| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 13, Number 2, February 2021, pages 92-100
Clinical Practice Changes After Post-Market Safety Reports on Desmopressin Orally Disintegrating Tablet in Japan: A Single-Center Retrospective Study
Takuma Yasudaa, d, Takaaki Murakamia, d, Akihiro Yasodab, Masakatsu Sonea, c, Norio Haradaa, Masahito Oguraa, Nobuya Inagakia, e
aDepartment of Diabetes, Endocrinology and Nutrition, Graduate School of Medicine, Kyoto University, Kyoto, Japan
bClinical Research Center, National Hospital Organization Kyoto Medical Center, Kyoto, Japan
cDivision of Metabolism and Endocrinology, Department of Internal Medicine, St. Marianna University School of Medicine, Kawasaki, Japan
dThe first two authors (T.Y. and T.M.) contributed equally to this work.
eCorresponding Author: Nobuya Inagaki, Department of Diabetes, Endocrinology and Nutrition, Graduate School of Medicine, Kyoto University, 54 Kawara-cho, Shogoin, Sakyo-ku, Kyoto 606-8507, Japan
Manuscript submitted November 27, 2020, accepted December 16, 2020, published online February 25, 2021
Short title: Usage and Safety of Desmopressin
doi: https://doi.org/10.14740/jocmr4399
| Abstract | ▴Top |
Background: Desmopressin orally disintegrating tablet (ODT) was approved in March 2012 in Japan; the post-market safety reports, which warned about adequate initial dose of desmopressin ODT, were published in 2014. However, it is unclear how the warning affected physician and patient behavior.
Methods: We performed a retrospective single-center study to compare the clinical situation of Japanese central diabetes insipidus patients before and after the report.
Results: Thirty-four patients before October 2014 and 16 patients after November 2014 switched from intranasal desmopressin to desmopressin ODT. The mean follow-up period after the switch to desmopressin ODT was 38 ± 3 months. Patients switching after November 2014 tended to have lower ratios of oral to nasal desmopressin dose at switching and 3 months after the switch (at switching; P = 0.20, 3 months; P = 0.42, respectively), and higher ratios from 6 to 12 months than before October 2014 (6 months; P = 0.93, 9 months; P = 0.52, 12 months; P = 0.80, respectively). Relative doses per initial desmopressin ODT at 9 and 12 months were significantly higher in patients switching after November 2014 than in patients switching before October 2014 (9 months; P = 0.02, 12 months; P = 0.04, respectively). Moreover, logistic regression analysis revealed that the incidence of hyponatremia was dependent on the ratio of nasal to oral desmopressin dose (P = 0.02). In addition, in four out of six patients who had serum sodium level reduced below 130 mEq/L, hyponatremia occurred within 1 month after the switch.
Conclusions: A more gradual dose titration after the safety reports was performed, which involved the long-term safety of desmopressin ODT use. Vigilance of hyponatremia in early phase of desmopressin ODT use should be noted.
Keywords: Central diabetes insipidus; Desmopressin; Oral disintegrating tablet; Hyponatremia
| Introduction | ▴Top |
Central diabetes insipidus (CDI) is a rare disease leading to polyuria and polydipsia [1, 2]. Deficiency of arginine vasopressin (AVP) causes CDI; desmopressin, an analogue of AVP, is widely used for patients with CDI. Desmopressin acts through the V2 receptor in the kidney and reabsorbs free water, which sometimes induces hyponatremia [3, 4]. Originally, desmopressin was prescribed as a nasal drop in the 1970s and as nasal spray in the 1990s. However, intranasal desmopressin can be inconvenient and embarrassing for the patient to use. Moreover, there are frequent side effects of intranasal desmopressin in those with rhinitis and visual disturbances [5, 6]. Desmopressin orally disintegrating tablet (ODT) was approved in North Europe in 2005, and it has been available in Japan since March 30, 2012. Because desmopressin ODT can be taken without water, it has been reported to reduce excessive intake of fluids. Moreover, it has been reported to have higher bioavailability than solid tablets [7].
After approval of ODT in Japan, a limited number of studies investigated the efficacy and safety of desmopressin ODT. The first post-market report in Japan published in May 2014 showed that the ratio of oral to nasal desmopressin dose depended on the intranasal dose during observation for 12 weeks [8]. In addition, the study reported that the mean ratio of oral to nasal desmopressin dose was 17.0, which was much smaller than that reported in the pre-market report published in 2013 [4, 8]. The following post-market report indicated that the mean ratio of oral to nasal desmopressin dose was 14.2, and that switching from intranasal desmopressin to desmopressin ODT significantly decreased the frequency of hyponatremia [9]. As the proposed ratio of oral to intranasal desmopressin in the pre-market reports was much higher than that in the post-market reports [4, 6], concerns about overdose of desmopressin ODT still remain, and it is unclear how the difference impacts physician and patient behavior in regard to desmopressin therapy.
