmed - Wong AL, Seng KY, Ong EM, Wang LZ, Oscar H, Cordero MT, Copones R, et al. Body fat composition impacts the hematologic toxicities and pharmacokinetics of doxorubicin in Asian breast cancer patients. Breast Cancer Res Treat. 2014;144(1):143-152.
doi pubmed - Gurunathan U, Myles PS. Limitations of body mass index as an obesity measure of perioperative risk. Br J Anaesth. 2016;116(3):319-321.
doi pubmed - Taylor J. The obesity paradox. Eur Heart J. 2011;32(13):1575-1576.
- Golledge J, Cronin O, Iyer V, Bradshaw B, Moxon JV, Cunningham MA. Body mass index is inversely associated with mortality in patients with peripheral vascular disease. Atherosclerosis. 2013;229(2):549-555.
doi pubmed - Collins J, Noble S, Chester J, Coles B, Byrne A. The assessment and impact of sarcopenia in lung cancer: a systematic literature review. BMJ Open. 2014;4(1):e003697.
doi pubmed - Prado CM, Baracos VE, McCargar LJ, Reiman T, Mourtzakis M, Tonkin K, Mackey JR, et al. Sarcopenia as a determinant of chemotherapy toxicity and time to tumor progression in metastatic breast cancer patients receiving capecitabine treatment. Clin Cancer Res. 2009;15(8):2920-2926.
doi pubmed - Gonzalez MC, Pastore CA, Orlandi SP, Heymsfield SB. Obesity paradox in cancer: new insights provided by body composition. Am J Clin Nutr. 2014;99(5):999-1005.
doi pubmed - Harimoto N, Shirabe K, Yamashita YI, Ikegami T, Yoshizumi T, Soejima Y, Ikeda T, et al. Sarcopenia as a predictor of prognosis in patients following hepatectomy for hepatocellular carcinoma. Br J Surg. 2013;100(11):1523-1530.
doi pubmed - Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006;119(6):526 e529-517.
doi pubmed - Kuroda K, Nakashima J, Kanao K, Kikuchi E, Miyajima A, Horiguchi Y, Nakagawa K, et al. Interleukin 6 is associated with cachexia in patients with prostate cancer. Urology. 2007;69(1):113-117.
doi pubmed - Lederle W, Depner S, Schnur S, Obermueller E, Catone N, Just A, Fusenig NE, et al. IL-6 promotes malignant growth of skin SCCs by regulating a network of autocrine and paracrine cytokines. Int J Cancer. 2011;128(12):2803-2814.
doi pubmed - Sullivan NJ, Sasser AK, Axel AE, Vesuna F, Raman V, Ramirez N, Oberyszyn TM, et al. Interleukin-6 induces an epithelial-mesenchymal transition phenotype in human breast cancer cells. Oncogene. 2009;28(33):2940-2947.
doi pubmed - Kushi LH, Doyle C, McCullough M, Rock CL, Demark-Wahnefried W, Bandera EV, Gapstur S, et al. American Cancer Society Guidelines on nutrition and physical activity for cancer prevention: reducing the risk of cancer with healthy food choices and physical activity. CA Cancer J Clin. 2012;62(1):30-67.
doi pubmed - Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569-578.
doi - De Pergola G, Silvestris F. Obesity as a major risk factor for cancer. J Obes. 2013;2013:291546.
doi pubmed - Taghizadeh N, Boezen HM, Schouten JP, Schroder CP, Elisabeth de Vries EG, Vonk JM. BMI and lifetime changes in BMI and cancer mortality risk. PLoS One. 2015;10(4):e0125261.
doi pubmed - Smith L, Brinton LA, Spitz MR, Lam TK, Park Y, Hollenbeck AR, Freedman ND, et al. Body mass index and risk of lung cancer among never, former, and current smokers. J Natl Cancer Inst. 2012;104(10):778-789.
doi pubmed - Prospective Studies C, Whitlock G, Lewington S, Sherliker P, Clarke R, Emberson J, Halsey J, et al. Body-mass index and cause-specific mortality in 900 000 adults: collaborative analyses of 57 prospective studies. Lancet. 2009;373(9669):1083-1096.
doi - Leung CC, Lam TH, Yew WW, Chan WM, Law WS, Tam CM. Lower lung cancer mortality in obesity. Int J Epidemiol. 2011;40(1):174-182.
doi pubmed - Yang Y, Dong J, Sun K, Zhao L, Zhao F, Wang L, Jiao Y. Obesity and incidence of lung cancer: a meta-analysis. Int J Cancer. 2013;132(5):1162-1169.
doi pubmed - Derks MGM, Bastiaannet E, van de Water W, de Glas NA, Seynaeve C, Putter H, Nortier JWR, et al. Impact of age on breast cancer mortality and competing causes of death at 10 years follow-up in the adjuvant TEAM trial. Eur J Cancer. 2018;99:1-8.
doi pubmed - Xu J. Trends in liver cancer mortality among adults aged 25 and over in the United States, 2000-2016. NCHS Data Brief. 2018;314:1-8.
- Quinn BA, Deng X, Colton A, Bandyopadhyay D, Carter JS, Fields EC. Increasing age predicts poor cervical cancer prognosis with subsequent effect on treatment and overall survival. Brachytherapy. 2019;18(1):29-37.
doi pubmed - CDC. An update on cancer deaths in the United States. Atlanta, GA: US Department of Health and Human Services. 2021. Accessed June 13, 2021, at: https://www.cdc.gov/cancer/dcpc/research/update-on-cancer-deaths/index.htm.
