| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 13, Number 2, February 2021, pages 113-120
Anti-Ro/SSA Antibodies May Be Responsible for Cerebellar Degeneration in Sjogren’s Syndrome
Syuichi Tetsukaa, b, Tomohiro Suzukia, Tomoko Ogawaa, Ritsuo Hashimotoa, Hiroyuki Katoa
aDepartment of Neurology, International University of Health and Welfare Hospital, 537-3, Iguchi, Nasushiobara, Tochigi 329-2763, Japan
bCorresponding Author: Syuichi Tetsuka, Department of Neurology, International University of Health and Welfare Hospital, 537-3, Iguchi, Nasushiobara, Tochigi 329-2763, Japan
Manuscript submitted January 16, 2021, accepted January 26, 2021, published online February 25, 2021
Short title: SSA Antibodies Cause Cerebellar Degeneration
doi: https://doi.org/10.14740/jocmr4429
| Abstract | ▴Top |
Background: Neurological disorders have been identified to be a common extraglandular manifestation of Sjogren’s syndrome (SjS). Central nervous system (CNS) symptoms appear in about 5% of patients with SjS. However, so far, only a few incidences of cerebellar degeneration have been reported, and the clinical features and pathological mechanisms associated with SjS remain to be unclear. Intramedullary production of anti-Ro/anti-SjS-related antigen A (SSA) has been observed in some patients with SjS patients who have CNS involvement, suggesting the involvement of anti- Ro/SSA antibodies as antineuronal antibodies in previous studies.
Methods: We recently treated cerebellar degeneration in a patient with SjS. We analyzed the serum and cerebrospinal fluid (CSF) in order to detect anti-Ro/SSA and anti-La/anti-SjS-related antigen B (SSB) antibodies. We also searched the literature for previous case reports to evaluate the characteristics of cerebellar degeneration in patients with SjS. First, we have studied in mouse brain tissue and examined whether the Ro/SSA (Ro52/tripartite motif protein (TRIM)21) protein was expressed in the cerebellum of mice using immunohistochemistry.
Results: Although all patients that we found in the literature review and our patient 1 were positive for anti-Ro/SSA antibodies, some patients were also negative for anti-La/SSB antibodies. Anti-Ro/SSA antibodies were observed in both serum and CSF; however, anti-Ro/SSA antibodies were negative in the CSF of patients with SjS without CNS involvement. Cerebellar atrophy was observed, and sequelae remained in the majority of patients. Autopsy findings indicated a selective loss of Purkinje cells. Ro52/TRIM21 expression was also detected throughout murine brains, including the hippocampus, cerebral cortex and cerebellum. High Ro52/TRIM21 expression was observed in the Purkinje cells.
Conclusions: We described the characteristics of cerebellar degeneration in patients with SjS and Ro52/TRIM21 expression in the Purkinje cells of murine cerebellar tissue sections. These outcomes indicate that anti-Ro/SSA antibodies were likely responsible for cerebellar degeneration in patients suffering from SjS.
Keywords: Anti-Ro/SSA antibodies; Cerebellar degeneration; Purkinje cell; Ro52/TRIM21; Sjogren’s syndrome
| Introduction | ▴Top |
Sjogren’s syndrome (SjS) has been defined as an autoimmune disease in which the exocrine glands, primarily the salivary glands, are damaged. In addition, SjS is known to affect a wide variety of organs, including the skin, joints, nervous system, lungs, kidneys and digestive tract [1]. In particular, peripheral and central neurological symptoms can be evident in about 15% and 5% of patients with SjS, respectively [2]. In the past decade, central nervous system (CNS) involvement in SjS has been observed more commonly than initially suspected, with disorders that include encephalitis, cognitive disorders, meningitis, myelitis and cerebellar degeneration. However, only a few reports of cerebellar degeneration have been described, and its clinical features and pathological mechanisms associated with SjS are yet to be determined.
Anti-Ro/anti-SjS-related antigen A (SSA) and anti-La/anti-SjS-related antigen B (SSB) antibodies have been identified to be essential for the classification of SjS during diagnostic workups [3]. On the basis of molecular weights, Ro/SSA and La/SSB antibodies target three cellular proteins, namely, Ro52 (also referred to as tripartite motif protein (TRIM)21), Ro60 and La48 [3]. Intramedullary production of anti-Ro52/TRIM21 antibodies has been observed in some patients with SjS who have CNS involvement, suggesting the involvement of anti-Ro52/TRIM21 antibodies as antineuronal antibodies, and it has been reported that cerebrospinal fluid (CSF) anti-Ro/SSA antibodies can serve as a biomarker for SjS-related CNS involvement [4]. However, an understanding on the molecular and pathological mechanisms behind autoantibodies in CNS manifestations of SjS, including cerebellar degeneration, remains to be lacking; thus, further investigations are required to clarify their associations.
We recently treated cerebellar degeneration in a patient with SjS. We analyzed serum and CSF to determine any presence of anti-Ro/SSA and anti-La/SSB antibodies. We also performed a literature review to assess the clinical characteristics, diagnostic methods and therapeutic strategies used for patients with SjS who have cerebellar degeneration. Moreover, we examined the expression of autoantigens (potential autoantibody target sites) in the murine cerebellar tissue sections to elucidate the molecular and pathological mechanisms of cerebellar degeneration in these patients.
| Materials and Methods | ▴Top |
Patients
Written informed consent was obtained from the patients in these case presentations, including for the accompanying images in the figures.