This study aims to clarify how clinical practice, including frequency of use, dose, and consideration for the safety of desmopressin ODT was changed after the safety reports in Japan [8, 9] as well as what concerns remain. Here, we report a retrospective single-center study of Japanese CDI patients to compare the clinical characteristics before and after November 2014.
| Materials and Methods | ▴Top |
Patients
We enrolled CDI patients who were treated at the outpatient clinic of the Department of Diabetes, Endocrinology and Nutrition, Kyoto University Hospital (Kyoto, Japan) between April 2010 and March 2019. In this study, CDI was diagnosed based on the criteria shown in the previous reports, which included a history of polyuria and polydipsia, a deficiency in plasma AVP responses to osmotic stimulation with hypertonic saline, response to administration of vasopressin, and/or lack of responses to a water-deprivation test [10, 11]. The exclusion criteria included presence of uncorrected hypothyroidism or adrenal dysfunction as well as presence of hypothalamus abnormalities such as tumor, infarction, and hemorrhage. Patients with dementia leading to thirst disorder were also excluded. The following data were collected from our clinical records: sex, age, blood pressure, body mass index, smoking status, urine volume, etiology of CDI, laboratory data, and medications for CDI. As for smoking status, patients were classified into never smokers, ex-smokers, or current smokers. Urine volume was measured the day before the initiation of desmopressin ODT. The diagnosis of anterior pituitary deficiency was determined by the basal levels of anterior pituitary hormones or the responses of adrenocorticotropic hormone (ACTH), growth hormone (GH), thyroid-stimulating hormone (TSH)/prolactin (PRL), and luteinizing hormone (LH)/follicle-stimulating hormone (FSH) to corticotropin-releasing hormone (CRH), growth hormone-releasing hormone (GHRH), thyrotropin-releasing hormone (TRH) and gonadotropin-releasing hormone (GnRH), respectively. Patients treated with replacement therapy of cortisol, growth hormone, thyroid hormone, or sex hormone was also considered to have anterior pituitary deficiency. The following medications were investigated from our clinical records: Desmopressin Intranasal 0.01% Kyowa® (Kyowa Kirin Co., Ltd. Tokyo, Japan), Desmopressin Spray 2.5 Kyowa® (Kyowa Kirin Co., Ltd. Tokyo, Japan), or Desmopressin Spray 10 Kyowa® (Kyowa Kirin Co., Ltd. Tokyo, Japan) as intranasal desmopressin, and Minirin Melt® OD Tablet (Ferring Pharmaceuticals Co., Ltd. Tokyo, Japan, and Kyowa Kirin Co., Ltd. Tokyo, Japan) as desmopressin ODT.
Study protocol
In this retrospective, single-center study, CDI patients whose treatment was started with intranasal desmopressin or desmopressin ODT were identified and divided according to the date when desmopressin therapy was started. Subsequently, patients switching from intranasal desmopressin to desmopressin ODT were identified. We collected data of serum sodium levels up to 53 months after initiation of desmopressin ODT.
As for the analysis plan, since the latest post-market safety report about desmopressin ODT was published online on November 7, 2014, patients have been classified into two groups: patients who started desmopressin ODT before October 2014 and patients who started the drug after November 2014 [9]. In addition, the characteristics of patients with and without hyponatremia were compared. We defined hyponatremia as serum sodium level lower than 135 mEq/L, referring to the latest safety report [9]. Patients with hyponatremia were defined as those experiencing hyponatremia even once during the observation period. In patients with hyponatremia, the doses of desmopressin ODT when they had hyponatremia were analyzed. In patients without hyponatremia, the maximum doses of desmopressin up to 53 months after the switch from intranasal desmopressin were analyzed.
Ethical issues and informed consent
This study is a retrospective observational study, carried out by the opt-out method of our hospital website. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments, or comparable ethical standards. The protocol of this study was approved by the Kyoto University Graduate School and Faculty of Medicine, Ethics Committee (R1977-1).