- Pal SK, Katheria V, Hurria A. Evaluating the older patient with cancer: understanding frailty and the geriatric assessment. CA Cancer J Clin. 2010;60(2):120-132.
doi pubmed - Harding JL, Andes LJ, Gregg EW, Cheng YJ, Weir HK, Bullard KM, Burrows NR, et al. Trends in cancer mortality among people with vs without diabetes in the USA, 1988-2015. Diabetologia. 2020;63(1):75-84.
doi pubmed - Currie CJ, Poole CD, Jenkins-Jones S, Gale EA, Johnson JA, Morgan CL. Mortality after incident cancer in people with and without type 2 diabetes: impact of metformin on survival. Diabetes Care. 2012;35(2):299-304.
doi pubmed - Currie CJ, Poole CD, Gale EA. The influence of glucose-lowering therapies on cancer risk in type 2 diabetes. Diabetologia. 2009;52(9):1766-1777.
doi pubmed - Xiu W, Huang Y, Li Y, Yu M, Gong Y. Comorbidities and mortality risk among extensive-stage small-cell lung cancer patients in mainland China: impacts of hypertension, type 2 diabetes mellitus, and chronic hepatitis B virus infection. Anticancer Drugs. 2021.
doi pubmed - Kelkar S, Oyekunle T, Eisenberg A, Howard L, Aronson WJ, Kane CJ, Amling CL, et al. Diabetes and prostate cancer outcomes in obese and nonobese men after radical prostatectomy. JNCI Cancer Spectr. 2021;5(3):pkab023.
doi pubmed - Wu M, Lu J, Yang Z, Shen P, Yu Z, Tang M, Jin M, et al. Longitudinal changes in fasting plasma glucose are associated with risk of cancer mortality: A Chinese cohort study. Cancer Med. 2021.
doi pubmed - Jousheghany F, Phelps J, Crook T, Hakkak R. Relationship between level of HbA1C and breast cancer. BBA Clin. 2016;6:45-48.
doi pubmed - Erickson K, Patterson RE, Flatt SW, Natarajan L, Parker BA, Heath DD, Laughlin GA, et al. Clinically defined type 2 diabetes mellitus and prognosis in early-stage breast cancer. J Clin Oncol. 2011;29(1):54-60.
doi pubmed - van de Poll-Franse LV, Houterman S, Janssen-Heijnen ML, Dercksen MW, Coebergh JW, Haak HR. Less aggressive treatment and worse overall survival in cancer patients with diabetes: a large population based analysis. Int J Cancer. 2007;120(9):1986-1992.
doi pubmed - Meyerhardt JA, Catalano PJ, Haller DG, Mayer RJ, Macdonald JS, Benson AB, 3rd, Fuchs CS. Impact of diabetes mellitus on outcomes in patients with colon cancer. J Clin Oncol. 2003;21(3):433-440.
doi pubmed - Fiotti N, Altamura N, Cappelli C, Schillan M, Guarnieri G, Giansante C. Long term prognosis in patients with peripheral arterial disease treated with antiplatelet agents. Eur J Vasc Endovasc Surg. 2003;26(4):374-380.
doi - Yannoutsos A, Fontaine M, Galloula A, Damotte D, Chatellier G, Paterlini-Brechot P, Meyer G, et al. Peripheral arterial disease and systematic detection of circulating tumor cells: rationale and design of the DETECTOR prospective cohort study. BMC Cardiovasc Disord. 2019;19(1):212.
doi pubmed - Nicolajsen CW, Dickenson MH, Budtz-Lilly J, Eldrup N. Frequency of cancer in patients operated on for acute peripheral arterial thrombosis and the impact on prognosis. J Vasc Surg. 2015;62(6):1598-1606.
doi pubmed - Harthun NL, Lau CL. The incidence of pulmonary neoplasms discovered by serial computed tomography scanning after endovascular abdominal aortic aneurysm repair. J Vasc Surg. 2011;53(3):738-741.
doi pubmed - El Sakka K, Gambhir RP, Halawa M, Chong P, Rashid H. Association of malignant disease with critical leg ischaemia. Br J Surg. 2005;92(12):1498-1501.
doi pubmed - Valentine RJ, Pearson AS, McIntire DD, Hagino RT, Turnage RH, Clagett GP. Abdominal aortic aneurysms and malignant neoplasia: double jeopardy. Surgery. 1998;123(2):228-233.
doi - Truijers M, Pol JA, Kurvers H, Bredie S, Oyen WJ, Blankensteijn JD. Incidental finding of malignancy in patients preoperatively evaluated for aneurysm wall pathology using PET/CT. J Vasc Surg. 2009;49(5):1313-1315.
doi pubmed - Kaschwich M, Peters F, Hischke S, Riess HC, Gansel M, Marschall U, L'Hoest H, et al. Long-term incidence of cancer after index treatment for symptomatic peripheral arterial disease - a health insurance claims data analysis. Vasa. 2020;49(6):493-499.
doi pubmed - Sundboll J, Veres K, Horvath-Puho E, Adelborg K, Sorensen HT. Risk and prognosis of cancer after lower limb arterial thrombosis. Circulation. 2018;138(7):669-677.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


