| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Review
Volume 12, Number 12, December 2020, pages 758-772
COVID-19 and Possible Pharmacological Preventive Options
Pontus Dunera, Albert Salehib, c
aDepartment of Clinical Science, SUS, Experimental Cardiovascular research, University of Lund, Sweden
bDepartment of Clinical Science, Division of Islet Cell Physiology, University of Lund, Sweden
cCorresponding Author: Albert Salehi, Department of Clinical Science, Division of Islet Cell Physiology, Jan Waldenstromsgata 35, Building 91, Floor 11, SE-205 02 Malmo, Sweden
Manuscript submitted October 27, 2020, accepted November 11, 2020, published online December 18, 2020
Short title: Pharmacological Aspect for COVID-19
doi: https://doi.org/10.14740/jocmr4383
- Abstract
- Introduction
- A Brief Summary of Common Strategies to Fight Viruses
- Coronavirus Structure and Potential Drugs to Target the SARS-CoV-2 Infection
- Therapies That Do Not Directly Target the Coronavirus
- Convalescent Plasma as a Treatment for COVID-19
- Generation of Monoclonal Antibodies Against COVID-19 as a Possible Treatment
- The Possible Effect of SARS-CoV-2 on the Erythrocytes
- COVID-19 and Blood Thromboembolism
- Other Preventive Therapies
- SARS-CoV-2-Induced Pneumonia
- Conclusions
- References
| Abstract | ▴Top |
The dreadful fear of the coronavirus disease 2019 (COVID-19), which is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), with the deadly consequences, requires rapid development of pharmacological cures. The objective of this review is to speculate about possible pharmacological options, already available today to prevent or treat the COVID-19 in the early stage of its outbreak. A literature search across PubMed and internet was conducted. A number of studies dealing with COVID-19 were identified. The data elucidated that increased pro-inflammatory and decreased anti-inflammatory cytokines in combination with hypoxia, thromboembolism and pneumonia are involved in the pathogenesis of SARS-CoV-2 infection. Although many drugs has been tested in monotherapy regimen with varying outcome or without desirable effect, there is still hope for better results by simultaneously targeting the virus itself and its symptoms. Theoretically, a mixture of at least two available antiviral drugs in combination with other anti-pathogenic and immune system-enhancing drugs or combination of antiviral drugs with convalescent plasma seems likely to have much better effect than the monotherapy regimen of either of these drugs.
Keywords: Viral infection; Respiratory infection; RNA virus
| Introduction | ▴Top |
Current status of coronavirus disease 2019 (COVID-19)
The COVID-19 pandemic and the fear that it has brought to the society have emphasized the need for a rapid scientific communication to increase knowledge about the virus itself and how it can be combated pharmacologically. Historically severe acute respiratory syndrome (SARS) coronavirus 2 (SARS-CoV-2) (Table 1 [1-5]) is a descendant of the SARS-CoV, which was a virus that caused the first major pandemic of the new millennium [1-4]. The current SARS-CoV-2 infection has strong homology to the SARS-CoV virus that was responsible for the SARS pandemic in 2002 [2, 4, 5]. Clinically, SARS-CoV-2 causes a respiratory infection similar to that previously reported for SARS-CoV or Middle Eastern respiratory syndrome coronavirus MERS-CoV [2, 4, 5]. The initial symptoms are almost identical to other viral diseases, such as a common cold, influenza with fever, muscle pain, malaise, headache and fatigue [2, 4, 5]. The digestive tract is commonly involved in the viral infections and it is consequently also affected by these three closely related coronaviruses, so symptoms such as nausea, vomiting and back pain can also occur during SARS-CoV-2 infection [2, 4, 5]. The rapid spread of the COVID-19 pandemic around the world together with its severe and life-threatening respiratory infection has resulted in a situation that requires a rapid treatment strategy to save human lives, even if specific drug candidates are still missing [2, 4, 5]. An unusual feature of coronaviruses is that they can easily be conveyed between mammalian species as closely related coronaviruses could be found in bats, civets, camels, cows and humans [2, 4, 5]. Like many other viral infections targeting respiratory organs such as the flu, SARS, MERS-CoV as well as other airborne viral infections, SARS-CoV-2 spreads through tiny droplets released from the nose and mouth by infectious carriers via a cough. The released virus particles normally remain in the surrounding air for some time and can be a direct route of transmission of the SARS-CoV-2 infection to other people through breathing. Released SARS-CoV-2 virus particles by infected subjects can also land on the surfaces around them, providing an additional path for the virus to spread.
 Click to view | Table 1. Characteristics of COVID-19, SARS and MERS |
The COVID-19 has spread rapidly around the world in line with a pandemic feature. The disease has brought fear for both close social contacts, as well as contact with material things (surfaces) surrounding us [6]. There is an urgent need for effective treatments to prevent the aggressive and exaggerated SARS-CoV-2 replication within infected cells that consequently could decrease the duration of the SARS-CoV-2 infection and the transmission rate in the community. The purpose of this review is to summarize the available information regarding possible pharmacological tools that could be used for treatment of COVID-19. We have carried out a literature and internet search for relevant published material on COVID 19 and other closely related viral infections.
Characterization of the SARS-CoV-2 virus
The origin of the highly contagious SARS-CoV-2 virus that causes the COVID-19 disease is not known. SARS-CoV-2 belongs to a family of coronaviruses causing a severe acute respiratory syndrome which can be life-threatening most commonly for elderly male and individuals with a preexisting (underlying) medical condition such as diabetes, high blood pressure as well as other cardiovascular disease [5]. SARS-CoV-2 is a positive-sense single-stranded RNA ((+)ssRNA) virus with the ability of an aggressive way of replication using the host cell machinery [7]. Sequencing of the SARS-CoV-2 revealed that it shares 88% sequence identity with two bat-derived SARS-like CoV, indicative of a bat origin (Table 1 [1-5]), while genetically more distant to SARS-CoV (about 79%) and MERS-CoV (about 50%) regarding the sequence similarity [8]. Interestingly, the genome sequences of SARS-CoV-2 obtained from infected humans with severe acute respiratory syndrome, showed an extreme (more than 99.98%) sequence similarity, indicating that they were infected by a common pathogenic viral strain [8]. Coronavirus family possesses the largest genomes (ranging from 26.4 to 31.7 kb in length) among all known RNA viruses [7].
Previous reports have revealed that SARS-CoV-2 penetrates the target cells through binding to angiotensin-converting enzyme 2 (ACE2), which is expressed on the surface of epithelial cells in the lung, intestine, kidney, and blood vessels in humans [9]. ACE2 is a type I transmembrane metallocarboxypeptidase with homology to ACE1, an enzyme known to be a key player in the renin-angiotensin system (RAS) and a pharmacological target for the treatment of hypertension [9].
Brief summary of other coronaviruses as human and animal pathogens (SARS, MERS, COVID-19)
SARS-CoV-2, SARS-CoV and MERS are viruses (Table 1 [1-5]) that belong to the coronaviruses family from the order known as Nidovirales [1]. Members of this coronavirus family including SARS-CoV-2 are medium-sized viruses, they are enveloped and have a positive-sense, single-stranded large RNA composition as genetic code [1]. SARS-CoV-2 has the largest RNA known in the coronavirus family [1]. Generally there are four types of coronaviruses denoted as α-, β-, γ-, and δ-coronaviruses, where only members of the α- and β-coronaviruses could cause infection with various severity in humans, while γ-, and δ-coronaviruses are only pathogen in animals [1-5]. In the α-coronavirus group, there are two members that are causing illness in human, e.g., HCoV-229E discovered in 1967, which mostly infects immunocompromised subjects and the other member, e.g., HCoV-NL63, discovered in 2003 which is the second most common coronavirus [1-5]. Both of these coronaviruses are worldwide distributed having a seasonality pattern, i.e., highest number of infection in winter and lowest number of infections during the summer period [1-5]. The major infection symptoms are a common cold, acute rhinitis and nasal congestion, but they can also cause bronchiolitis and pneumonia in infant or immunocompromised subjects [1-5]. In the β-coronavirus group, there are three subgroups denoted as lineage A, lineage B and lineage C [1-5]. HCoV-OC43 and HCoV-HKU1 detected in 1967 and 2005 respectively are two important pathogenic members of in lineage A [1-5]. Although both viruses are worldwide distributed causing common cold symptoms, it seems that HCoV-OC43 causes a more severe disease compared to HCoV-HKU1 [1-5]. Concerning lineage B, the most important members are SARS-CoV discovered in 2002 - 2003 in Guangdong, China and SARS-CoV-2 discovered in 2019 also known as COVID-19, in Wuhan, China [1-5]. Both SARS-CoV and SARS-CoV-2 are very infectious to human and have fatal symptoms such as severe acute respiratory syndrome which is also reflected in their name and is the basis for the given names of these two viruses [1-5]. The clinical symptoms of SARS-CoV and COVID-19 vary from asymptomatic to fatal. However, due to the concerted efforts of global community SARS-CoV has been almost expelled from human population in 2003 (Table 1 [1-5]), whereas COVID-19 is still appearing highly effective and in some individuals causes life-threatening symptoms [1-5]. The basic reproduction number (Table 1 [1-5]) or basic reproductive ration (R0) for SARS-CoV-2 seems to be around 2 - 3 range [1-5] that mean one infected patient can spread the virus to two or three new individuals [1-5].