Statistical analysis
In this study, data are presented as mean ± standard error (SE). We used Wilcoxon signed-rank test for continuous variables. Dose ratios of oral to nasal desmopressin were set as an indication for the primary analysis and serum sodium levels were set as an indication for the safety analysis. The Chi-square test and Fisher’s exact test were used for binary variables. Logistic regression analyses were performed to determine the significance of association between the dependent and independent variables after adjusting for age, a potentially confounding independent variable. A two-sided P value lower than 0.05 was considered significant. JMP Pro®, version 14.0.0 (SAS Institute Inc., Cary, NC, USA) was used to perform the statistical analyses.
| Results | ▴Top |
Intranasal and oral desmopressin use in patients with CDI for initial therapy
Figure 1 shows the number of CDI patients according to the initial type of desmopressin formulations. Eleven of 33 patients (33.3%) used desmopressin ODT from April 2012 to October 2014, and 83 of 95 patients used desmopressin ODT (87.4%) from November 2014 to March 2019. The rate of patients who used desmopressin ODT for initial therapy from November 2014 to March 2019 was significantly increased compared to that of April 2012 to October 2014 (P < 0.01). Of 34 patients from April 2012 to March 2019 who used intranasal desmopressin for initial therapy, 11 patients switched to desmopressin ODT afterward. Moreover, in seven of these 34 patients, intranasal desmopressin was started as an initial therapy after operation for pituitary tumor, and was discontinued within 3 months.
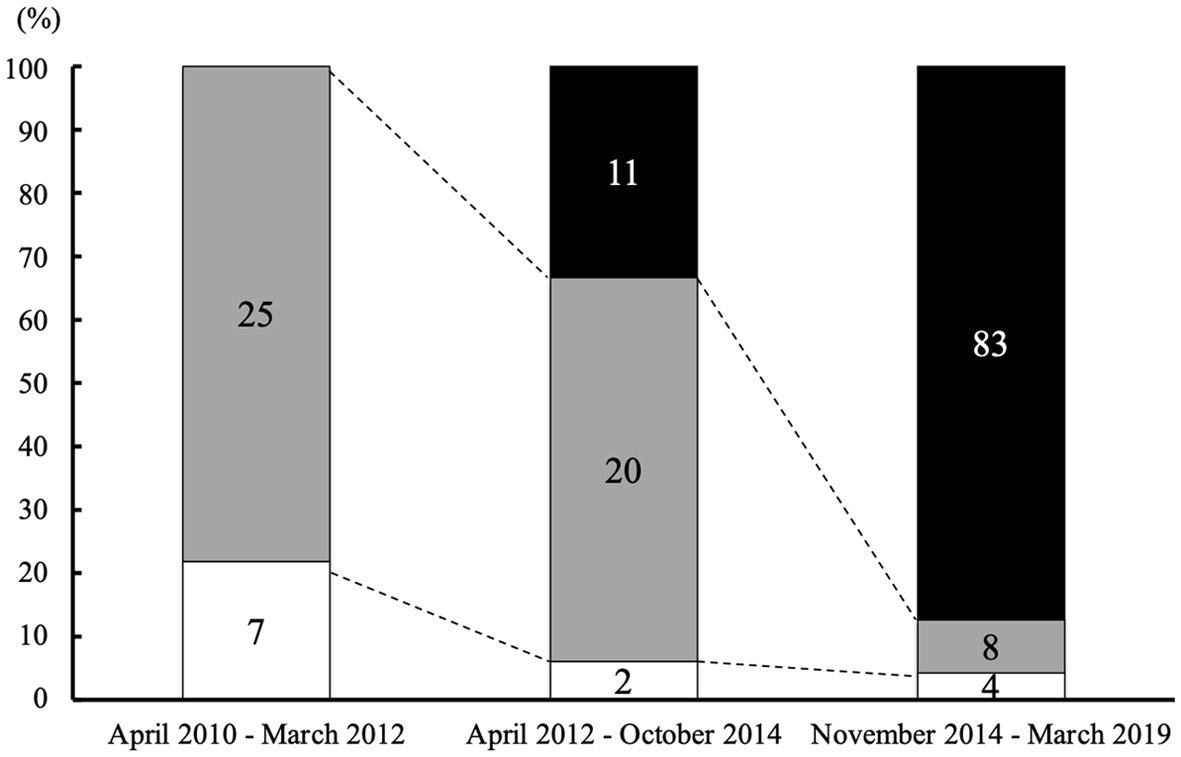 Click for large image | Figure 1. Type of desmopressin according to the date starting treatment for CDI. The percentage of patients who received desmopressin ODT as initial desmopressin therapy was continuously increased. The numbers of patients who started each therapy are indicated in the column (black: patients who started treatment with desmopressin ODT; gray: patients who started treatment with desmopressin spray; white: patients who started treatment with desmopressin nasal drop). CDI: central diabetes insipidus; ODT: orally disintegrating tablet. |
Characteristics of patients switching from intranasal desmopressin to desmopressin ODT
Fifty patients switching from intranasal desmopressin to desmopressin ODT for CDI were identified from April 2010 to March 2019, as shown in Table 1. The mean follow-up period after the switch to desmopressin ODT was 38 ± 3 months (range, 1 - 53 months). Twenty patients were male, and the mean age was 51.2 ± 2.8 years. No significant difference was found in blood pressure, body mass index, smoking status, or urine volume between the two groups. Craniopharyngioma was the most common among etiologies of CDI, which accounted for 30% of the patients (n = 15). Forty-one patients had anterior pituitary deficiency, and 28 were treated with replacement therapy for anterior pituitary deficiency. TSH deficiency was the most common among the anterior pituitary deficiencies, and occurred in 68% of the patients (n = 34). In the analysis of the timing of switching desmopressin formulations, 34 patients switching to desmopressin ODT before October 2014 were compared with 16 patients switching after November 2014. There was no significant difference between the two groups except for the rate of TSH deficiency (58.8% (n = 20) vs. 87.5% (n = 14), P = 0.04).