Patient 1 (SjS with cerebellar degeneration)
A 36-year-old male patient with progressive gait imbalance for 2 weeks was admitted to our neurology department. He had no family history of gait disturbance and neurological disorders and no history of exposure to toxins or drugs. The neurological examination revealed dysarthria, dysmetria in both legs, ataxic gait and inability to walk without assistance due to several cerebellar ataxia affecting all limbs and trunk. His scale for the assessment and rating of ataxia score, in which ≤ 8 points indicates the ability to walk unassisted, was 24.5. His muscle strength and sensory examinations were normal, and his deep tendon reflexes were normoactive. He did not have nystagmus or cognitive impairment. Laboratory evaluation revealed high serum anti-Ro/SSA and anti-La/SSB antibody levels (≥ 1,200 and 198 U/mL, respectively) and antinuclear antibody (ANA) positivity (1:80). He was negative for anti-dsDNA, anti-Sm and anti-phospholipid (cardiolipin and β2 glycoprotein) antibodies. Paraneoplastic (anti-Hu, nti-Ri, anti-Yo, anti-Tr, anti-PNMA2 and anti-CV2), anti-GAD and anti-thyroglobulin antibodies were not detected. Both Schirmer’s and fluorescein tests were positive, indicating that the patient also had dry eye. A salivary gland biopsy was then performed, revealing a lymphocytic infiltration around the salivary gland duct (Fig. 1a, b). The CSF showed increased anti-Ro/SSA antibodies at 15.9 U/mL (normal range: < 7.0 U/mL) and negative anti-La/SSB antibodies (< 1.0 U/mL). This patient was then diagnosed with SjS according to the American-European Consensus Group’s criteria [5]. Intravenous methylprednisolone (1 g/day) was then administered for 3 days, and maintenance therapy with oral methylprednisolone was continued for 4 months. In addition, the patient received intravenous immunoglobulin treatments twice during his hospitalization. Four months later, the patient had significant clinical improvement, with a scale for the assessment and rating of ataxia score of 12. After 1 year, brain magnetic resonance imaging (MRI) showed cerebellar atrophy (Fig. 1c), compared with the MRI obtained at the time of the patient’s hospital admission (Fig. 1d).
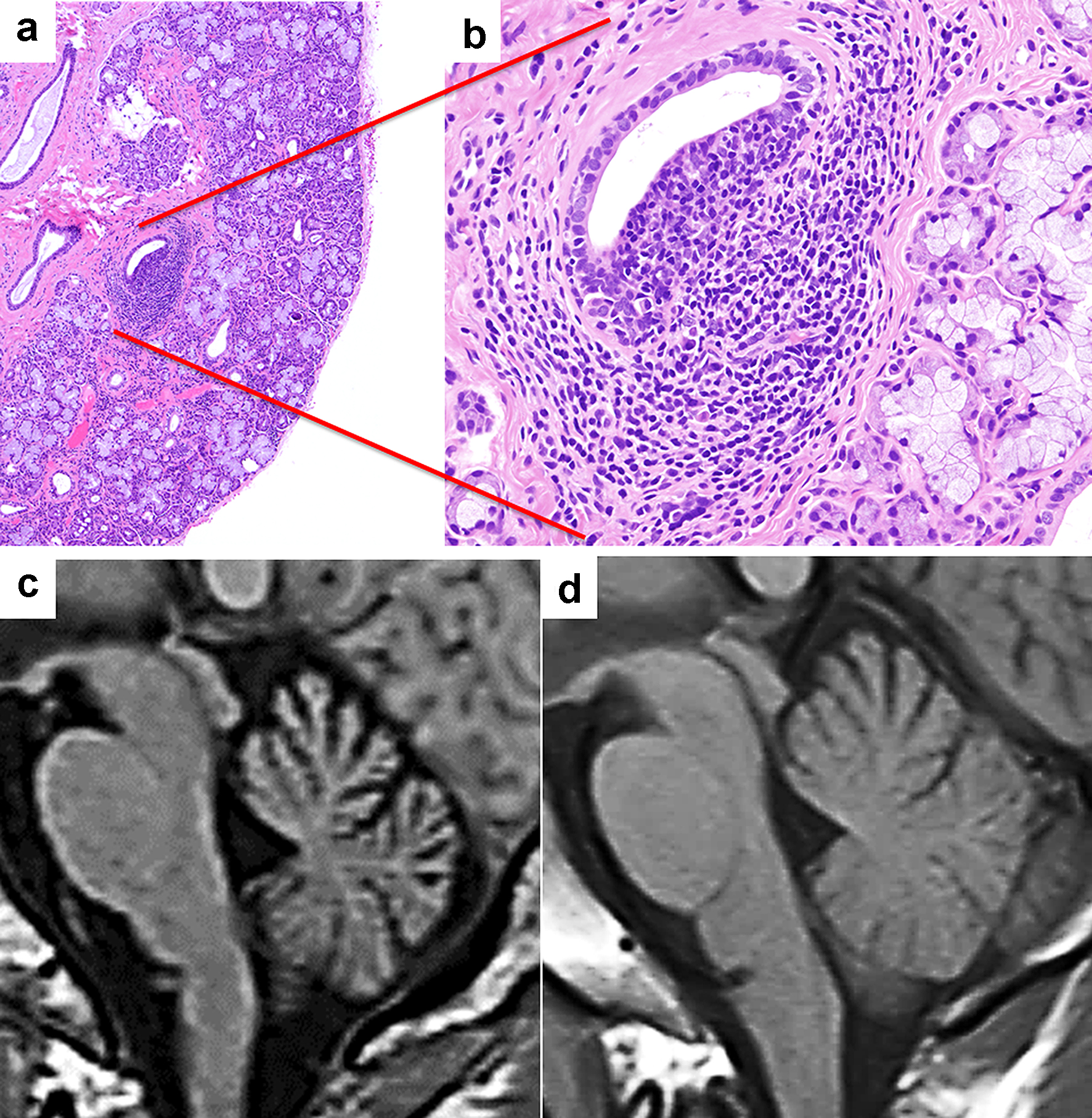 Click for large image | Figure 1. Patient 1 (Sjogren’s syndrome with cerebellar degeneration). (a, b) Photomicrographs of a salivary gland tissue section showing mild lymphocytic infiltration between the acini and glands (hematoxylin and eosin stain, magnification for a: × 40, b: × 200). (c, d) Sagittal views of the brain using magnetic resonance imaging T1 sequences. (c) The MRI performed 1 year after the initial presentation revealed cerebellar atrophy. (d) The initial MRI was unremarkable. |
Patient 2 (SjS with chronic inflammatory demyelinating polyradiculoneuropathy (CIDP))
A 53-year-old male patient was admitted to our neurology department with complaints of numbness and weakness in all limbs, which emerged 3 years earlier and gradually worsened. He had a history of hypertension and hyperlipidemia. His cranial nerves were intact, and there were no overt symptoms of cerebellar ataxia by neurological examination. Deep tendon reflexes were not present in the upper and lower extremities, and there were no pathological reflexes. Muscle atrophy and weakness (Medical Research Center muscle scale, grade: R4-/L4) were noted in the distal muscles of lower extremities. Sensory disturbance (dysesthesia and hypoesthesia) was observed along the dermatomes of the left chest, left abdomen, left back and both extremities. A slight decrease in position and vibration sense was observed in both feet. He showed a wadding gait and inability to walk with a tandem gait. The serum anti-Ro/SSA and anti-La/SSB antibody levels were 26.5 and 24.5 U/mL, respectively. The levels of ANA, anti-dsDNA, anti-GM1 and anti-GQ1b were also within the normal limits. He was also negative for serum anti-neutrophil cytoplasmic antibodies (ANCAs) targeting myeloperoxidase (MPO) and proteinase 3 (PR3). A lip biopsy showed lymphocyte infiltration around the salivary gland duct. A series of electromyographic examinations showed polyradiculoneuropathy with demyelination, consistent with the electrodiagnostic criteria of CIDP. CSF specimens showed increased total protein levels (96 mg/dL), and both anti-Ro/SSA and anti-La/SSB antibodies were determined to be absent (< 1.0 U/mL). Brain MRI did not reveal abnormalities, and the patient was diagnosed with SjS with CIDP.