The MERS-CoV which was discovered in 2012 in Saudi Arabia/Korea (Table 1 [1-5]) belongs to lineage C of β-coronavirus that similar to SARS-CoV and SARS-CoV-2, and is pathogenic to human causing a severe deep airway infection, also known as Middle East respiratory syndrome [10]. The MERS-CoV has not received much attention worldwide since it was only localized to Saudi Arabia although some outbreak in Korea has been reported [10]. The basic reproductive ratio (R0) for MERS-CoV seems to be low, around 0.3 - 0.7 range [10]. A common problem with all pathogenic coronaviruses is that in great numbers of infected people, they are asymptomatic and can easily transmit the infection. The clinical symptoms vary and can be perceived as a common cold, cough associated with chills and myalgia as well as severe respiratory tract infection in some individuals [10].
Although coronavirus family members infect both birds and mammals, bats have been shown to be the host with the greatest number of coronavirus genotypes and thus have the ability to transmit the virus to other birds and mammals. An epidemic generally could evolve when the coronavirus is transmitted from one species to another. Coronaviruses typically gain or cultivate mutation in their envelope proteins, allowing them to bind to cell surface protein in other species and infect other species more easily [3]. Transmission between species allows coronavirus to acquire new abilities to infect cells. Coronaviruses could cause illness by both respiratory tract infections and gastrointestinal infections in adults and children [8]. Signs and symptoms may vary widely, but elderly people as well as individuals with an underlying diseases such as diabetes, obesity, chronic cardiovascular disease or those treated with immune suppressants are the most severely affected by complications and mortality [11]. In this review we aim to present pharmacological treatments that in the absence of a properly functioning vaccine may be conceivable to combat the complications and spreading of COVID-19.
| A Brief Summary of Common Strategies to Fight Viruses | ▴Top |
Vaccines
For the development of a well-functioning vaccine against COVID-19, the three-dimensional structure of the membrane proteins (antigenic part) in the virus, that trigger an immune recognition response, must be revealed. There are several ongoing studies suggesting the spike protein (S-protein) part of the SARS-CoV-2 which interacts with the host cell membrane proteins is suitable as antigen in the development of a vaccine against SARS-CoV-2 [12, 13].
Since the development of a vaccine normally is time-consuming, no studies have so far completed a clinical trial with a clear and reliable result from a SARS-CoV-2 vaccine. While trials are underway to develop a vaccine, there are some epidemiological studies suggesting that the vaccine against Bacille Calmette-Guerin (BCG) could have some preventive effect against SARS-CoV-2 infection, although a relevant and well-designed study is still missing [14, 15]. The assumption is based on the observations that in countries routinely using the BCG vaccine in neonates have less SARS-CoV-2 infection been reported [16, 17]. However, it should be kept in mind that epidemiological studies are prone to substantial biases from many aspects of insidious interference in the investigation, including the stage of pandemic in each country, the test rates for SARS-CoV-2 infections as well as the usual health condition for the population.
Antiviral therapies: a pharmacological avenue for COVID-19 therapies
A well-functioning vaccine is the best option for prevention of a SARS-CoV-2 infection and avoiding its lethal respiratory consequences; however in the absence of a vaccine, we must find an alternative route of COVID-19 treatment among the existing and proven drugs on the market today. We have the opportunity to use drugs that normally is used for other infective conditions to prevent the SARS-CoV-2 virus action in the body. Antiviral drugs have successfully been used in the treatment of other viral infections such as chronic hepatitis B infection, although the hepatitis B virus contains double-stranded DNA (dsDNA) [18]. We speculate that an alternative antiviral drug could have an impact on the prevention/treatment of a SARS-CoV-2 infection. There are a number of established antiviral drugs on the market today, as well as those not yet clinically tested compounds that in various ways, i.e., by reducing the virus infection could be attractive tools in the prevention/treatment of SARS-CoV-2. These drugs could be used as monotherapy or in a different combination which makes the treatment even more effective.
| Coronavirus Structure and Potential Drugs to Target the SARS-CoV-2 Infection | ▴Top |
Coronaviruses have been categorized into four different classifications, i.e., α-, β-, γ- and δ-coronavirus where the coronaviruses that are causing infections in humans belongs to α- or β-coronaviruses [19]. SARS-CoV-2 is a β-coronavirus and is a medium-size virus enveloping a positive-stranded RNA with a large RNA genome [7].
Like other coronaviruses, the SARS-CoV-2 genome encodes four major structural proteins identified so far, i.e., S-protein, membrane (M) protein, the envelope protein (E) protein as well as a nucleocapsid (N) protein, which encapsulate a single stranded RNA. Some coronaviruses including SARS-CoV-2 also contain a hemagglutinin-esterase protein (HE) [20]. SARS-CoV-2 exhibit a number of open read frame (ORF) proteins such as ORF1a, ORF1b, ORF3a, ORF8 and ORF10. The importance and functionality of ORF proteins in the virus life cycle remains to be determined (Fig. 1 [8, 19]). Structurally the S-protein is composed of a short intracellular tail, a transmembrane anchor and a large ectodomain that consists of a receptor binding S1 subunit and a membrane-fusing S2 subunit that is used for the entry into the target cells [9, 19, 20]. From a pathogenic point of view, S-protein is an interesting part of SARS-CoV-2, since a naturally occurred mutation in the S-protein would possibly alter the infectivity of SARS-CoV-2 in both directions, i.e., by either increasing or decreasing the binding capability of the virus to the host cells. However, it is more likely that the naturally occurring mutations in the Spike genes mostly favor a more pathogenic phenotype of the virus. Although the clinical consequences of Spike gene mutations (antigenic drift) for SARS-CoV-2 need to be established, point mutations have been reported for other members of coronavirus family, e.g., MERS-CoV and SARS-CoV-1 [21, 22].
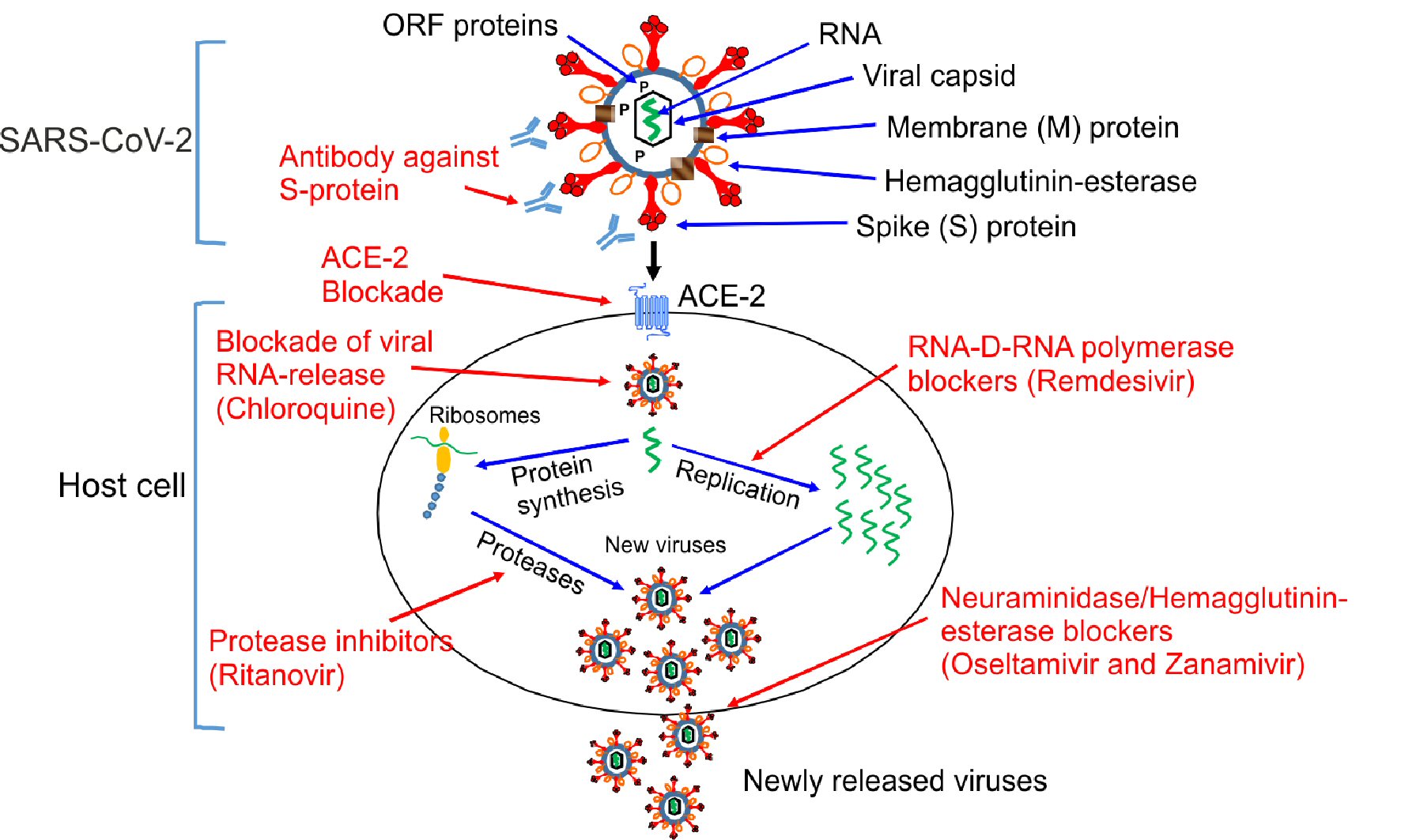 Click for large image | Figure 1. Mechanisms of action of different antiviral drugs. A schematically diagram of SARS-CoV-2 structure and different pathway used by SARS-CoV-2 for the replication in the host cells. Theoretical applicable inhibitory action of certain antiviral drugs used in the treatment of other viral infections, as well as specific SARS-CoV-2 neutralizing antibodies would in combination interfere with the SARS-CoV-2 life cycle exerting a possible inhibitory effect on the virus replication, which is shown in red color. ORF proteins: ORF1a, ORF1b, ORF3a, ORF8 and ORF10 [8, 19]. SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; ACE2: angiotensin-converting enzyme 2; ORF: open read frame. |
However, a more detailed investigation on the structural biology as well as the functional properties of SARS-CoV-2 is needed to successfully develop an antidote to prevent the COVID-19 pandemic.