 Click to view | Table 1. Characteristics of CDI Patients Who Switched From Intranasal Desmopressin to Desmopressin ODT |
Comparison of desmopressin dose between patients switching before October 2014 and after November 2014
The switching dose ratios of desmopressin were compared between the patients who switched from intranasal desmopressin before October 2014 and those who switched after November 2014 (Fig. 2a). Although a statistically significant difference was not found, the dose ratio of initial switching in patients switching after November 2014 tended to be lower than the ratio in those switching before October 2014 (12.2 ± 1.5 vs. 14.9 ± 1.2, P = 0.20). Moreover, the ratios of oral to nasal desmopressin doses 3 months after the switch tended to be lower in patients switching after November 2014 than in patients switching before October 2014 (Fig. 2a). Conversely, these ratios turned higher from 6 to 12 months after the switch in patients switching after November 2014 than in patients switching before October 2014. In addition, the relative doses per initial desmopressin ODT at 9 and 12 months were significantly higher in patients switching after November 2014 than in those switching before October 2014 (9 months; 2.03 ± 0.33 vs. 1.23 ± 0.12, P = 0.02, 12 months; 2.03 ± 0.33 vs. 1.26 ± 0.12, P = 0.04, respectively) (Fig. 2b). Moreover, the increments of desmopressin ODT dose at 9 and 12 months from initiation were significantly higher in patients switching after November 2014 than in those switching before October 2014 (9 months; 70.9 ± 21.1 vs. 17.3 ± 10.0, P = 0.02, 12 months; 70.9 ± 21.1 vs. 20.4 ± 10.5, P = 0.03, respectively) (Fig. 2c).
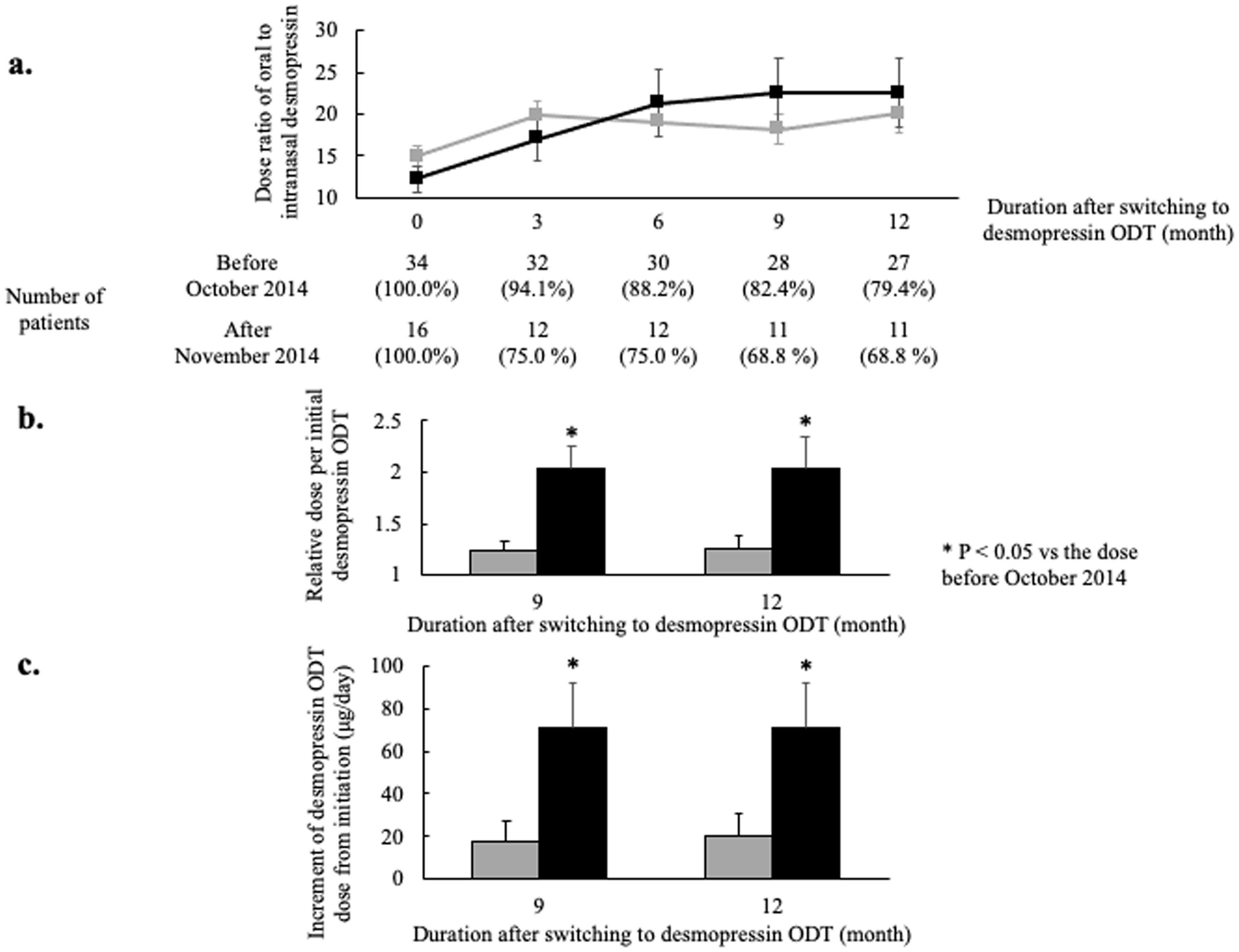 Click for large image | Figure 2. Comparison of dose ratios of oral to intranasal desmopressin between patients switching before October 2014 and those switching after November 2014. (a) The ratios of oral to intranasal desmopressin dose. Patients were divided into two groups; patients switching from intranasal desmopressin to desmopressin ODT before October 2014 (gray line) and patients switching after November 2014 (black line). More gradual titration but increased final dose is shown in patients switching after November 2014 (dose ratio: the ratio of oral to nasal desmopressin dose; error bar: standard error). (b) The relative dose per initial oral desmopressin