We present patient 2 to show the differences between patients with SjS with peripheral nervous system (PNS) involvement and those with CNS involvement (patient 1). Anti-Ro/SSA antibodies were positive in the serum, but negative in the CSF, in the patient with SjS who had PNS involvement without CNS involvement.
Literature review
We conducted a literature review by searching PubMed for articles from 1990 to 2019 using the keywords “Sjogren’s syndrome”, “cerebellar” or “cerebellum” and “Purkinje”. Several patients with SjS who also developed cerebellar degeneration were previously reported to undergo autopsy, including the examination of cerebellum. We reprinted and modified the photomicrographs of cerebellar tissue sections from a patient whose neuropathological findings in cerebellum were reported by Nanri et al, with permission from the publisher ([6], figure 4, Creative Commons CC BY). We compared the cerebellar tissue section of that patient with a healthy human cerebellar tissue section.
Immunohistochemistry
This experimental study used healthy 8-week-old C57BL/6N male mice. Animal experiments were approved by the Institutional Animal Experiment Committee of the Genostaff Co., Ltd, Tokyo, Japan, and complied with the Institutional Regulations for Animal Experiments and Fundamental Guidelines for the Proper Conduct of Animal Experiments and Related Activities in Academic Research Institutions under the Ministry of Education, Culture, Sports, Science, and Technology. Using immunohistochemistry, paraffin-embedded murine sagittal sections of the brain tissue were de-paraffined with xylene and rehydrated using graded ethanol and phosphate-buffered saline (PBS). Antigen retrieval was performed in a citrate buffer at pH 6 and given microwave treatment. Endogenous peroxidase was blocked with 0.3% H2O2 in methanol for 30 min, followed by incubation with G-Block (Genostaff) and an avidin/biotin blocking kit (Vector). Sections were incubated with an anti-Ro52/TRIM21 rabbit polyclonal antibody (Affinity Biosciences) at 4 °C overnight; these were further incubated with biotin-conjugated anti-rabbit Ig (Dako) for 30 min at room temperature, followed by the addition of peroxidase-conjugated streptavidin (Nichirei) for 5 min. Peroxidase activity was visualized using diaminobenzidine staining. The sections were counterstained with Mayer’s hematoxylin (MUTO), were dehydrated and then were mounted with malinol (MUTO).
| Results | ▴Top |
Characteristics of cerebellar degeneration in patients with SjS
We found 14 patients with previously reported SjS, including our patient 1, who also had developed cerebellar degeneration [6-18]. The characteristics of cerebellar degeneration in patients with SjS are provided in Table 1. The average age of onset was 48.9 ± 19.4 years. The male/female ratio was overwhelmingly female, with three men and 11 women. Cerebellar atrophy was observed with brain MRI in the majority of the patients as the disease progressed (12/14 cases). Three main symptoms suggested cerebellar disturbances: ataxia (14/14 cases), nystagmus (6/14 cases) and dysarthria (10/14 cases). A few patients had been diagnosed with SjS (2/14 cases), and many patients presented with cerebellar degeneration as the first manifestation of SjS, as did our patient 1.
 Click to view | Table 1. The Clinical Manifestations of Sjogren Syndrome Patients With Cerebellar Degeneration From Our Literature Review |
In many case reports, steroid or immunosuppressive therapies were provided, and, despite the relatively good therapeutic responses to this type of therapy, sequelae remained in 11 of the 14 patients, including our patient 1. In the majority of patients, cerebellar atrophy was observed after the onset of neurological symptoms (12/14 cases). Interestingly, Nanri et al reported on their SjS patient with cerebellar degeneration found on autopsy [6]. This patient had a selective loss of Purkinje cells, had no apparent degenerative changes in the efferent pathways, such as the dentate or vestibular nuclei and had no prominent inflammatory reaction (Fig. 2).