Since there are currently no well-defined and specific antiviral drugs that targets the SARS-CoV-2 virus, we investigated the literature for the possibility of other antiviral drugs as well as other anti-pathogenic agents used for the treatment of infectious diseases, to address a possible way of pharmacological treatment of the SARS-CoV-2 infection. Based on available information and publications, it seems that some existing drugs in clinical use can be effective against the SARS-CoV-2 virus. Below we shortly present a review of some reliable drugs and their mechanism of action in this respect.
Antiviral drugs targeting coronavirus proteins
The whole structure as well as individual membrane protein of a virus is the easiest step in the recognition by the host immune cells when combating a viral infection. This however does not rule out the idea of pharmacologically targeting additional intracellular viral proteins in a combination strategy with membrane proteins for an effective prevention of SARS-CoV-2 replication and infection of new host cells. With no time for experimental and preclinical studies and the urgent situation, drug discovery against the SARS-CoV-2 will be a very challenging task. We hope to shed some light on this difficult situation that has suddenly arisen by emphasising the promising effects of the following remedies that theoretically or even in some causes practically could be useful for the combat of SARS-CoV-2.
RNA-dependent RNA polymerase inhibitors as a target in COVID-19 treatment
SARS-CoV-2 is a (+)ssRNA virus, utilizes its own genetic material (mRNA) directly by using the host ribosomal machinery and translating it into functional proteins [7]. One of these viral proteins is the RNA-dependent RNA polymerase that copies the viral RNA, which is subsequently translated to a number of functional proteins [7]. The generated SARS-CoV-2 proteins will then be packed into new functioning viral particles, which are released by the infected cells and thus a new cycle of viral infection targeting additional cells will continue.
Fortunately, there are drugs that can effectively prevent this viral pathway by blocking the RNA-dependent RNA polymerase. One such a RNA-dependent RNA polymerase inhibitor is remdesivir (a nucleotide analog mimicking adenosine) which has initially been developed for the treatment and prevention of the Ebola virus [23]. This could also be an interesting drug candidate for the treatment of SARS-CoV-2. Remdesivir accordingly, incorporates into the nascent RNA chain causing a premature termination of viral RNA genes [24]. There are several clinical trials initiated to investigating the effect of remdesivir on SARS-CoV-2 infection with positively reported results [25-27]. Remdesivir overcomes the proofreading capacity of the SARS-CoV-2 while other similar RNA-dependent RNA polymerase inhibitors such as ribavirin may fail to do. So conclude, remdesivir potentially represents a promising drug candidate to treat COVID-19 patients.
Proteases as a target to be used in COVID-19 treatment
Proteases are an additional attractive and highly interesting target for treatment of SARS-CoV-2 since they are involved in the generation of structurally and functionally important virus proteins [7]. Recently, in a clinical trial performed in China, two protease inhibitors developed specifically to treat human immunodeficiency virus (HIV), e.g., lopinavir and ritonavir were tested in hospitalized adult patients with severe COVID-19. These drugs did not add substantial benefit beyond the standard care [28]. However, the mortality rate and organ failure was lower in the treated patients if the drug was given early in the SARS-CoV-2 infection process, especially in the acute respiratory distress syndrome (ARDS) [28]. Despite the negative outcome of this trial we believe that more clinical trials are needed to evaluate the beneficial effects of lopinavir and ritonavir on SARS-CoV-2. A major consideration in this regard is that the drugs should be given in combination with other viral suppressor and in an early stage of infection.
Neuraminidase/hemagglutinin-esterase inhibitors as a target in COVID-19 treatment
Earlier studies have shown good effect of oseltamivir (a neuraminidase inhibitor approved for the treatment of influenza A and B) in combination with antibiotics in the treatment of MERS-CoV [29]. Since SARS-CoV-2 displays certain structural similarities with MERS-CoV the neuraminidase inhibitors could have positive effects in COVID-19 treatment, although the neuraminidase protein seems to be scarcely or not at all expressed by SARS-CoV-2. The SARS-CoV-2 virus expresses another membrane esterase, i.e., hemagglutinin-esterase which could to some extent also be inhibited by neuraminidase inhibitors. Administration of several of commercially available neuraminidase inhibitors on the market, e.g., oseltamivir and peramivir have not shown any impressive effect in the treatment of SARS-CoV-2 when they have been used alone [29]. This has caused considerations whether neuraminidase inhibitors could be effective in the prevention of SARS-CoV-2 proliferation [29]. On the other hand, there are clinical studies showing that oseltamivir in combination with other antiviral drugs have effect on SARS-CoV-2 [29]. This indicates that due to an intense intracellular virus production some of the produced virus bypasses neuraminidase and is released through other pathways/mechanisms and therefore neuraminidase inhibitors in monotherapy cannot exert the desirable effect in SARS-CoV-2 treatment. It should also be noted that most coronaviruses express hemagglutinin-esterase, thus inhibitors of this enzyme might be even more an attractive candidate in the treatment of SARS-CoV-2.
| Therapies That Do Not Directly Target the Coronavirus | ▴Top |
As we said earlier, the SARS-CoV-2 is a respiratory virus and the pulmonary tissue and cardiovascular system are the primary targets of infection [2, 4]. However, there is also a concern that there might be additional underlying not yet identified etiology in SARS-CoV-2 infection that is worsening the illness. It has been reported that a severe infection of SARS-CoV-2 could in some individuals cause a great increase in locally and systemically produced cytokines, a phenomenon also called a “cytokine storm”, which is an overreaction of the body’s immune system [30].
COVID-19 and the cytokine storm
Evidence has been presented that some SARS-CoV-2-infected patient displays a dramatically increased cytokine production/release both at systemic level and importantly also within the pulmonary tract which is referred to as a cytokine storm [30]. Post-mortem analysis of pulmonary tissues and hearts from SARS-CoV-2-infected patients has revealed interstitial macrophages and monocytes infiltration as well as tissue necrosis [30]. The cytokine storm has also been reported in leukemia patients who are refractory to chemotherapy. Unfortunately, there is no comparable evidence whether the SARS-CoV-2-induced cytokine storm is identical, equivalent or different to the cytokine storm that occurs in the leukemia patients who are subjected to chemotherapy [31].
It is well known that typical pro-inflammatory cytokines such as interleukin (IL)-β, IL-6, interferon (IFN)γ, tumor necrosis factor (TNF)α and the chemokine (C-C motif) ligand 5 (CCL5) also known as RANTES (regulated on activation, normal T expressed and secreted) are released initially by activated immune cells at the site of infection [30]. Thus, RANTES that is released by T lymphocytes and tissue macrophages at the cardiopulmonary site of SARS-CoV-2 infection would play an important role in the severity of COVID-19 disease. Interestingly, increasing levels of these cytokines have been reported in many clinical investigations with a strong correlation with disease severity and mortality in COVID-19 patients [30]. It seems that the body’s normal immunological homeostasis following a viral or bacterial infection is dysregulated by SARS-CoV-2 (Fig. 2 [29, 32]). An explanation could be the durability of the SARS-CoV-2 infection titer compared to other seasonally dependent and normally occurring viral infections, in parallel with the ability of the SARS-CoV-2 to suppress both host cell defense causing an excessive viral replication resulting in an excessive pro-inflammatory cytokine release. While the virus must be combated, the excessive immune response must also be mitigated to avoid damage to pulmonary and cardiovascular cells (Fig. 2 [29, 32]).
