| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 12, Number 9, September 2020, pages 612-617
Serum Irisin Levels and Clinical Implication in Elderly Patients With Type 2 Diabetes Mellitus
Xianfa Xuana, e, Jincheng Linb, e, Yiqin Zhanga, Lina Zhoua, Liping Xua, Junlu Jiac, Benhua Zhaoc, Zhiyang Lind, Qiong Zhud, Lianmeng Lib, Ting Wua, Siyu Zhanga, Hanxiang Jiangb, e, f, Yuxin Wanga, e, f
aDepartment of Nephrology, The Second Affiliated Hospital of Xiamen Medical College, Xiamen, Fujian, China
bDepartment of General Practice, Guankou Hospital, Jimei District, Xiamen City, Fujian, China
cState Key Laboratory of Molecular Vaccinology and Molecular Diagnostics, School of Public Health, Xiamen University, Xiamen, Fujian, China
dClinical Laboratory, Guankou Hospital, Jimei District, Xiamen City, Fujian, China
eThey contributed equally to this work.
fCorresponding Author: Hanxiang Jiang, Department of General Practice, Guankou Hospital, Jimei District, Xiamen City, Fujian, China; Yuxin Wang, Department of Nephrology, The Second Affiliated Hospital of Xiamen Medical College, Xiamen, Fujian, China
Manuscript submitted June 10, 2020, accepted July 2, 2020, published online August 15, 2020
Short title: Serum Irisin in Elderly Patients With T2DM
doi: https://doi.org/10.14740/jocmr4261
| Abstract | ▴Top |
Background: The aim of this study is to evaluate the level and role of serum irisin in elderly patients with type 2 diabetes mellitus (T2DM) using case-control study.
Methods: A total of 71 patients with T2DM were selected as the case group according to the inclusion criteria and exclusion criteria; and the ratio of 1:1 was calculated according to the inclusion rate of the residents. The cohort established in Guankou Town, Jimei District, Xiamen City, Fujian Province, China and the residents of this cohort were selected at the age of 60 and above. A total of 71 healthy subjects were included as the control group with the same gender and the age with a difference of ± 5 years old. The clinical data of the subjects were collected to determine their previous history, blood pressure, body mass index (BMI), hemoglobin (HB), liver function test, renal function test, fasting blood glucose and serum lipid. The irisin level in serum was measured by enzyme-linked immunosorbent assay (ELISA). The data were analyzed by using SPSS17.0 software. Single factor analysis using Chi-square test or t-test was performed to compare the differences between T2DM patients with the control group of the general data, clinical indicators and irisin level in serum. Logistic regression was used to analyze the protective factors and risk factors of diabetes mellitus.
Results: The results of single factor analysis showed that the level of irisin in T2DM group was significantly lower than that in the control group (703.37 ± 241.51 ng/mL and 800.22 ± 275.59 ng/mL, respectively). The levels of BMI, systolic blood pressure (SBP), diastolic blood pressure (DBP), triglyceride (TG) and fasting plasma glucose (FPG) in T2DM group were higher than those in control group, and differences were statistically significant. Logistic regression analysis indicated that irisin may be a protective factor for type 2 diabetes (odds ratio (OR) = 0.997, 95% confidence interval (CI): 0.994 - 0.999).
Conclusions: The serum irisin level in T2DM group was significantly lower than that in control group, suggesting that irisin may be a protective factor for type 2 diabetes.
Keywords: Irisin; Type 2 diabetes mellitus; Elderly
| Introduction | ▴Top |
Type 2 diabetes mellitus (T2DM) has become a common global epidemic chronic endocrine and metabolic disease, which can cause a variety of acute and chronic complications and seriously endanger human health. It is one of the important public health problems in the world. Lack of exercise, obesity, dyslipidemia and insulin resistance are considered as the main risk factors for T2DM development. Irisin is a newly discovered muscle factor released by skeletal muscles after exercise in 2012 [1], which can induce the conversion of white fat with the function of storage into brown fat with the function of heat generation, increase energy consumption of the body, promote metabolism, and reduce body weight. As a result, irisin can improve insulin sensitivity. Studies have shown that reduced irisin level is related to insulin resistance and the occurrence of T2DM [2]. Arhire et al pointed out in a systemic review, that irisin is a new hope to better understand and mange obesity and metabolic syndrome especially diabetes [3]. Serum irisin level of T2DM patients is lower than that of normal individuals [4, 5], but there have been some controversial conclusions [6]. Moreover, there are few studies so far specifically focusing on serum irisin level of elderly people. Most recently, a study demonstrated that the irisin levels were significantly lower in patients with hypertension, T2DM, overweight and obesity than those in the controls [7].