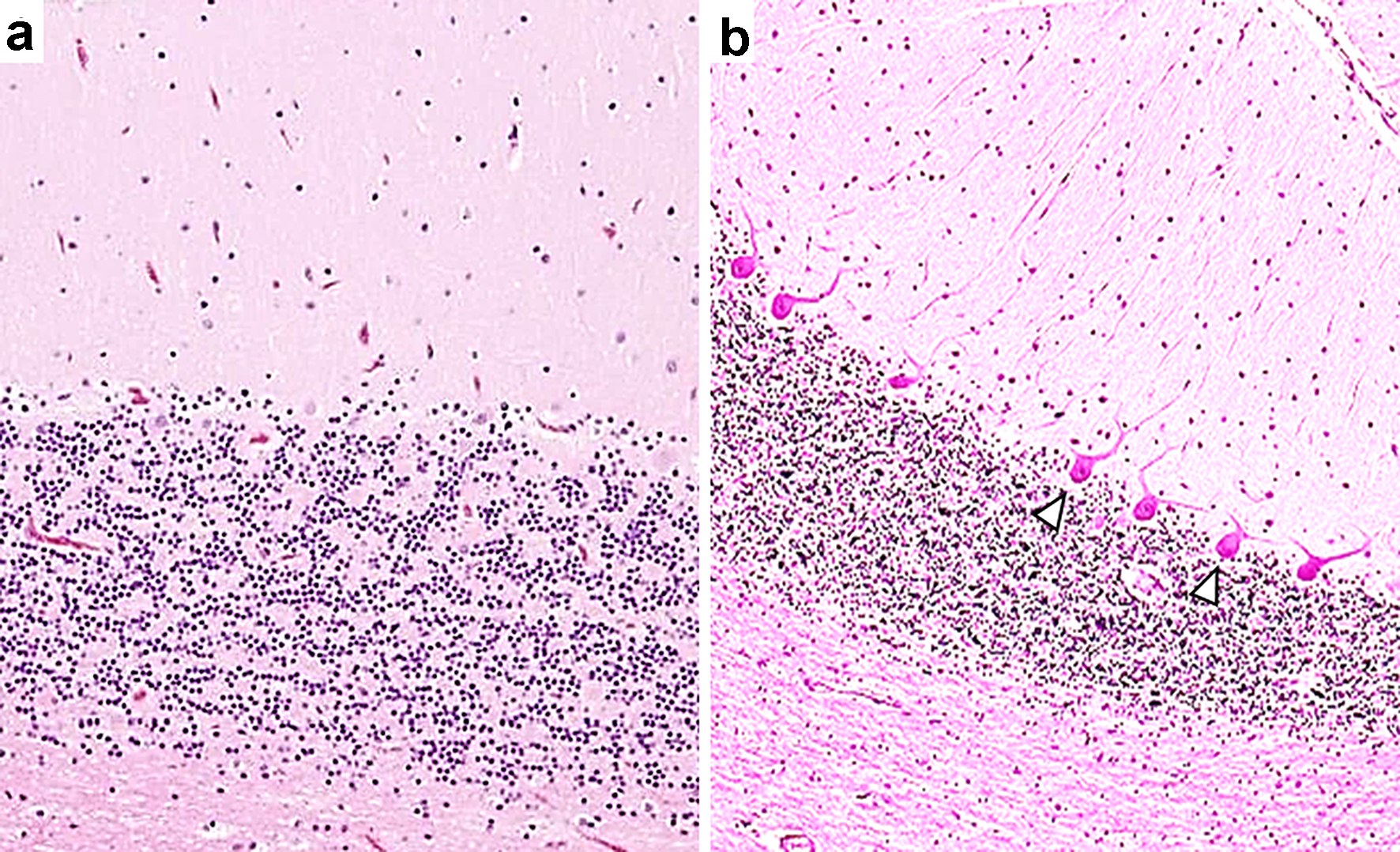 Click for large image | Figure 2. A selective loss of Purkinje cells in a patient with Sjogren’s syndrome with cerebellar degeneration. The patient was an 84-year-old woman who presented with nystagmus, dysarthria and ataxia in all limbs. Anti-Ro/SSA antibodies concentrations were determined to be > 500 U/mL, and anti-La/SSB antibodies were 41.1 U/mL. Intravenous immunoglobulin therapy was moderately effective. The patient died of pneumonia at age 85 years; an autopsy was then performed [6]. (a) A photomicrograph shows a mild-to-moderate decrease in the number of Purkinje cells in association with mild Bergman gliosis elsewhere in the cerebellar cortex. (b) This photomicrograph shows a normal human cerebellar tissue section with an appropriate number of Purkinje cells. Hematoxylin and eosin stain. The photomicrographs of tissue sections from this patient were reprinted and modified with permission from the publisher ([6], figure 4, Creative Commons CC BY). Arrowheads indicate Purkinje cells. |
The most notable finding in the literature review was the positive rate of autoantibody detection (Table 1). Although anti-Ro/SSA antibodies in the serum were positive in all patients, anti-La/SSB antibodies in the serum were negative in five of the 14 patients [7, 12, 16-18]. In addition, our patient 2, who had SjS with PNS involvement but no CNS involvement, was negative for both anti-Ro/SSA and anti-La/SSB antibodies in the CSF. These findings suggest that anti-Ro/SSA antibodies, but not anti-La/SSB antibodies, are involved in the pathology of SjS-related cerebellar degeneration. Therefore, first, we have studied in mouse brain tissue and examined whether the Ro/SSA (Ro52/TRIM21) protein was expressed in the cerebellum of mice using immunohistochemistry.
Ro52/TRIM21 expression in the murine brain
We demonstrated that Ro52/TRIM21 expression was easily detected throughout the brain, including the hippocampus, cerebral cortex and cerebellum (Fig. 3a) and that the control antibody did not exhibit any specific staining patterns (Fig. 3b). Ro52/TRIM21 expression was clearly detected in the murine cerebellum using anti-Ro52/TRIM21 antibodies (Fig. 4a) as compared with the negative staining using normal rabbit Ig (Fig. 4b). In particular, high Ro52/TRIM21 expression was observed in the cytoplasm of Purkinje cells (Fig. 4a), suggesting that Ro52/TRIM21-expressing cells in the cerebellum are major targets for anti-Ro52/TRIM21. In addition, Ro52/TRIM21 expression also was detected in the cytoplasm of the neurons on the cortex (Fig. 5a) and the hippocampus (Fig. 5b), and high levels of staining were observed using anti-Ro52/TRIM21 antibodies in the cytoplasm of the neurons in the CA3 region of the hippocampus on high magnification image (Fig. 5c).