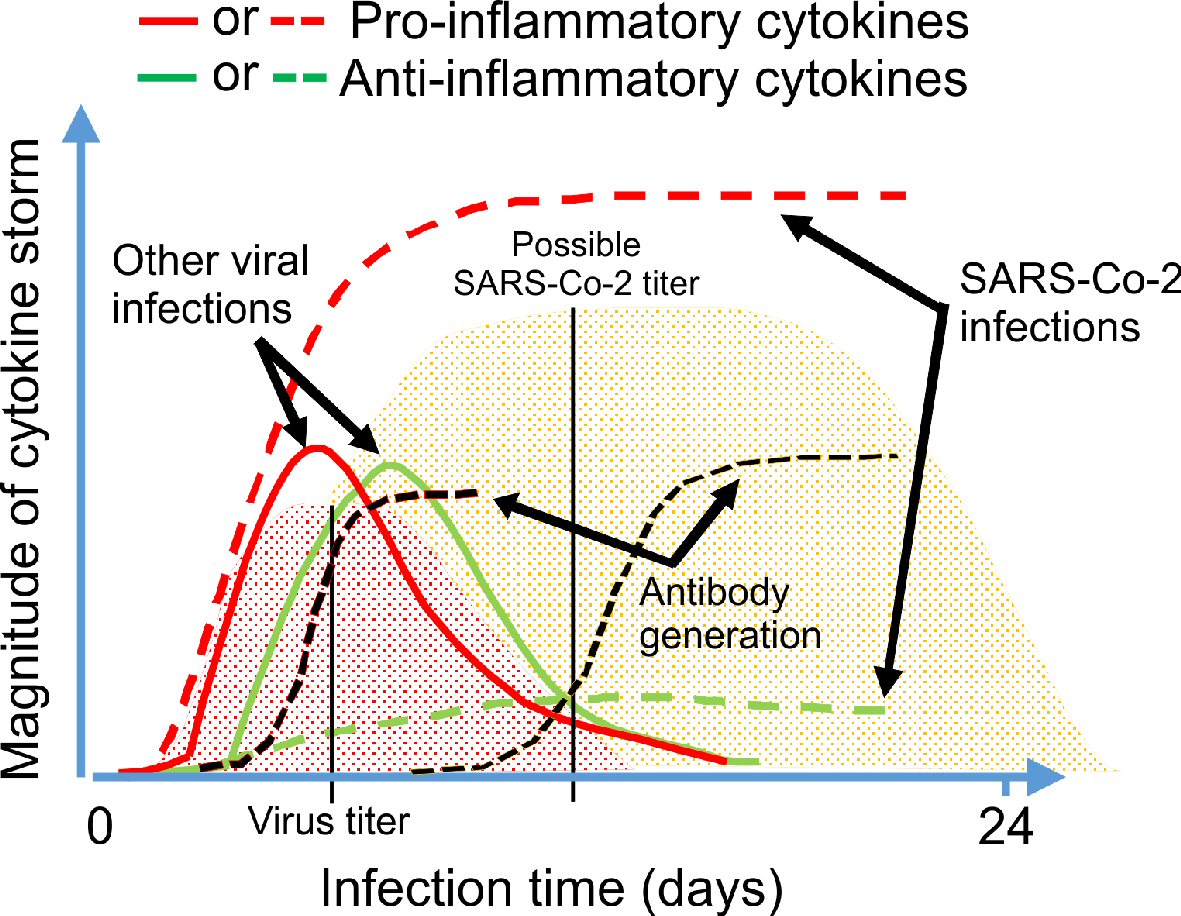 Click for large image | Figure 2. Schematic illustration of the immune response to a viral infection. Based on the previous knowledge of immune system, we proposed a comparing graphic image for the virally-induced pro-inflammatory and anti-inflammatory cytokine generation (with the obvious suppressive effect of SARS-CoV-2 on the anti-inflammatory cytokine production resulting in cytokine storm), as well as development of antibody against SARS-CoV-2 compared to other viral infections by the body [29, 32]. |
Anti-inflammatory drugs of steroidal or nonsteroidal origin could have counteracting effects when treating any viral infections in an early stage of the disease. It should be better to prevent the SARS-CoV-2-induced cytokine storm by other appropriable drugs, ideally by inhibitors of cytokine receptors at the very early stage of SARS-CoV-2 infection, to avoid a cytokine storm that occurs at later stage of infection. However, cytokine-receptor blockers could only reduce one of the SARS-CoV-2-induced effects and should be considered as a supplementary to other antiviral drugs. Tocilizumab an IL-6 receptor blocker or anakinra an IL-1 receptor blocker as well as leronlimab, a CCR5 antagonist [32, 33], that have been investigated for the treatment of other cytokine-related diseases could be good supplemental candidates to be used together with other remedies which is directly targeting the SARS-CoV-2 infection (Table 2 [26-29, 32-52]). There are ongoing trials testing the effect of tocilizumab, anakinra, leronlimab as well as canakinumab on SARS-CoV-2 patients, and the outcome will show whether any of these could be of benefit to SARS-CoV-2 patients [34]. Conversely, suppression of cytokine production in the later stage of the disease by low dose of anti-inflammatory drugs of steroidal origin, i.e., glucocorticoids such as hydrocortisone, dexamethasone or prednisolone could have some beneficial effect via suppression of the exaggerated immune response in the SARS-CoV-2 patients. However, it should be kept in mind that the use of glucocorticoids in diabetic patients is not without any risks as it impairs an already dysregulated blood glucose metabolism which consequently leads to higher blood glucose levels.
 Click to view | Table 2. Medications/Treatments Against COVID-19 |
ACE2 manipulation as a target in COVID-19 treatment
In the first step of the SARS-CoV-2 infection, the virus target ACE2 on the cell surface. This follows by the S-protein being cleaved to S1 and S2 by the host cell membrane proteases, one of which is transmembrane protein 72 that is known as TMPRSS2 [53], The main function of S1 subunit is thus binding to the host cell membrane ACE2, whereas the main function of S2 subunit is to provide membrane fusion [53]. One therapeutic approach of interest is to target the host cell transmembrane protease serine 2, e.g., TMPRSS2. One potential and presently existing inhibitor of TMPRSS2 [53] is camostat mesylate, which could be considered as an anti-SARS-CoV-2 candidate.
The structure of the S-protein is known [53], and it is a potential target for SARS-CoV-2 prevention by developing a vaccine that contains antigen derived from the S-protein which could initiate and/or increase the recognition of virus by immune cells. Another potential target is to develop monoclonal antibodies that bind to the S-protein of virus which could block the interaction of SARS-CoV-2 with the host cell’s ACE2.
Since previous protein-modeling investigations on the S-protein have revealed that SARS-CoV-2 has a strong binding affinity to human ACE2 and likely uses the ACE2 as mechanism for the cell entry, a possible target for drug development against SARS-CoV-2 could be the interaction site between S-protein and ACE2. Interestingly, the naturally occurring flavonoid called hesperidin, which is richly found in citrus fruits [54], is a compound that has been found during recent computational investigations and has been predicted to bind with the binding interface of S-protein-ACE2 complex.
ACE2 is highly expressed in the human respiratory, vascular and gastrointestinal tract and since SARS-CoV-2 with high affinity is binding ACE2 to enter target cells [53, 54], the use of a manipulated and easily accessible circulating recombinant variant of human ACE2, theoretically and possibly could be possible to prevent or reduce the free amount of SARS-CoV-2 available for binding to the naturally occurring ACE2 expressed on the cell surface. It should be emphasized that a normal and adequate expression of ACE2 is important for the cell function in the lungs and down-regulation of ACE2 during SARS-CoV-2 infection, i.e., ACE2-COVID-19 complex internalization, exerts tremendous damage to the vascular endothelial and alveolar epithelial cells in the lung [35]. This suggests that in humans, exogenously delivered excessive form of soluble ACE2 has the potential to bind to SARS-CoV-2 preventing the virus to binding naturally expressed ACE2 on the cell surface and rescuing the lung cells from injury. One small study has already tested and found that recombinant human ACE2 (rhACE2) is safe to be used in human subjects [36]. Theoretically, a rhACE2 treatment (Table 2) for SARS-CoV-2 infection should be used in the very early stage of infection. One challenge is to test if the rhACE2 protein is efficient to prevent the SARS-CoV-2 infection given as monotherapy or if it needs to be combined together with other antiviral drugs. Another interesting alternative for the prevention of SARS-CoV-2 binding to ACE2 is the use of non-specific ACE1 inhibitors in the early stage of SARS-CoV-2 infection to protect the ACE2 from SARS-CoV-2 binding. Since the use of ACE1 inhibitors is associated with a lowering of the blood pressure, a strategy like this is not without the risk of hypotension and need to be taken in the consideration.
Blockers of SARS-CoV-2 RNA release from hosted cells
There are still no approved drugs or therapeutic modalities as blocker of viral RNA releasing capability of SARS-CoV-2. However, hydroxychloroquine and chloroquine, two drugs that have been used for the treatment of malaria and rheumatoid arthritis have been shown to block coronavirus replication in the experimental studies [54]. Hydroxychloroquine and chloroquine exert their antiviral activity in part by increasing the pH in the host cell’s lysosomes which in turn inhibit the hydrolytic activity of protease enzymes that are required for processing of viral gluco-protein that is needed for the viral RNA transcription and translation (Table 2). Caution should be taken when treating patients suffering from for the type 2 diabetes as treatment with these drugs could be associated with hypoglycemia [37].