To this end, we used case-control study method to analyze serum irisin levels and significance in elderly patients with T2DM, providing a theoretical basis for the prevention and treatment of T2DM and its complications such as diabetic nephropathy.
| Materials and Methods | ▴Top |
Subjects
A total of 71 patients with T2DM were selected from a study cohort of permanent residents aged over 60 years old. All participants were enrolled from five communities and 12 administrative villages in Guankou Town, Jimei District, Xiamen City, Fujian Province, China as case group (67.38 ± 4.85 years old, including 28 males and 43 females). Clinical manifestations and laboratory test results were in accordance with the 2011 edition of the American Diabetes Association (ADA) diagnostic criteria of T2DM, and other types of diabetes were ruled out. Healthy cohort individuals of the same sex and similar age (± 5 years old) were randomly selected as the control group (66.99 ± 4.66 years old, 28 males and 43 females) in a 1:1 ratio from the cohort. No metabolic diseases such as diabetes, hypertension, hyperlipidemia and obesity were found in the control group. None of the subjects had a recent hospitalization, other major diseases (such as liver and kidney insufficiency, tumor or immune system disease), or other major treatments (such as oral glucocorticoids). This study was approved by the Research Ethics Committee of our hospital, and all participants were informed of the purpose of the study in advance and approved to sign the consent forms. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Collection of clinical data
Physical examination data of all subjects were collected. Their general condition and medical history were recorded, and their height, weight and blood pressure (BP) including systolic BP (SBP) and diastolic BP (DBP) were measured. Body mass index (BMI) was calculated according to a formula: BMI = body weight (kg) divided by height (m) square (kg/m2). Peripheral blood was collected from the cubital vein of all subjects in the early morning (fasting and without exercise for more than 8 h). Some of them were used for the detection of hemoglobin (HB), liver function including alanine aminotransferase (ALT), aspartate aminotransferase (AST), albumin (ALB), renal function including serum creatinine (Cr), blood urea nitrogen (BUN), fasting plasma glucose (FPG), blood lipid (total cholesterol (CHOL), triglyceride (TG)) and other indicators. In addition, serum samples were left at room temperature for 2 h for centrifugation (1,000 g, 20 min) (to reduce the adhesion between red blood cells and serum and reduce the incidence of hemolysis during centrifugation). The upper serum was placed in an EP tube and stored in a freezer at -80 °C for detection of irisin level.
Detection of serum irisin level by enzyme-linked immunosorbent assay (ELISA)
Serum irisin levels were determined by ELISA using human ELISA kit which was purchased from Sino Best Biological Technology Co., Ltd. This kit was proved to be highly sensitive to human irisin. The sensitivity was 1.0 ng/mL, and the correlation coefficient R value between the standard linear regression and the expected concentration was not lower than 0.9900.
Statistical analysis
SPSS17.0 statistical software was used for data analysis. For measurement data, mean ± standard deviation (SD) was used. Univariate analysis: continuous variables were tested by t-test; categorical variables were tested by χ2 (dichotomous variables). The influencing factors of diabetes were analyzed by logistic regression. All the statistics were bilateral tests, and P < 0.05 was considered as statistically significant.
| Results | ▴Top |
Comparison of irisin level and clinical index between T2DM group and control group
Irisin level of T2DM group was lower than that of control group (703.37 ± 241.51 ng/mL and 800.22 ± 275.59 ng/mL, respectively), and the difference was statistically significant. BMI, SBP, DBP, TG and FPG in the T2DM group were higher than those in the control group, with statistically significant differences (P < 0.05 for each comparison). There was no significant difference in HB, ALT, AST, ALB, Cr, BUN and CHOL between the two groups (P > 0.05) (Fig. 1, Tables 1 and 2).