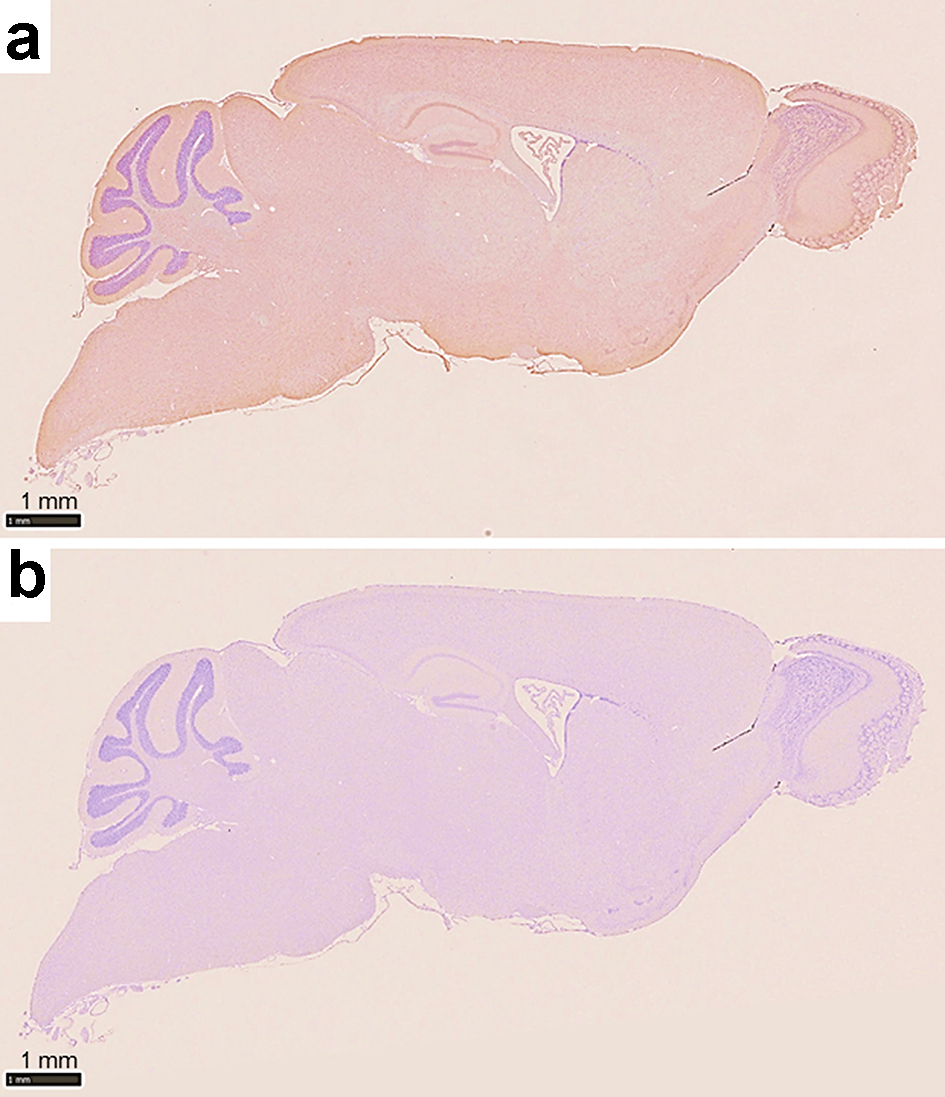 Click for large image | Figure 3. Photomicrographs of immunohistochemistry of a sagittal section of the murine brain. (a) The section is stained with a commercially available anti-Ro52/TRIM21 antibody showing Ro52/TRIM21 expression throughout the entire brain, including the hippocampus, cerebral cortex and cerebellum. (b) Negative control using an unrelated control antibody (anti-rabbit Ig) showing a lack of background staining (counterstain: hematoxylin). |
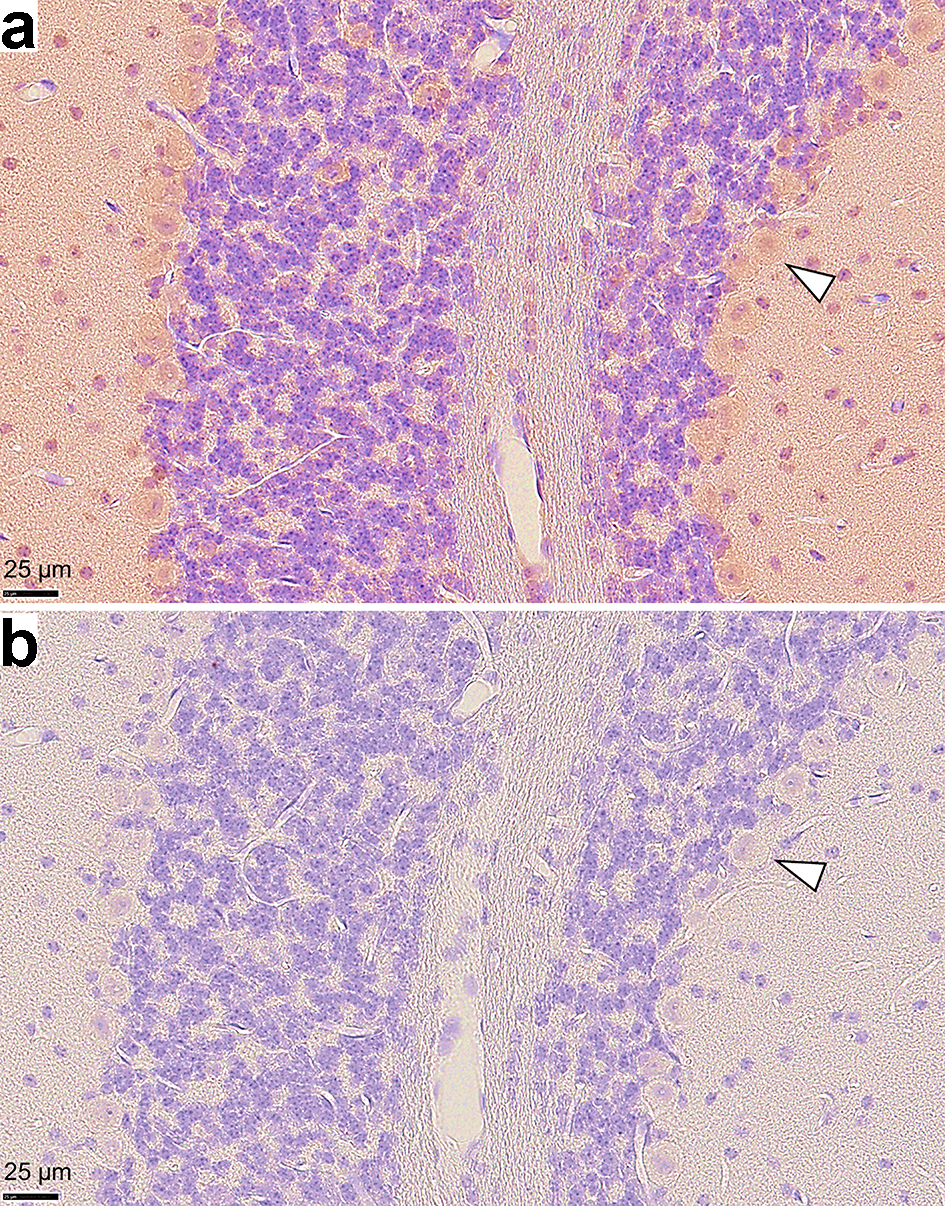 Click for large image | Figure 4. Photomicrographs of immunohistochemistry on murine cerebellar tissue sections. (a) Ro52/TRIM21 expression is shown using an anti-Ro52/TRIM21 antibody, but (b) is not present after staining with an anti-rabbit Ig (negative control). High Ro52/TRIM21 expression was observed in the cytoplasm of Purkinje cells. Arrowheads indicate representative Purkinje cells (magnification: × 40, counterstain: hematoxylin). |
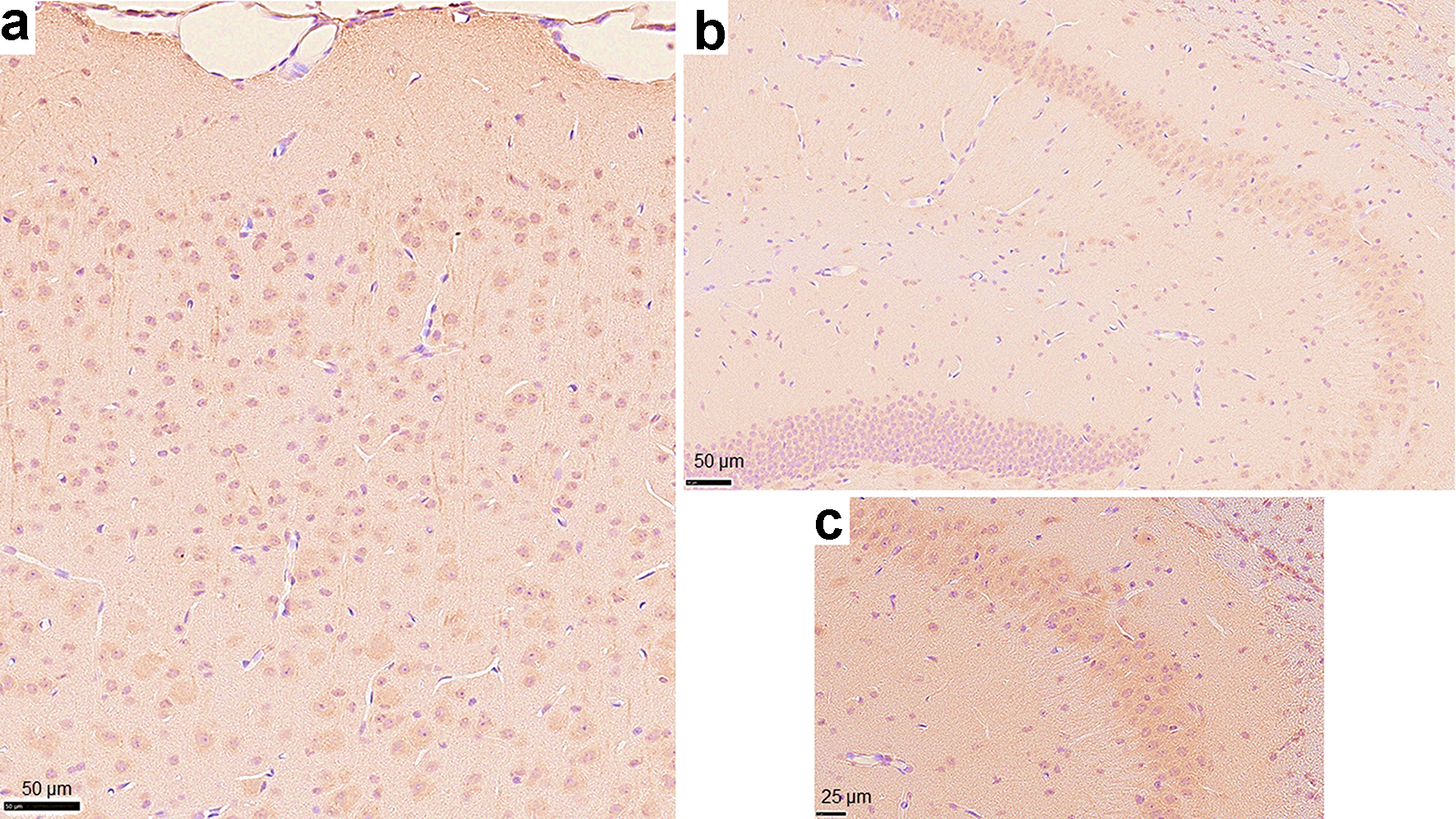 Click for large image | Figure 5. Photomicrographs of immunohistochemistry on other regions of the murine brain using the anti-Ro52/TRIM21 antibody. Cells that stained positively with the anti-Ro52/TRIM21 antibody were observed throughout (a) the cerebral cortex and (b) the hippocampus (magnification: × 20). (c) High magnification image representing the CA3 region of the hippocampus (magnification: × 40, counterstain: hematoxylin). |