Promising result has been demonstrated in an open-label non-randomized clinical trial using hydroxychloroquine in combination with azithromycin resulting in a more dramatic suppression of SARS-CoV-2 levels in infected patients [55]. There have been suggestions that both chloroquine and its derivative hydroxychloroquine could interact with ACE2 and thus preventing SARS-CoV-2 binding; on the other hand a more recent hypotheses has challenged this and indicate that both drugs could act as ionophore for the zinc (Zn2+) to enter the cell, where Zn2+ enhances the inhibitory action of hydroxychloroquine and chloroquine on viral RNA release into the intracellular space [56]. The ionophoric action of the chloroquine compound has previously been documented [57]. It remains to be clarified whether other compounds acting as Zn2+ ionophore also could exert a similar effect on RNA replication of SARS-CoV-2 as hydroxychloroquine and chloroquine (Fig. 1 [8, 19]). Quercetin (a flavonoid), is another ionophore for Zn2+ [53]. Unfortunately, no preclinical studies or clinical trial have so far documented the effect in the prevention of COVID-19.
Both hydroxychloroquine and chloroquine exert anti-inflammatory property and could be capable of preventing the SARS-CoV-2-induced increase of proinflammatory cytokines in circulation [30], and are thus be beneficial in reducing the disease severity. Claims have been raised by some researchers that the effect of hydroxychloroquine or chloroquine alone or in combination with Zn2+ is insufficient. The claim is based on a clinical observation where the compounds were administered to patients with severely developed symptoms without any substantial effect [38].
Concerning the treatment protocol for hydroxychloroquine and chloroquine, they need to be administered early in the process of COVID-19 infection. Long-term treatment with hydroxychloroquine and chloroquine should be performed with caution since they could be associated with unwanted side effects such as hypersensitivity, porphyria or a prolonged QTc interval. However the duration of COVID-19 treatment with these compounds might not last more than 2 - 3 weeks and the risk of develop any side effects should be limited.
In a recent clinical trial with patients treated for COVID-19 [55], it has been shown that patients treated with hydroxychloroquine in combination with azithromycin (a macrolide antibiotic) were virologically cured to a higher degree compared to patients only treated with hydroxychloroquine, although both groups showed a marked better virus elimination than the vehicle treated control group. Higher doses of hydroxychloroquine used in COVID-19 treatment compared to standard indications could be associated with a different or at least partially different mechanism and needs to be exercised with caution. It is known that in clinically relevant doses, azithromycin increases antiviral IFNs production in rhinovirus-infected bronchial epithelial cells from asthmatics but had no effect in cells from healthy donors [39]. This might theoretically explain why the effect of azithromycin is only seen in a subpopulation of COVID-19 patients where SARS-CoV-2 of some unknown reason might be still harmful. Hence, drugs inducing lung IFNs production would be warranted in the treatment of COVID-19 patients as adjacent to other drugs with at least partially different mechanistic actions.
Recently there have been reports showing that a worldwide used anti-parasitic drug, e.g., ivermectin, known to have antiviral activity in vitro, strongly inhibits the replication of SARS-CoV-2 in SARS-CoV-2-infected Vero-hSLAM cells [58]. The antiviral action of ivermectin has been previously documented for dengue virus type 2 (DENV-2) [59]. The mode of action and therapeutically relevant doses to block a SARS-CoV-2 infection need to be further validated in preclinical studies. Our recommendation is that ivermectin should be considered as a possible treatment for SARS-CoV-2 infection in a randomized clinical study, both alone and in combination with hydroxychloroquine.
| Convalescent Plasma as a Treatment for COVID-19 | ▴Top |
Most patients develop SARS-CoV-2 neutralizing antibodies after recovery from COVID-19. Their convalescent plasma is an economically reasonable tool that could be used as treatment for COVID-19. On an emergency basis the Food and Drug Administration (FDA) has approved convalescent plasma (CP) for the treatment of severely ill with immediately life-threatening symptom due to COVID-19 infection [40]. CP has been tested in a pilot study including a small group of patients and the result was promising, however further studies with a larger cohort is needed to establish the potential clinical benefit and exclude the possible risk of convalescent blood products in the treatment of COVID-19 patients. If the CP hypothesis is confirmed in a larger cohort studies, CP treatment could be the best COVID-19 treatment to date.
| Generation of Monoclonal Antibodies Against COVID-19 as a Possible Treatment | ▴Top |
Neutralizing antibodies are important components of the immunity against viral infections. Engineered antibodies (immunoglobulins) have been developed for a wide spectrum of therapeutic indications and are being used in recent years as an efficient way of treating certain diseases such as cancer and rheumatoid arthritis [32]. The antibody concept as a treatment is exciting with a potential to revolutionize the treatment of many aggressive viral conditions such as COVID-19 [41]. The structure of S-protein is known [26] and could be used as antigen to produce antibodies. Monoclonal antibodies targeting the receptor-binding domain aim to block the virus entry into target cells, and have a great potential to be used in COVID-19 prevention/treatment while we are waiting for a successful vaccine to be developed.
The S-protein of SARS-CoV-2 seems to be the most promising part of the virus particle for antibody development. By binding the S-protein of the virus, the antibody inhibits the virus ability to interact with the host cells ACE2, which prevents the viral invasion and pathogenicity and at the same time the antibody-virus complex making the virus a identifiable targets for the host immune defense cells.
| The Possible Effect of SARS-CoV-2 on the Erythrocytes | ▴Top |
Multiple clinical evidences as well as pathological reports have suggested that most COVID-19-associated mortalities have been due to multi-organ failure, pneumonia and ARDS [5, 25]. The difficulty in breathing and the low blood oxygenation in SARS-CoV-2-infected patients have been linked to a reduced gas exchange in the alveoli due to an exacerbated released of inflammatory factors and local inflammation [30].
A computational-modeled hypothesis for additional mechanism of action of the SARS-CoV-2 virus involves hemoglobin in red blood cells, i.e., erythrocytes [60]. According to a reported study by Wenzhong et al, SARS-CoV-2 could have the ability to interact with the heme group in hemoglobin [60]. This is an important hypothesis, since previous reports have revealed that a high percentage of COVID-19 patients suffer from hypoxia despite a well functioning cardio/pulmonary system [2, 5]. Hypoxia alone or in combination with other SARS-CoV-2-induced inflammatory factors can cause multi-organ failure [61]. Previous reports have also revealed that hemoglobin levels in most COVID-19 patients are decreased, while ferritin levels as well as the erythrocyte sedimentation rate, C-reactive protein (CRP) and lactate dehydrogenase, all markers of hemolysis, in parallel with CRP (markers of inflammation) are significantly increased [62].
If SARS-CoV-2 targets red blood cells causing damage, they will probably lose the capability of carrying oxygen in sufficient quantities as during normal conditions. Some recent reports [61] show that in severely ill patients during hospitalization, even the use of a ventilator does not help, indicating that despite a still functioning lung the blood cells might not be able to bind and carry oxygen in a sufficient way.
A majority of patients who have died due to COVID-19 in the western world, have died from hypoxia as well as hypoxia-induced multi-organ failure [61]. A logical explanation for the observed hypoxia, elevated levels of ferritin as well as multi-organ failure in COVID-19 patients is that coronavirus interacts with the heme group of hemoglobin in the erythrocytes causing hemolysis. This hypothesis needs to be confirmed by more preclinical and clinical cohort studies. Treatment with erythropoietin (EPO), a widely tested supplementary medication for the management of anemia [42], could also be considered as a constituent medication for COVID-19 treatment alone or as an adjacent to other antiviral agents.
| COVID-19 and Blood Thromboembolism | ▴Top |
SARS-CoV-2 is aggressively attacking the pulmonary tissues and causes an atypical pneumonia that in some patients is converted into a typical bacterial pneumonia. Some cases have reportedly been successfully treated with antibiotics while others did not respond to antibiotics and were converted to ARDS, culminating in septic shock and consequently death [5, 29].
There have been reports showing that 20-25% of the hospitalized patients in the intensive care unit (ICU) with COVID-19-associated pneumonia have markedly increased blood clotting problems (hypercoagulability) [63, 64]. The actual mechanisms behind SARS-CoV-2-induced blood clotting is not fully understood, but it seems to be in part dependent on local inflammation as a consequence of a cytokine storm within the lungs, which specifically affecting type II alveoli as well as damaging of adjacent microvascular blood vessels, trigging blood clotting (thromboembolism) to develop [65]. However, this scenario has also been reported earlier for ARDS patients hospitalized under ICU [66] where multiple small and tiny clots, so called microthrombi could be detected in the microvasculature of lung.
As pulmonary epithelial cells and vascular endothelial cells in the lungs express high levels of ACE2 and since SARS-CoV-2 enters the cell by binding ACE2, this would successively lead to a dramatically reduced cell surface expression of ACE2 due to internalization [67]. This in turn causes elevated levels of angiotensin II and consequently to an insufficient quantity of angiotensin I - VII. Angiotensin II is a powerful vasoconstrictor exerting inflammatory and oxidative effects on the vascular endothelial and smooth muscle cells that are associated with atherosclerosis and thrombosis [68].
An adequate and sufficient cell membrane expression of ACE2 is of importance for normal physiology of the blood, pulmonary and cardiovascular system [69]. Down-regulation of ACE2 per se leads to an increased pneumonia as well as microthrombi formation and pulmonary vasoconstriction, i.e., constriction of the all small pulmonary blood vessels [43]. Each of these two conditions, i.e., lack of presence of functioning ACE2 or an imbalance between angiotensin I - VII and angiotensin II by itself is reportedly associated with increased hypoxia and combination of these conditions due to COVID-infection, will dramatically increase hypoxia intensity and duration. Administration of angiotensin I - VII to counteract the unbeneficial effect of the elevated angiotensin II, could have some positive effects on preventing vasoconstriction, generation of oxygen radicals and pulmonary cell damages; however the effects might not be as efficient as the presence of anticoagulants [70].