dose. The relative doses 9 and 12 months after switching from intranasal desmopressin to ODT were significantly higher in patients switching after November 2014 (gray bar: patients switching from intranasal desmopressin to desmopressin ODT from April 2012 to October 2014; black bar: patients switching from intranasal desmopressin to desmopressin ODT from November 2014 to March 2019; error bar: standard error; *P < 0.05). (c) The increment of desmopressin ODT dose from initiation. The increments 9 and 12 months after switching from intranasal desmopressin to ODT were significantly higher in patients switching after November 2014 (gray bar: patients switching from intranasal desmopressin to desmopressin ODT from April 2012 to October 2014; black bar: patients switching from intranasal desmopressin to desmopressin ODT from November 2014 to March 2019; error bar: standard error; *P < 0.05). ODT: orally disintegrating tablet. |
Comparison between patients with and without hyponatremia
Subsequently, we investigated hyponatremia in the 50 patients who switched from intranasal desmopressin to desmopressin ODT (Table 1). The rate of patients who experienced hyponatremia below 135 mEq/L was 28.0 % (n = 14) and the rate of patients below 130 mEq/L was 12.0 % (n = 6), which were higher than those in the previous safety reports [8, 9]. Regarding the date of the switch to desmopressin ODT, the rate of patients with hyponatremia was not significantly different between in those who switched before publication of the latest safety report and in those who switched after its publication, as shown in Table 1 [9].
Next, we compared the frequencies of anterior pituitary deficiency, the dose of intranasal desmopressin, the dose of desmopressin ODT, and the ratio of oral to nasal desmopressin dose between the patients who did not experience hyponatremia and the patients who experienced hyponatremia below 135 mEq/L or 130 mEq/L (Table 2). The frequency of LH deficiency was significantly higher in patients whose serum sodium levels below 135 mEq/L than in patients above 135 mEq/L (85.7% (n = 12) vs. 55.6% (n = 20), P = 0.04). Although no significant difference was found, the rates of patients with other hormonal deficiencies tended to be higher in patients with hyponatremia than patients without hyponatremia, regardless of the cut-off value of serum sodium level.