| Discussion | ▴Top |
The most notable findings in this study were as follows: 1) Although anti-Ro/SSA antibodies were found in all patients, there were patients in whom anti-La/SSB antibodies were negative; 2) Anti-Ro/SSA antibodies were observed in both the serum and CSF; 3) In the SjS patient who had PNS involvement without CNS involvement (our patient 2), anti-Ro/SSA antibodies were negative in the CSF; 4) Cerebellar atrophy was observed after the onset of cerebellar degeneration in patients with SjS, and sequelae have remained in most cases; 5) A selective loss of Purkinje cells was found in the histological sections of the brain in one patient with cerebellar degeneration; 6) Ro52/TRIM21 expression was detected throughout the murine brain, including the hippocampus, cerebral cortex and cerebellum; and 7) High Ro52/TRIM21 expression was observed in Purkinje cells.
In the literature review, we were able to determine that all patients had anti-Ro/SSA antibodies, but some of those were negative for anti-La/SSB antibodies. This finding was counter to studies showing that anti-La/SSB antibodies are usually present with anti-Ro/SSA antibodies in serum, while anti-Ro/SSA antibodies could be detected without the presence of anti-La/SSB antibodies, suggesting the latter antibodies are not associated with the pathophysiological mechanism of cerebellar degeneration in SjS.