We suggest that, as for patients admitted to ICU for other ailments receiving anticoagulants, COVID-19 patients should also be considered to be given a low dose of anticoagulants for prophylactic purposes along with other treatments, unless a contraindicated medical situation is prohibitive, i.e., if there is a risk of bleeding.
| Other Preventive Therapies | ▴Top |
Although not scientifically established, specific vitamins and antioxidants could have positive effect on a COVID-19 infection. From a general perspective view some vitamins, e.g., vitamins C and D, as well as antioxidants such as N-acetylcysteine (NAC) are important for a proper function of the immune system. There are ample of evidence-based studies showing that vitamin C, vitamin D or NAC supplementation reduces the incidence of clinically apparent disease (NAC), the probability of sepsis progression (vitamin C), contracting respiratory tract infection (vitamin D) caused by either bacterial or viral origin [44, 70-73]. Hence, obtaining the recommended levels of vitamins and antioxidant in blood would certainly have some effect on the prevention or mitigation of COVID-19 symptoms by improving the functionality of the immune system.
The use of vitamin C in COVID-19 treatment
It has been reported that people deficient in vitamin C (ascorbic acid) are more prone to develop pneumonia with respiratory infections [10, 71]. Furthermore, it is well documented that vitamin C exerts an antioxidant activity capable of reducing oxidative stress, suppressing the generation of inflammatory markers and enhancing immune cell function that subsequently should shorten the infection period, as it has been reported for the influenza epidemic [45]. It should also be recalled that an adequate daily uptake of vitamin C to maintain a normal blood/plasma level is important for a proper collagen production by the pulmonary- and vascular-cells [46]. Vitamin C can neither prevent nor cure SARS-CoV-2, but due to other function such as helping the immune system, vitamin C could play an important role in the overall recovery of the body during a SARS-CoV-2 infection. We believe that daily doses of 70 - 90 mg/kg of vitamin C during hospitalization of SARS-CoV-2 patients could shorten the disease period and speed up the recovery in a similar way that has been reported for the influenza virus [29, 45, 47]. It should be mentioned that some clinical trials have demonstrated promising data on prevention of mortality in sepsis, but more extensive studies would be necessary to validate our conclusion regarding the effect of vitamin C on SARS-CoV-2 treatment.
Vitamin D and COVID-19 treatment
Vitamin D (after its activation by two hydroxylations) is known to be involved in the regulation of Ca2+/PO4- homeostasis in the body; and there is ample of evidence showing that vitamin D is an important player in immunomodulation [48, 49]. The normally low expressed vitamin D receptor (VDR) on immune cells, i.e., macrophages, monocytes, as well as in B and T lymphocytes increases considerably as a result of inflammatory and immunological stimuli [48, 49]. Activation of VDR by vitamin D is an important factor in promoting innate immune response, by enhancing the production of anti-pathogenic agents such as cathelicidin, beta defensins and NFkB by tissue macrophages [72]. Vitamin D deficiency or down-regulation of VDR allows pathogens to accumulate in blood [72] and tissue, and the weakened innate defense, due to less developed immune cells further causes susceptibility to additional infections.
Although previous epidemiological and in vitro data have shown that vitamin D supplementation could decrease the risk of acute respiratory tract infection in randomized controlled trials [73], no direct antiviral effect of vitamin D has been reported. This however, does not rule out the possible immunomodulatory action of vitamin D during a viral infection. Patients suffering from chronic obstructive pulmonary disease who have lower baseline vitamin D have reportedly responded with great clinical benefit from supplementation [50, 51]. In addition to its modulatory effect on the immune system, vitamin D also plays an important role in membrane stabilization of endothelial/epithelial cells in the respiratory organ, which make them more resistant to pathogenic infections [72]. Vitamin D could not exert any antiviral action directly, but we believe that the presence of an adequate level of vitamin D is necessary for combat of any pathogenic infection and could shorten the duration of SARS-CoV-2 infection.
NAC supplementation
It has long been known that NAC supplementation is associated with a relief of flu-like symptoms and shorten the period of illness and severity [44]. In a previous report, it was shown that in a randomized double-blind trial NAC supplementation (600 mg) twice daily for 6 months, significantly attenuated the severity of influenza-like symptoms especially in high-risk individuals, although it did not reduce the prevalence of influenza in the treated population [37]. In the NAC-treated group only 25% compared to 80% in the placebo group suffered from influenza symptoms [44]. NAC is known to be essentially an antioxidant and also to be able to compensate and replenish a reduced level of glutathione in the liver that occurs during oxidative stress when the body is exposed to toxins or pathogenic infections [44]. We believe that NAC, not alone but in combination to other treatment remedies could be of importance in the relief of COVID-19 symptoms and severity.
Traditional herbal supplementation and COVID-19
The possible immunomodulatory effects of certain herbals such as β-glucans as well as several phytopharmacs and Chinese medicines have been previously documented. Of these substances, β-glucans are of particular interest since they have been well studied with demonstrated immunomodulatory effects. The β-glucans are a heterogeneous group of glucose polymers that are the major cell wall structural components in fungi as well as plants and some bacteria [74]. It has been reported that in vivo administration of β-glucans would potentiate host responses against a variety of conditions, including tumor development and infection with fungal, bacterial, viral, and protozoal pathogens [75]. Consequently, β-glucans have also been used as prophylactic agent for the prevention of infections in surgical patients, with promising results. In another study it was showed that β-glucans can enhance immunity by increasing the levels of cytotoxic cells such as natural killer (NK) cells and macrophages, which will be the actual line of defense against the viruses [74, 75]. There is also reported that NK cell activity was significantly increased by β-glucans in patients with Leishmania amazonensis infection [74]. Although, β-glucans have not yet been subjected to a clinical study in COVID-19-infected subjects, its supplementation can be a potential strategy against COVID-19 infection, due to its immune-enhancing activity in terms of IFN-γ-increasing capability [76] whose suppression is characteristic pattern of SARS-CoV-2 infection [77]. It seems that β-glucans exert a general immune-boosting activity and thus can have a beneficial and protective effect against a wide range of septic conditions. We believe that daily supplementation of recommended amounts of β-glucans should have some preventing effects not only on SARS-CoV-2 but also on other viral infections as well.
| SARS-CoV-2-Induced Pneumonia | ▴Top |
SARS-CoV-2 infection induces immune hyper activation which in turn causes an increased release of pro-inflammatory cytokines in parallel with the suppression of anti-inflammatory cytokines (Fig. 2 [29, 32]), attracting immune cells, especially neutrophils, to infiltrate pulmonary capillaries [5, 29, 61, 65, 66]. Increase in neutrophil extracellular traps (NETs) that mainly consist of released double-stranded DNA as well as proteins and hyaline membrane brings an acute fibrin deposition and extravasation in the alveolar space. Normally endogenous deoxyribonucleases (DNases) is capable of degrading this extracellular DNA and NETs, however during the SARS-CoV-2-induced cytokine storm it becomes overwhelmed by the massive influx of NETs. The sudden increase of the viscous and thick mucus in the alveolar space of the pulmonary tissue makes gas exchange and ventilation very difficult for COVID-19 patients, in a similar way as it has been reported for cystic fibrosis. Hence, medicament used in the treatment of cystic fibrosis could also be of great benefit for COVID-19 patients. One such substance is the recombinant human DNase I (rhDNase), e.g., dornase alpha [52], which should be considered as a supplement in the treatment of COVID-19 pneumonia, which is one underlying cause of respiratory failure and death.
| Conclusions | ▴Top |
Although an effective and well-functioning vaccine is highly desirable for the prevention of the SARS-CoV-2 infection, in anticipation of such a vaccine, a number of already existing drugs used to prevent similar viral diseases reasonably might be considered for the treatment of COVID-19 patients. However, in terms of a pharmacological treatment method for COVID-19, it is important to focus on a combination therapy, i.e., using antiviral agents that target different part of the viral life cycle in combination with other drugs targeting pulmonary tissue to restore respiratory function and anti-inflammatory drugs to counteract pro-inflammatory cytokines. However, at an early stage of the infection, treatment with remdesivir in combination with SARS-CoV-2 neutralizing antibodies seems promising. Unfortunately in a vast majority of clinical trials performed so far, the treatment was based on a monotherapy regimen (the use of only one drug candidate) while for the combat of the aggressive SARS-CoV-2 virus a combination of at least two to three drugs that are targeting different path of the viral replication in the body could be more effective and should be introduced in an early stage of infection. In support of our hypothesis, there are several ongoing clinical trials on COVID-19 patients [78-80] demonstrating a more satisfactory treatment effects when using a combination of the above mentioned drugs that targets different part of the SARS-CoV-2 life cycle (Table 3 [78-83]).
 Click to view | Table 3. Combination Medications/Treatments Against COVID-19 |
In addition, since hypoxia and thromboembolism are also common features of severely ill COVID-19 patients, if necessary drugs that prevent such a harmful pathophysiological conditions should also be considered as supplements to the direct-acting antiviral drugs.
