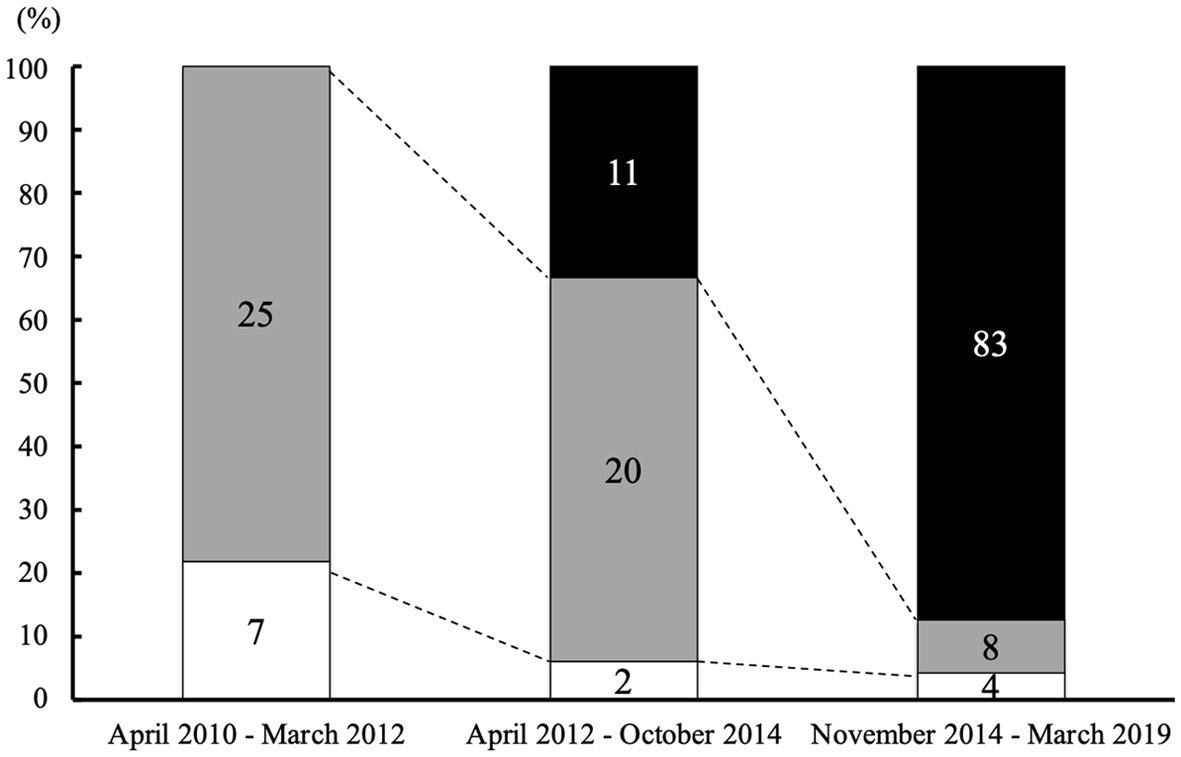
Figure 1. Type of desmopressin according to the date starting treatment for CDI. The percentage of patients who received desmopressin ODT as initial desmopressin therapy was continuously increased. The numbers of patients who started each therapy are indicated in the column (black: patients who started treatment with desmopressin ODT; gray: patients who started treatment with desmopressin spray; white: patients who started treatment with desmopressin nasal drop). CDI: central diabetes insipidus; ODT: orally disintegrating tablet.