Furthermore, anti-Ro/SSA antibodies were observed in both the serum and CSF of our patient 1 but were negative in the CSF of our patient 2 who did not have CNS involvement. It is essential to detect antineuronal autoantibodies in the CSF of patients with autoimmune diseases of the CNS. The presence of anti-Ro/SSA antibodies in the CSF suggests that there is a disruption of the blood-brain barrier, that autoantibody-mediated neuroinflammation is involved in the pathophysiological mechanisms of CNS disease, and that an immune-mediated neurological disease is likely [19].
Anti-Ro/SSA antibodies are typically described as being associated with SjS; they have been determined to have differential actions by two target proteins, Ro52/TRIM21 (52 kDa) and Ro60 (60 kDa), that are biochemically and immunologically distinct [20]. The seropositivity prevalence rates were approximately 70% for anti-Ro52/TRIM21 antibodies, 40% for anti-Ro60 antibodies and 50% for anti-La/SSB antibodies; in addition, 63.2% of the patients with anti-Ro52/TRIM21 antibodies in serum also had anti-Ro60 antibodies [3, 21].
Clinical findings have been shown to be different between the patients with anti-Ro52/TRIM21 and anti-Ro60 antibodies [20]. The Ro52/TRIM21 gene is 8.8 kb in size, located on chromosome 11, and is a cytoplasmic protein that belongs to the TRIM family. This protein has been identified to be involved in protein ubiquitination, proinflammatory states (interleukin 2) and apoptotic mechanisms; is suggested to play an important role in the regulation of inflammatory responses; and is upregulated at sites of autoimmune inflammation [22]. According to one study, intrathecal production of anti-Ro52/TRIM21 antibodies was observed in patients with SjS with CNS involvement [4]. In addition, Ro52/TRIM21 was recognized by anti-Hu antibodies, which are onconeural antibodies for paraneoplastic neurological syndromes, including paraneoplastic cerebellar degeneration [23].
Although these results suggest that the Ro52/TRIM2 protein is involved to a greater extent in SjS-related cerebellar degeneration compared with the Ro60 protein, to the best of our knowledge, the distribution of this protein in the human brain remains to be unknown. In this study, we demonstrated that Ro52/TRIM21 was expressed throughout the murine brain, including the hippocampus, cerebral cortex and cerebellum. Moreover, because the histopathological hallmark of autoimmune cerebellar degeneration is a severe loss of Purkinje cells [24], we might be able to confirm this point from the results of our immunohistochemistry analysis and previous autopsy case report [6]. Although the pathogenic mechanisms of autoantibodies against Ro/SSA in autoimmune disease has remained unclear, the hypothesis exists that anti-Ro/SSA antibodies might cause direct damage to cells and that anti-Yo antibodies, which are onconeural antibodies, are associated with paraneoplastic cerebellar degeneration [25, 26].
Some studies have reported that anti-Ro52/TRIM21 autoantibodies can penetrate the cytoplasm and inhibit the function of the Ro52/TRIM21 protein, which negatively regulates proinflammatory cytokines and degrades Ro52/TRIM21 E3 ubiquitin ligase activity [27, 28]. Furthermore, as anti-Ro/SSA antibodies were identified in the CSF of the patients with SjS with cerebellar degeneration, it is possible that these antibodies might attack Purkinje cells, reducing Purkinje cell functions and activities, and can cause symptoms of neurological degeneration, resulting in cerebellar atrophy. Several reports have shown the presence of serum anti-Ro/SSA antibodies in patients with SjS with limbic encephalitis [11, 29]. We demonstrated that Ro52/TRIM21 was expressed in the neurons of the cortex and hippocampus, which might indicate that anti-Ro/SSA antibodies are involved in other CNS diseases in addition to cerebellar degeneration.
The limitations of this study include the need for proof that anti-La/SSB antibodies are completely unrelated with SjS with cerebellar degeneration. The number of cases is so small that it is difficult to present the difference in positive rates between anti-Ro/SSA and anti-La/SSB as statistically significant. However, the observation that anti-Ro/SSA antibodies are positive in all patients with cerebellar degeneration while anti-La/SSB are negative in a minority is thus especially important. The strengths of this study include experimental results that high Ro/SSA expression was observed in Purkinje cells, which supports the hypothesis that Ro/SSA is involved in the pathology of cerebellar degeneration with SjS. Further studies are required to clarify the relevance of SjS to cerebellar degeneration and the pathogenic mechanisms of the antineuronal antibodies against Ro/SSA to determine how they may cause CNS dysfunction.
Conclusion
In this literature review and the analysis of our patients in the case studies, we evaluated the characteristics of cerebellar degeneration associated with SjS. We found high Ro52/TRIM21 expression in the Purkinje cells in the histological sections of the cerebellum. Thus, we can conclude that anti-Ro/SSA antibodies are the antineuronal antibodies involved in the cerebellar degeneration of patients with SjS and that the finding of anti-Ro/SSA antibodies in CSF might serve as a useful biomarker for SjS-related CNS diseases in the clinical setting. Additional studies are warranted to confirm these observations and to clarify the molecular and pathological mechanisms of Ro/SSA antibodies as antineuronal antibodies. Finally, determining how these autoantibodies cause CNS dysfunction is also necessary.
Acknowledgments
The authors would like to thank the Genostaff Co., Ltd for technical support of immunohistochemistry.
Financial Disclosure
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
Written informed consent for publication was obtained and is available upon request.