 Click to view | Table 2. The Comparison Between Patients With and Without Hyponatremia in the Early Phase |
As for desmopressin dose, there was no significant difference between patients whose serum sodium levels below 135 mEq/L and above 135 mEq/L. On the other hand, the mean ratios of oral to nasal desmopressin dose tended to be higher in patients whose serum sodium levels below 130 mEq/L than in patients above 135 mEq/L (37.0 ± 11.0 vs. 20.7 ± 1.3, P = 0.28). Furthermore, we analyzed the mean ratios of oral to nasal desmopressin dose after adjusting age because age is a potentially confounding independent variable for the incidence of hyponatremia [12]. Logistic regression analysis revealed that the incidence of serum sodium levels below 130 mEq/L was dependent on the ratio of oral to nasal desmopressin dose after adjusting for age (P = 0.02, Table 2). The model comparison of ODT/intranasal dose ratio after adjusting age between Na ≥ 135 mEq/L and Na < 135 mEq/L was not established because the P value in the whole model test was 0.10, which indicated that the overall model was not significant. Therefore, we focused on the characteristics of patients whose serum sodium levels below 130 mEq/L.
Characteristics of patients whose serum sodium levels below 130 mEq/L
Table 3 shows the characteristics of the patients with serum sodium levels below 130 mEq/L. Five patients had anterior pituitary deficiency, including five patients with central hypothyroidism and four patients with central adrenocortical insufficiency as a comorbidity. Of the six patients, four had serum sodium level below 130 mEq/L within 1 month after the switch (Case No. 1, 4, 5, and 6 in Table 3). Interestingly, four of the six patients were inpatients with recurrent pituitary tumor or recurrent hypophysitis (Case No. 3, 4, 5, and 6). No hospitalizations due to hyponatremia occurred. The other two patients (Case No. 1 and 2) were outpatients and did not have any symptoms of hyponatremia. As for continuation of desmopressin ODT, five patients continued to take desmopressin ODT after improvement of hyponatremia. Only one patient (Case 4) discontinued desmopressin ODT and started desmopressin spray 5 months after the switch due to the patient’s request. When the patient discontinued desmopressin ODT, she had not experienced hyponatremia. Fifty-four months after the discontinuation, she restarted desmopressin ODT during hospitalization after surgery for recurrent craniopharyngioma. She had hyponatremia after the restart, which was improved by reduction of desmopressin ODT dose.
 Click to view | Table 3. Characteristics of the Patients With Serum Sodium Levels Below 130 mEq/L |
| Discussion | ▴Top |
Notwithstanding the widespread use of desmopressin ODT for CDI, long-term safety and proper use of desmopressin ODT have not fully been investigated after the limited numbers of the previous safety reports were published [8, 9]. Although the two previous post-market safety reports warned about the initial switching dose ratio of desmopressin ODT, the dose and safety of desmopressin ODT were analyzed only in a relatively short duration. Moreover, it was not resolved how clinical practice for desmopressin ODT changed after the two reports and what pitfalls still remain in clinical settings. The clinical significance of such investigations is also supported by increased frequency of desmopressin ODT as an initial therapy for CDI as shown in Figure 1.
One of the most prominent finding of this paper was that a more gradual dose titration of desmopressin ODT was performed in clinical settings after the latest safety report than before (Fig. 2a). This is also proved by the fact that the ratios of the doses 9 and 12 months after the switch to the initial dose and the increments of the doses 9 and 12 months after the switch were significantly higher after the latest safety report than before (Fig. 2b, c) [8, 9]. On the other hand, the initial switching dose ratios tended to be lower after the latest safety report than before (Fig. 2a). This finding might contribute to the similar frequency of hyponatremia in spite of higher ratios and increments 9 and 12 months after the switch (Table 1). The prior suggestion of usefulness of the gradual titration method in a previous review may result in the present finding [4]. Therefore, this indicates that physicians got more cautious at the time of starting desmopressin ODT after the safety reports than before, even with an accumulation of physician usage experience with desmopressin ODT.