Limitations of proposed pharmacological therapy
A vaccine is the best option to prevent the COVID-19 pandemic. In the absence of a vaccine, existing pharmacological agents are of high value in the treatment of COVID-19 symptoms. The main limitation for the proposed combined pharmacological therapy is the limited experience in testing pharmaceutical compounds both in mono- and as a combination-therapy in a suitable SARS-CoV-2 cell system or animal models. In particular, there is a need for in-depth analysis of those clinical studies/trials that have been performed so far regarding the effect of combination therapies compared to already implemented monotherapy.
Acknowledgments
None to declare.
Financial Disclosure
Albert Salehi’s scientific research was supported by the grant from Swedish Research Council (01353), Forget Diabetes Foundation, Mats Paulson Foundation and Swedish Diabetes Foundation.
Conflict of Interest
The authors declare that there is no conflict of interest.
Author Contributions
Albert Salehi wrote the first draft of the manuscript. Pontus Duner contributed with comment and correction to the last version of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Estola T. Coronaviruses, a new group of animal RNA viruses. Avian Dis. 1970;14(2):330-336.
doi pubmed - Fung SY, Yuen KS, Ye ZW, Chan CP, Jin DY. A tug-of-war between severe acute respiratory syndrome coronavirus 2 and host antiviral defence: lessons from other pathogenic viruses. Emerg Microbes Infect. 2020;9(1):558-570.
doi pubmed - Hu T, Zhang Y, Li L, Wang K, Chen S, Chen J, Ding J, et al. Two adjacent mutations on the dimer interface of SARS coronavirus 3C-like protease cause different conformational changes in crystal structure. Virology. 2009;388(2):324-334.
doi pubmed - Yang Y, Peng F, Wang R, Yange M, Guan K, Jiang T, Xu G, et al. The deadly coronaviruses: The 2003 SARS pandemic and the 2020 novel coronavirus epidemic in China. J Autoimmun. 2020;109:102434.
doi pubmed - Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8(4):420-422.
doi - Shuja KH, Aqeel M, Jaffar A, Ahmed A. COVID-19 Pandemic and Impending Global Mental Health Implications. Psychiatr Danub. 2020;32(1):32-35.
doi pubmed - Belouzard S, Millet JK, Licitra BN, Whittaker GR. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4(6):1011-1033.
doi pubmed - Lu R, Zhao X, Li J, Niu P, Yang B, Wu H, Wang W, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565-574.
doi - Li F. Structure, Function, and Evolution of Coronavirus Spike Proteins. Annu Rev Virol. 2016;3(1):237-261.
doi pubmed - Alshahrani MS, Sindi A, Alshamsi F, Al-Omari A, El Tahan M, Alahmadi B, Zein A, et al. Extracorporeal membrane oxygenation for severe Middle East respiratory syndrome coronavirus. Ann Intensive Care. 2018;8(1):3.
doi pubmed - Venerito V, Lopalco G, Iannone F. COVID-19, rheumatic diseases and immunosuppressive drugs: an appeal for medication adherence. Rheumatol Int. 2020;40(5):827-828.
doi pubmed - A phase II clinical trial to evaluate the recombinant vaccine for COVID-19 (Adenovirus Vector) (CTII-nCoV). National Library of Medicine (US). 2020. https://clinicaltrials.gov/ct2/show/NCT04341389?term=NCT04341389&draw=2&rank=1.
- Duddu P. Coronavirus treatment: Vaccines/drugs in the pipeline for COVID-19. 2020. https://www.clinicaltrialsarena.com/analysis/coronavirus-mers-cov-drugs/.
- de Bree LCJ, Marijnissen RJ, Kel JM, Rosendahl Huber SK, Aaby P, Benn CS, Wijnands MVW, et al. Bacillus calmette-guerin-induced trained immunity is not protective for experimental influenza A/Anhui/1/2013 (H7N9) infection in mice. Front Immunol. 2018;9:869.
doi pubmed - Arts RJW, Moorlag S, Novakovic B, Li Y, Wang SY, Oosting M, Kumar V, et al. BCG vaccination protects against experimental viral infection in humans through the induction of cytokines associated with trained immunity. Cell Host Microbe. 2018;23(1):89-100 e105.
doi pubmed - Reducing health care workers absenteeism in COVID-19 pandemic through BCG vaccine (BCG-CORONA). National Library of Medicine (US). 2020.
- BCG vaccination to protect healthcare workers against COVID-19 (BRACE). 2020 National Library of Medicine (US). https://clinicaltrials.gov/ct2/show/NCT04327206.
- Zoulim F, Durantel D. Antiviral therapies and prospects for a cure of chronic hepatitis B. Cold Spring Harb Perspect Med. 2015;5.
doi pubmed - Mousavizadeh L, Ghasemi S. Genotype and phenotype of COVID-19: Their roles in pathogenesis. J Microbiol Immunol Infect. 2020.
doi pubmed - de Haan CA, Kuo L, Masters PS, Vennema H, Rottier PJ. Coronavirus particle assembly: primary structure requirements of the membrane protein. J Virol. 1998;72(8):6838-6850.
doi pubmed - Tang XC, Agnihothram SS, Jiao Y, Stanhope J, Graham RL, Peterson EC, Avnir Y, et al. Identification of human neutralizing antibodies against MERS-CoV and their role in virus adaptive evolution. Proc Natl Acad Sci U S A. 2014;111(19):E2018-2026.
doi pubmed - Sui J, Aird DR, Tamin A, Murakami A, Yan M, Yammanuru A, Jing H, et al. Broadening of neutralization activity to directly block a dominant antibody-driven SARS-coronavirus evolution pathway. PLoS Pathog. 2008;4(11):e1000197.
doi pubmed - Cardile AP, Downey LG, Wiseman PD, Warren TK, Bavari S. Antiviral therapeutics for the treatment of Ebola virus infection. Curr Opin Pharmacol. 2016;30:138-143.
doi pubmed - Konings DA, Bredenbeek PJ, Noten JF, Hogeweg P, Spaan WJ. Differential premature termination of transcription as a proposed mechanism for the regulation of coronavirus gene expression. Nucleic Acids Res. 1988;16(22):10849-10860.
doi pubmed - Holshue ML, DeBolt C, Lindquist S, Lofy KH, Wiesman J, Bruce H, Spitters C, et al. First case of 2019 novel coronavirus in the United States. N Engl J Med. 2020;382(10):929-936.
doi pubmed - Beigel JH, Tomashek KM, Dodd LE. Remdesivir for the treatment of COVID-19 - preliminary report. Reply. N Engl J Med. 2020;383(10):994.
doi - Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A, Feldt T, et al. Compassionate use of remdesivir for patients with severe COVID-19. N Engl J Med. 2020;382(24):2327-2336.
doi pubmed - Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, et al. A trial of lopinavir-ritonavir in adults hospitalized with severe COVID-19. N Engl J Med. 2020;382(19):1787-1799.
doi pubmed - Arabi YM, Fowler R, Hayden FG. Critical care management of adults with community-acquired severe respiratory viral infection. Intensive Care Med. 2020;46(2):315-328.
doi pubmed - Ye Q, Wang B, Mao J. The pathogenesis and treatment of the 'Cytokine Storm' in COVID-19. J Infect. 2020;80(6):607-613.
doi pubmed - Khosravi M, Taghvaye Masoumi H, Gholami K, Vaezi M, Hadjibabaei M, Ghavamzadeh A. The Relationship between Fatigue and Cytokine Levels in Patients with Acute Myeloid Leukemia. Int J Hematol Oncol Stem Cell Res. 2018;12(4):318-321.
doi pubmed - Siebert S, Tsoukas A, Robertson J, McInnes I. Cytokines as therapeutic targets in rheumatoid arthritis and other inflammatory diseases. Pharmacol Rev. 2015;67(2):280-309.
doi pubmed - Cavalli G, De Luca G, Campochiaro C, Della-Torre E, Ripa M, Canetti D, Oltolini C, et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study. Lancet Rheumatol. 2020;2(6):e325-e331.
doi - Ucciferri C, Auricchio A, Di Nicola M, Potere N, Abbate A, Cipollone F, Vecchiet J, et al. Canakinumab in a subgroup of patients with COVID-19. Lancet Rheumatol. 2020;2(8):e457-ee458.
doi - Uhal BD, Li X, Xue A, Gao X, Abdul-Hafez A. Regulation of alveolar epithelial cell survival by the ACE-2/angiotensin 1-7/Mas axis. Am J Physiol Lung Cell Mol Physiol. 2011;301(3):L269-274.
doi pubmed - Khan A, Benthin C, Zeno B, Albertson TE, Boyd J, Christie JD, Hall R, et al. A pilot clinical trial of recombinant human angiotensin-converting enzyme 2 in acute respiratory distress syndrome. Crit Care. 2017;21(1):234.
doi pubmed - Winter EM, Schrander-van der Meer A, Eustatia-Rutten C, Janssen M. Hydroxychloroquine as a glucose lowering drug. BMJ Case Rep. 2011;2011.
doi pubmed - Damle B, Vourvahis M, Wang E, Leaney J, Corrigan B. Clinical pharmacology perspectives on the antiviral activity of azithromycin and use in COVID-19. Clin Pharmacol Ther. 2020;108(2):201-211.
doi pubmed - Menzel M, Akbarshahi H, Tufvesson E, Persson C, Bjermer L, Uller L. Azithromycin augments rhinovirus-induced IFNbeta via cytosolic MDA5 in experimental models of asthma exacerbation. Oncotarget. 2017;8(19):31601-31611.
doi pubmed - Administration USFaD. U.S. Food and Drug Administration. 2020.