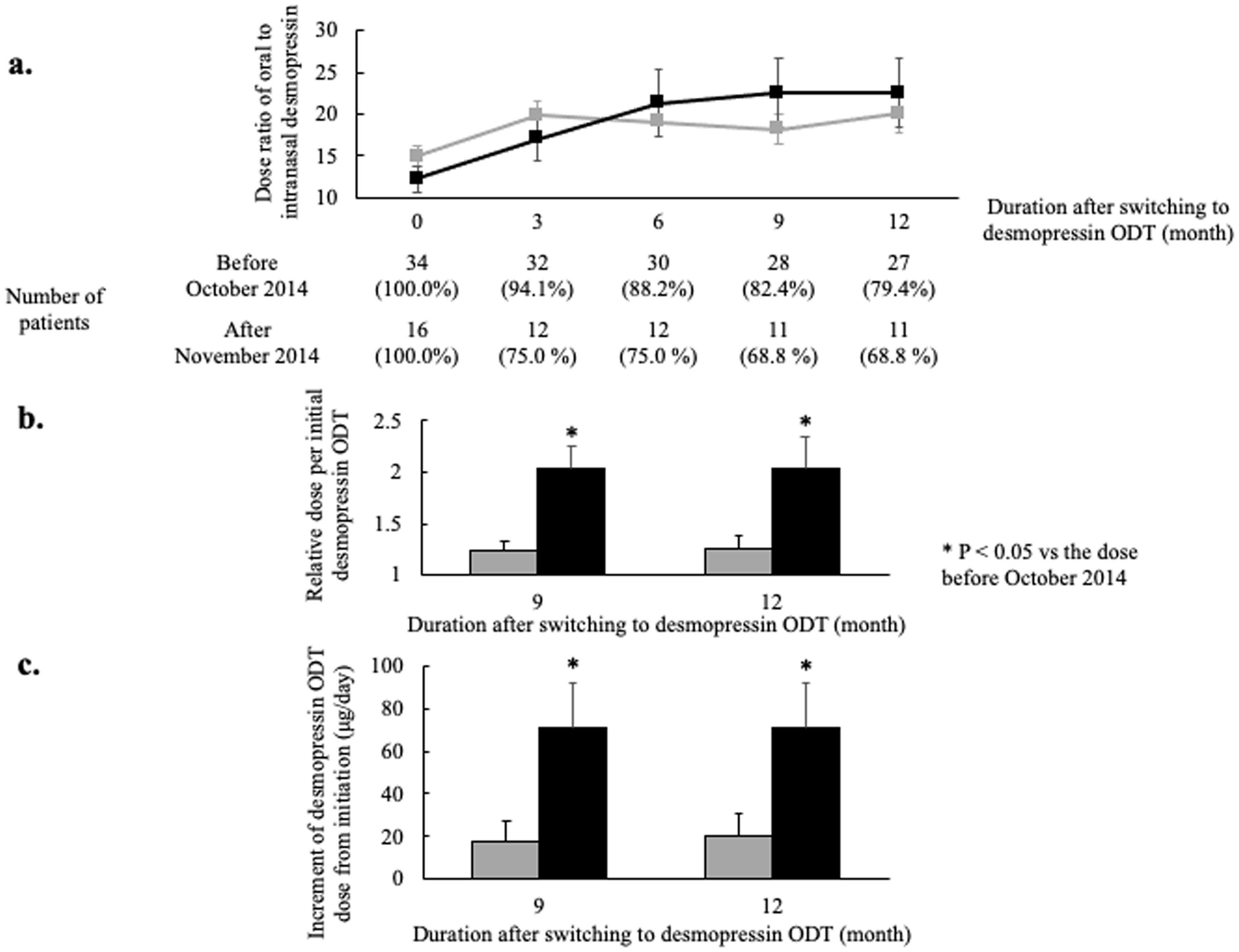
Figure 2. Comparison of dose ratios of oral to intranasal desmopressin between patients switching before October 2014 and those switching after November 2014. (a) The ratios of oral to intranasal desmopressin dose. Patients were divided into two groups; patients switching from intranasal desmopressin to desmopressin ODT before October 2014 (gray line) and patients switching after November 2014 (black line). More gradual titration but increased final dose is shown in patients switching after November 2014 (dose ratio: the ratio of oral to nasal desmopressin dose; error bar: standard error). (b) The relative dose per initial oral desmopressin dose. The relative doses 9 and 12 months after switching from intranasal desmopressin to ODT were significantly higher in patients switching after November 2014 (gray bar: patients switching from intranasal desmopressin to desmopressin ODT from April 2012 to October 2014; black bar: patients switching from intranasal desmopressin to desmopressin ODT from November 2014 to March 2019; error bar: standard error; *P < 0.05). (c) The increment of desmopressin ODT dose from initiation. The increments 9 and 12 months after switching from intranasal desmopressin to ODT were significantly higher in patients switching after November 2014 (gray bar: patients switching from intranasal desmopressin to desmopressin ODT from April 2012 to October 2014; black bar: patients switching from intranasal desmopressin to desmopressin ODT from November 2014 to March 2019; error bar: standard error; *P < 0.05). ODT: orally disintegrating tablet.