While safety and tolerability of desmopressin ODT were observed in this study, the ratio of developing hyponatremia in this report was higher than that in the latest safety report (below 135 mEq/L; 28.0% vs. 7.6%, below 135 mEq/L; 12.0% vs. 1.3%) [9]. Importantly, hyponatremia occurred within 1 month after the switch to desmopressin ODT in four patients out of six with hyponatremia. Moreover, in terms of the relationship between the incident of hyponatremia and dose of desmopressin ODT, the doses of desmopressin ODT in patients whose serum sodium levels below 130 mEq/L tended to be higher than the doses in patients whose serum sodium levels above 135 mEq/L (Table 2: 215.0 ± 108.6 vs. 130.6 ± 10.0, P = 0.72). Although statistical difference was not observed because of large SE of desmopressin ODT doses and small sample size in patients whose serum sodium levels below 130 mEq/L, the dose of desmopressin ODT itself might also affect the incident of hyponatremia. In addition, the dose ratio of oral to nasal desmopressin in patients whose serum sodium levels below 130 mEq/L exceeded the indicated level of the previous report, which tended to be higher than in patients above 135 mEq/L (Table 2: 37.0 ± 11.0 vs. 20.7 ± 1.3, P = 0.28) [13]. Logistic regression analysis after adjusting for age revealed that the dose ratio of oral to nasal desmopressin was significantly related to risk of serum sodium levels below 130 mEq/L (P = 0.02). A previous cohort study demonstrated that among 114 nocturia patients who developed hyponatremia under newly prescribed desmopressin treatment, 77 patients were diagnosed with hyponatremia within the first 30 days of the therapy [14]. Another review indicated that a small dose of oral desmopressin should be used initially and then increased as necessary [15]. The changes in clinical practice with smaller initial dose and more gradual dose titration after the previous post-market reports might contribute to lower the risk of hyponatremia. The dose ratio of oral to intranasal desmopressin might therefore be a useful clinical indicator to prevent hyponatremia, especially the first 1 - 3 months after initiation of desmopressin ODT.
With regard to other characteristics of patients, this study also reveals that concomitant anterior pituitary deficiency and unstable comorbidities in the hypophysis can be a risk factor for hyponatremia in desmopressin ODT treatment. Five out of six patients with hyponatremia had hypothyroidism and four patients had adrenocortical insufficiency [16]. In addition, four of the six patients were inpatients who had recovered from recurrent pituitary tumor or recurrent hypophysitis. Both of the patients with hyponatremia after the previous safety report were hospitalized, which might cause hyponatremia within 1 month after the switch and lead to higher rate of hyponatremia after the safety report was published than before (Tables 1, 3) [9]. Therefore, physicians should note the possibility of hyponatremia especially under concomitant anterior pituitary deficiency. Moreover, a switch should only be performed in stable and well controlled comorbidities in order to avoid hyponatremia in the early phase of desmopressin ODT therapy.
Widespread use of desmopressin ODT was expected because of its convenience, and this study shows that desmopressin ODT is frequently used in current Japanese clinical practice, about one-third of patients having used intranasal desmopressin and then switched to desmopressin ODT. In fact, intranasal desmopressin was frequently used temporarily in these patients after pituitary surgery, which may be attributed to the relative ease of dose adjustment. Based on the possibility of frequent hyponatremia in patients using desmopressin ODT after surgery (Table 3), intranasal desmopressin can be useful for postoperative management of CDI.
Finally, there are several limitations in this study. First, the period and frequency of follow-up were not fixed in the 50 patients because CDI is a rare disease and this is a retrospective study. However, this study is the first report clarifying clinical practice change after post-market reports and revealing a remaining pitfall for clinical use. Second, this is a single-center study. However, this study did not include patients treated by clinicians who were related with the previous post-market reports in order to avoid selection bias [8, 9]. Further large-scale, multicenter studies are warranted to validate our results.
In conclusion, a more gradual dose titration after switching from intranasal desmopressin to desmopressin ODT was performed after the previous post-market studies had warned about an excessive initial dose of desmopressin ODT, which might contribute to the decreased frequency of hyponatremia. In addition, although desmopressin ODT showed safety and efficacy even for longer duration compared with the previous reports, physicians still should be careful for a possible excessive dose of desmopressin ODT, especially within the first 1 - 3 months. It is important to recognize the high dose ratio of oral to intranasal desmopressin as well as the increment of desmopressin ODT dose from initiation to prevent hyponatremia in CDI patients with desmopressin ODT.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
N. I. received clinical commissioned/joint research grants from Daiichi Sankyo, Terumo, and Drawbridge Inc.; speaker honoraria from Kowa, MSD, Astellas Pharma, Novo Nordisk Pharma, Ono Pharmaceutical, Nippon Boehringer Ingelheim, Takeda, Sumitomo Dainippon Pharma and Mitsubishi Tanabe Pharma; scholarship grants from Kissei Pharmaceutical, Sanofi, Daiichi Sankyo, Mitsubishi Tanabe Pharma, Takeda, Japan Tobacco, Kyowa Kirin, Sumitomo Dainippon Pharma, Astellas Pharma, MSD, Eli Lilly Japan, Ono Pharmaceutical, Sanwa Kagaku Kenkyusho, Nippon Boehringer Ingelheim, Novo Nordisk Pharma, Novartis Pharma, Teijin Pharma, and Life Scan Japan.