Author Contributions
ST, TS, RH and HK developed the main conceptual idea and research design. ST and TO performed experiment and data acquisition. ST, RH and HK analyzed the data. ST wrote the manuscript. ST, TO and HK edited manuscript. All authors approved the final version of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article. The datasets are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Fox RI. Sjogren's syndrome. Lancet. 2005;366(9482):321-331.
doi - Carvajal Alegria G, Guellec D, Mariette X, Gottenberg JE, Dernis E, Dubost JJ, Trouvin AP, et al. Epidemiology of neurological manifestations in Sjogren's syndrome: data from the French ASSESS Cohort. RMD Open. 2016;2(1):e000179.
doi pubmed - Jonsson R, Brokstad KA, Jonsson MV, Delaleu N, Skarstein K. Current concepts on Sjogren's syndrome - classification criteria and biomarkers. Eur J Oral Sci. 2018;126(Suppl 1):37-48.
doi pubmed - Megevand P, Chizzolini C, Chofflon M, Roux-Lombard P, Lalive PH, Picard F. Cerebrospinal fluid anti-SSA autoantibodies in primary Sjogren's syndrome with central nervous system involvement. Eur Neurol. 2007;57(3):166-171.
doi pubmed - Vitali C, Bombardieri S, Jonsson R, Moutsopoulos HM, Alexander EL, Carsons SE, Daniels TE, et al. Classification criteria for Sjogren's syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61(6):554-558.
doi pubmed - Nanri K, Shibuya M, Taguchi T, Hasegawa A, Tanaka N. Selective loss of Purkinje cells in a patient with anti-gliadin-antibody-positive autoimmune cerebellar ataxia. Diagn Pathol. 2011;6:14.
doi pubmed - Terao Y, Sakai K, Kato S, Tanabe H, Ishida K, Tsukamoto T. Antineuronal antibody in Sjogren's syndrome masquerading as paraneoplastic cerebellar degeneration. Lancet. 1994;343(8900):790.
doi - Owada K, Uchihara T, Ishida K, Mizusawa H, Watabiki S, Tsuchiya K. Motor weakness and cerebellar ataxia in Sjogren syndrome—identification of antineuronal antibody: a case report. J Neurol Sci. 2002;197(1-2):79-84.
doi - Wong S, Pollock AN, Burnham JM, Sherry DD, Dlugos DJ. Acute cerebellar ataxia due to Sjogren syndrome. Neurology. 2004;62(12):2332-2333.
doi pubmed - Ichikawa H, Ishihara K, Fujimoto R, Katoh T, Arai M, Kawamura M, Nakano I. An autopsied case of Sjogren's syndrome with massive necrotic and demyelinating lesions of the cerebellar white matter. J Neurol Sci. 2004;225(1-2):143-148.
doi pubmed - Collison K, Rees J. Asymmetric cerebellar ataxia and limbic encephalitis as a presenting feature of primary Sjogren's syndrome. J Neurol. 2007;254(11):1609-1611.
doi pubmed - Milic V, Ostojic P. Cerebellar ataxia in a patient with primary Sjogren's syndrome after treatment with chloroquine. Rheumatol Int. 2008;28(12):1295-1296.
doi pubmed - Kim MJ, Lee MC, Lee JH, Chung SJ. Cerebellar degeneration associated with Sjogren's syndrome. J Clin Neurol. 2012;8(2):155-159.
doi pubmed - Chen YW, Lee KC, Chang IW, Chang CS, Hsu SP, Kuo HC. Sjogren's syndrome with acute cerebellar ataxia and massive lymphadenopathy: a case report. Acta Neurol Taiwan. 2013;22(2):81-86.
- Sharma R, Chilukuri V, Sarma AK, Gokhale S. Primary Sjogren's syndrome presenting as acute cerebellitis. J Clin Neurosci. 2014;21(3):508-509.
doi pubmed - Farhat E, Zouari M, Abdelaziz IB, Drissi C, Beyrouti R, Hammouda MB, Hentati F. Progressive cerebellar degeneration revealing Primary Sjogren Syndrome: a case report. Cerebellum Ataxias. 2016;3:18.
doi pubmed - Maciel R, Camargos S, Cardoso F. Subacute cerebellar degeneration as the first manifestation of Sjogren's syndrome. Mov Disord Clin Pract. 2017;4(4):637-638.
doi pubmed - Heidary M, Alesaeidi S, Afshari K. Cerebellar degeneration in primary Sjgren syndrome. BMJ Case Rep. 2018;2018.
doi pubmed - Shimizu F, Nishihara H, Kanda T. Blood-brain barrier dysfunction in immuno-mediated neurological diseases. Immunol Med. 2018;41(3):120-128.
doi pubmed - Schulte-Pelkum J, Fritzler M, Mahler M. Latest update on the Ro/SS-A autoantibody system. Autoimmun Rev. 2009;8(7):632-637.
doi pubmed - Garberg H, Jonsson R, Brokstad KA. The serological pattern of autoantibodies to the Ro52, Ro60, and La48 autoantigens in primary Sjogren's syndrome patients and healthy controls. Scand J Rheumatol. 2005;34(1):49-55.
doi pubmed - Oke V, Wahren-Herlenius M. The immunobiology of Ro52 (TRIM21) in autoimmunity: a critical review. J Autoimmun. 2012;39(1-2):77-82.
doi pubmed - Manley G, Wong E, Dalmau J, Elkon K, Posner J, Furneaux H. Sera from some patients with antibody-associated paraneoplastic encephalomyelitis/sensory neuronopathy recognize the Ro-52K antigen. J Neurooncol. 1994;19(2):105-112.
doi pubmed - Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the CNS. Lancet Neurol. 2008;7(4):327-340.
doi - Yoshimi R, Ueda A, Ozato K, Ishigatsubo Y. Clinical and pathological roles of Ro/SSA autoantibody system. Clin Dev Immunol. 2012;2012:606195.
doi pubmed - Greenlee JE, Clawson SA, Hill KE, Wood B, Clardy SL, Tsunoda I, Carlson NG. Anti-Yo antibody uptake and interaction with its intracellular target antigen causes Purkinje cell death in rat cerebellar slice cultures: a possible mechanism for paraneoplastic cerebellar degeneration in humans with gynecological or breast cancers. PLoS One. 2015;10(4):e0123446.
doi pubmed - Mallery DL, McEwan WA, Bidgood SR, Towers GJ, Johnson CM, James LC. Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21). Proc Natl Acad Sci U S A. 2010;107(46):19985-19990.
doi pubmed - Espinosa A, Hennig J, Ambrosi A, Anandapadmanaban M, Abelius MS, Sheng Y, Nyberg F, et al. Anti-Ro52 autoantibodies from patients with Sjogren's syndrome inhibit the Ro52 E3 ligase activity by blocking the E3/E2 interface. J Biol Chem. 2011;286(42):36478-36491.
doi pubmed - Coban A, Ozyurt S, Meric K, Misirli H, Tuzun E, Turkoglu R. Limbic encephalitis associated with Sjogren's syndrome: report of three cases. Intern Med. 2016;55(16):2285-2289.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