- Shanmugaraj B, Siriwattananon K, Wangkanont K, Phoolcharoen W. Perspectives on monoclonal antibody therapy as potential therapeutic intervention for Coronavirus disease-19 (COVID-19). Asian Pac J Allergy Immunol. 2020;38(1):10-18.
- Hadadi A, Mortezazadeh M, Kolahdouzan K, Alavian G. Does recombinant human erythropoietin administration in critically ill COVID-19 patients have miraculous therapeutic effects? J Med Virol. 2020;92(7):915-918.
doi pubmed - Gaddam RR, Chambers S, Bhatia M. ACE and ACE2 in inflammation: a tale of two enzymes. Inflamm Allergy Drug Targets. 2014;13(4):224-234.
doi pubmed - De Flora S, Grassi C, Carati L. Attenuation of influenza-like symptomatology and improvement of cell-mediated immunity with long-term N-acetylcysteine treatment. Eur Respir J. 1997;10(7):1535-1541.
doi pubmed - Gorton HC, Jarvis K. The effectiveness of vitamin C in preventing and relieving the symptoms of virus-induced respiratory infections. J Manipulative Physiol Ther. 1999;22(8):530-533.
doi - Carr AC, Maggini S. Vitamin C and immune function. Nutrients. 2017;9(11).
doi pubmed - Hemila H, Chalker E. Vitamin C can shorten the length of stay in the ICU: a meta-analysis. Nutrients. 2019;11(4).
doi pubmed - Lin R. Crosstalk between Vitamin D Metabolism, VDR Signalling, and Innate Immunity. Biomed Res Int. 2016;2016:1375858.
doi pubmed - Vanherwegen AS, Gysemans C, Mathieu C. Regulation of immune function by vitamin D and its use in diseases of immunity. Endocrinol Metab Clin North Am. 2017;46(4):1061-1094.
doi pubmed - Lehouck A, Mathieu C, Carremans C, Baeke F, Verhaegen J, Van Eldere J, Decallonne B, et al. High doses of vitamin D to reduce exacerbations in chronic obstructive pulmonary disease: a randomized trial. Ann Intern Med. 2012;156(2):105-114.
doi pubmed - Martineau AR, James WY, Hooper RL, Barnes NC, Jolliffe DA, Greiller CL, Islam K, et al. Vitamin D3 supplementation in patients with chronic obstructive pulmonary disease (ViDiCO): a multicentre, double-blind, randomised controlled trial. Lancet Respir Med. 2015;3(2):120-130.
doi - Tarrant BJ, Le Maitre C, Romero L, Steward R, Button BM, Thompson BR, Holland AE. Mucoactive agents for chronic, non-cystic fibrosis lung disease: A systematic review and meta-analysis. Respirology. 2017;22(6):1084-1092.
doi pubmed - Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, Schiergens TS, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271-280 e278.
doi pubmed - Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, Shi Z, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30(3):269-271.
doi pubmed - Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M, Doudier B, et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56(1):105949.
doi pubmed - te Velthuis AJ, van den Worm SH, Sims AC, Baric RS, Snijder EJ, van Hemert MJ. Zn(2+) inhibits coronavirus and arterivirus RNA polymerase activity in vitro and zinc ionophores block the replication of these viruses in cell culture. PLoS Pathog. 2010;6(11):e1001176.
doi pubmed - Xue J, Moyer A, Peng B, Wu J, Hannafon BN, Ding WQ. Chloroquine is a zinc ionophore. PLoS One. 2014;9(10):e109180.
doi pubmed - Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787.
doi pubmed - Xu TL, Han Y, Liu W, Pang XY, Zheng B, Zhang Y, Zhou XN. Antivirus effectiveness of ivermectin on dengue virus type 2 in Aedes albopictus. PLoS Negl Trop Dis. 2018;12(11):e0006934.
doi pubmed - Wenzhong L HL. COVID-19: attacks the 1-beta chain of hemoglobin and captures the porphyrin to inhibit human heme metabolism. Chem Rxiv. 2020.
doi - Bhatraju PK, Ghassemieh BJ, Nichols M, Kim R, Jerome KR, Nalla AK, Greninger AL, et al. COVID-19 in critically ill patients in the seattle region - case series. N Engl J Med. 2020;382(21):2012-2022.
doi pubmed - Li LQ, Huang T, Wang YQ, Wang ZP, Liang Y, Huang TB, Zhang HY, et al. COVID-19 patients' clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92(6):577-583.
doi pubmed - Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost. 2020;18(6):1421-1424.
doi pubmed - Klok FA, Kruip M, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145-147.
doi pubmed - Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, Nigoghossian C, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC State-of-the-Art review. J Am Coll Cardiol. 2020;75(23):2950-2973.
doi pubmed - Wang J, Hajizadeh N, Moore EE, McIntyre RC, Moore PK, Veress LA, Yaffe MB, et al. Tissue plasminogen activator (tPA) treatment for COVID-19 associated acute respiratory distress syndrome (ARDS): A case series. J Thromb Haemost. 2020;18(7):1752-1755.
doi pubmed - Wu C, Liu Y, Yang Y, Zhang P, Zhong W, Wang Y, Wang Q, et al. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm Sin B. 2020;10(5):766-788.
doi pubmed - Dandona P, Dhindsa S, Ghanim H, Chaudhuri A. Angiotensin II and inflammation: the effect of angiotensin-converting enzyme inhibition and angiotensin II receptor blockade. J Hum Hypertens. 2007;21(1):20-27.
doi pubmed - Jiang F, Yang J, Zhang Y, Dong M, Wang S, Zhang Q, Liu FF, et al. Angiotensin-converting enzyme 2 and angiotensin 1-7: novel therapeutic targets. Nat Rev Cardiol. 2014;11(7):413-426.
doi pubmed - Connors JM, Levy JH. COVID-19 and its implications for thrombosis and anticoagulation. Blood. 2020;135(23):2033-2040.
doi pubmed - Fowler AA, 3rd, Truwit JD, Hite RD, Morris PE, DeWilde C, Priday A, Fisher B, et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA. 2019;322(13):1261-1270.
doi pubmed - Gombart AF. The vitamin D-antimicrobial peptide pathway and its role in protection against infection. Future Microbiol. 2009;4(9):1151-1165.
doi pubmed - Martineau AR, Jolliffe DA, Hooper RL, Greenberg L, Aloia JF, Bergman P, Dubnov-Raz G, et al. Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583.
- Yatawara L, Wickramasinghe S, Nagataki M, Takamoto M, Nomura H, Ikeue Y, Watanabe Y, et al. Aureobasidium-derived soluble branched (1,3-1,6) beta-glucan (Sophy beta-glucan) enhances natural killer activity in Leishmania amazonensis-infected mice. Korean J Parasitol. 2009;47(4):345-351.
doi pubmed - Ross GD, Vetvicka V, Yan J, Xia Y, Vetvickova J. Therapeutic intervention with complement and beta-glucan in cancer. Immunopharmacology. 1999;42(1-3):61-74.
doi - Ikewaki N, Fujii N, Onaka T, Ikewaki S, Inoko H. Immunological actions of Sophy beta-glucan (beta-1,3-1,6 glucan), currently available commercially as a health food supplement. Microbiol Immunol. 2007;51(9):861-873.
doi pubmed - Prompetchara E, Ketloy C, Palaga T. Immune responses in COVID-19 and potential vaccines: Lessons learned from SARS and MERS epidemic. Asian Pac J Allergy Immunol. 2020;38(1):1-9.
- Lauriola M, Pani A, Ippoliti G, Mortara A, Milighetti S, Mazen M, Perseghin G, et al. Effect of Combination Therapy of Hydroxychloroquine and Azithromycin on Mortality in Patients With COVID-19. Clin Transl Sci. 2020;13(6):1071-1076.
doi pubmed - Arshad S, Kilgore P, Chaudhry ZS, Jacobsen G, Wang DD, Huitsing K, Brar I, et al. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int J Infect Dis. 2020;97:396-403.
doi pubmed - Hung IF, Lung KC, Tso EY, Liu R, Chung TW, Chu MY, Ng YY, et al. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395(10238):1695-1704.
doi - ClinicalTrials.gov Identifier: NCT04492475. Adaptive COVID-19 Treatment Trial 3 (ACTT-3). 2020 National Library of Medicine (US). https://clinicaltrials.gov/ct2/show/NCT04492475.
- ClinicalTrials.gov Identifier: NCT04409262. A Study to Evaluate the Efficacy and Safety of Remdesivir Plus Tocilizumab Compared With Remdesivir Plus Placebo in Hospitalized Participants With Severe COVID-19 Pneumonia (REMDACTA). 2020 National Library of Medicine (US). https://clinicaltrials.gov/ct2/show/NCT04409262.
- ClinicalTrials.gov Identifier: NCT04546581. Inpatient Treatment With Anti-Coronavirus Immunoglobulin (ITAC). 2020 National Library of Medicine (US). https://clinicaltrials.gov/ct2/show/NCT04546581.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