Informed Consent
This study is a retrospective observational study, carried out by the opt-out method of our hospital website.
Author Contributions
T.Y. and T.M. planned the study, collected, analyzed and discussed the data, wrote and reviewed the manuscript. A.Y. contributed to the data analysis and discussion, and reviewed the manuscript. M.S., N.H., and M.O. contributed to the discussion and reviewed the manuscript. N.I. contributed to the discussion, and reviewed and edited the manuscript. All the authors approved the final manuscript.
Data Availability
The authors declare that the data supporting the findings of this study are available within this article.
| References | ▴Top |
- Robertson GL. Diabetes insipidus. Endocrinol Metab Clin North Am. 1995;24(3):549-572.
doi - Di Iorgi N, Napoli F, Allegri AE, Olivieri I, Bertelli E, Gallizia A, Rossi A, et al. Diabetes insipidus—diagnosis and management. Horm Res Paediatr. 2012;77(2):69-84.
doi pubmed - Vande Walle J, Stockner M, Raes A, Norgaard JP. Desmopressin 30 years in clinical use: a safety review. Curr Drug Saf. 2007;2(3):232-238.
doi pubmed - Arima H, Oiso Y, Juul KV, Norgaard JP. Efficacy and safety of desmopressin orally disintegrating tablet in patients with central diabetes insipidus: results of a multicenter open-label dose-titration study. Endocr J. 2013;60(9):1085-1094.
doi pubmed - Greiff L, Andersson M, Svensson C, Lundin S, Wollmer P, Persson CG. Reduced airway absorption in seasonal allergic rhinitis. Am J Respir Crit Care Med. 1997;156(3 Pt 1):783-786.
doi pubmed - Fukuda I, Hizuka N, Takano K. Oral DDAVP is a good alternative therapy for patients with central diabetes insipidus: experience of five-year treatment. Endocr J. 2003;50(4):437-443.
doi pubmed - Weiss JP, Zinner NR, Klein BM, Norgaard JP. Desmopressin orally disintegrating tablet effectively reduces nocturia: results of a randomized, double-blind, placebo-controlled trial. Neurourol Urodyn. 2012;31(4):441-447.
doi pubmed - Murakami T, Hatoko T, Nambu T, Matsuda Y, Matsuo K, Yonemitsu S, Muro S, et al. Desmopressin orally disintegrating tablet in Japanese patients with central diabetes insipidus: a retrospective study of switching from intranasal desmopressin. Endocr J. 2014;61(8):773-779.
doi pubmed - Kataoka Y, Nishida S, Hirakawa A, Oiso Y, Arima H. Comparison of incidence of hyponatremia between intranasal and oral desmopressin in patients with central diabetes insipidus. Endocr J. 2015;62(2):195-200.
doi pubmed - Miller M, Dalakos T, Moses AM, Fellerman H, Streeten DH. Recognition of partial defects in antidiuretic hormone secretion. Ann Intern Med. 1970;73(5):721-729.
doi pubmed - The Group of diencephalohypophysial dysfunction of Research on Measures for Intractable Diseases of Japan's Ministry of Health, Labour and Welfare. The report of Research on Measures for Intractable Diseases of Japan's Ministry of Health, Labour and Welfare. Folia Endocrinologica Japonica. 2019;95(Suppl):15-17.
- Hawkins RC. Age and gender as risk factors for hyponatremia and hypernatremia. Clin Chim Acta. 2003;337(1-2):169-172.
doi pubmed - Oiso Y, Robertson GL, Norgaard JP, Juul KV. Clinical review: Treatment of neurohypophyseal diabetes insipidus. J Clin Endocrinol Metab. 2013;98(10):3958-3967.
doi pubmed - Fralick M, Schneeweiss S, Wallis CJD, Jung EH, Kesselheim AS. Desmopressin and the risk of hyponatremia: A population-based cohort study. PLoS Med. 2019;16(10):e1002930.
doi pubmed - Di Iorgi N, Morana G, Napoli F, Allegri AE, Rossi A, Maghnie M. Management of diabetes insipidus and adipsia in the child. Best Pract Res Clin Endocrinol Metab. 2015;29(3):415-436.
doi pubmed - Non L, Brito D, Anastasopoulou C. Neurosarcoidosis-associated central diabetes insipidus masked by adrenal insufficiency. BMJ Case Rep. 2015;2015:bcr2014206390.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