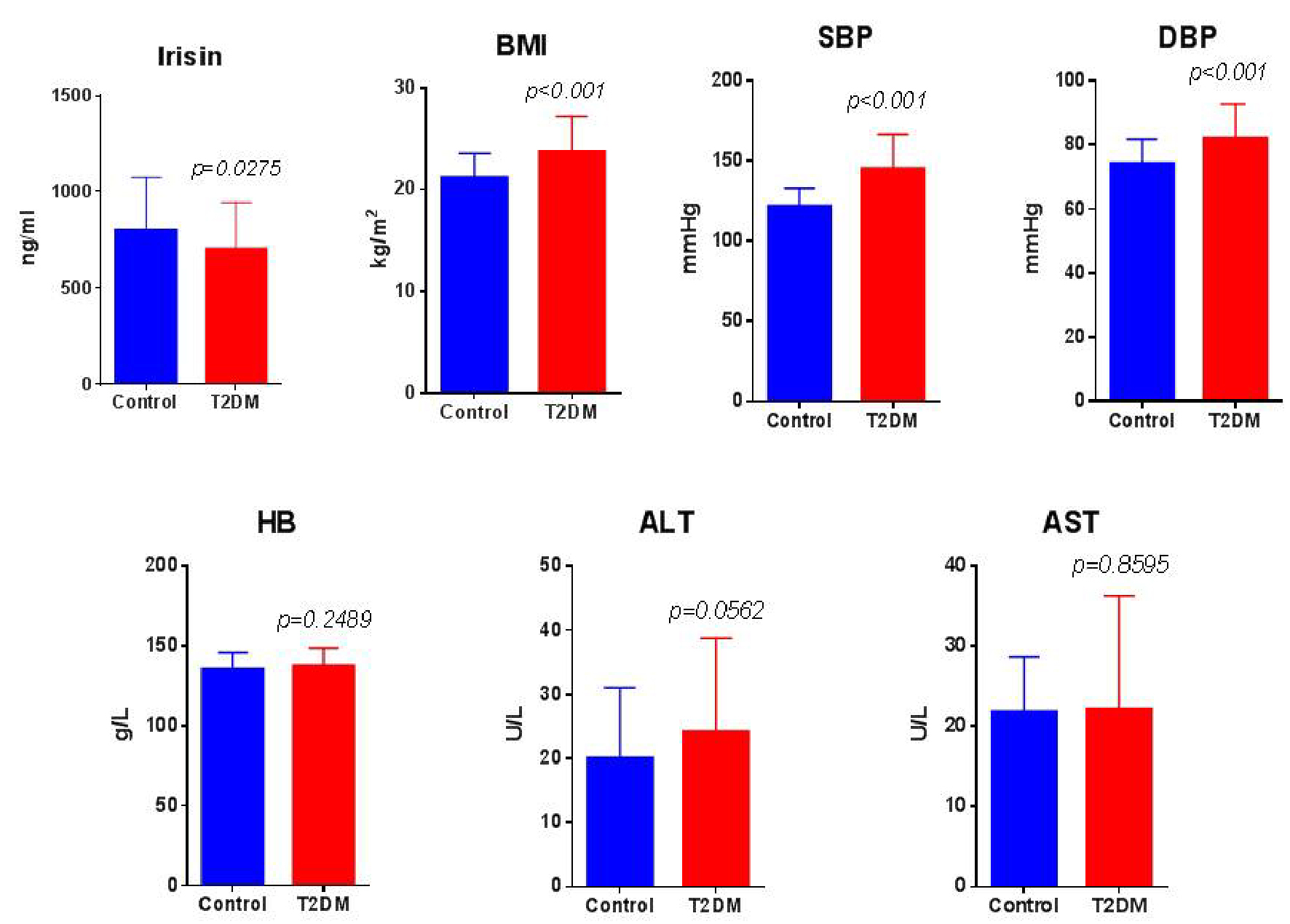 Click for large image | Figure 1. Irisin level in serum and clinical parameters between T2DM group and control group. Comparisons of irisin (ng/mL), BMI (kg/m2), systolic and diastolic blood pressure (SBP and DBP, mm Hg), hemoglobin (HB, g/L), alanine aminotransferase (ALT) and aspartate aminotransferase (AST, U/L) between groups. T2DM group: n = 71, and control group: n = 71. BMI: body mass index; T2DM: type 2 diabetes mellitus. |
 Click to view | Table 1. Comparison of Irisin Level and Clinical Indices Between T2DM Group and Control Group |
 Click to view | Table 2. Comparison of Laboratory Test Parameters Between T2DM Group and Control Group |
Logistic regression analysis of influencing factors of diabetes
The step by logistic regression analysis was conducted with or without diabetes as the dependent variable (Y) and serum irisin level, BMI, SBP, DBP, HB, ALT, AST, ALB, Cr, BUN, CHOL, TG and FPG as the independent variables (X). The results showed that irisin may be a protective factor for the development of diabetes mellitus (odds ratio (OR) = 0.997, 95% confidence interval (CI): 0.994 - 0.999). SBP and FPG may be risk factors for diabetes (SBP OR = 1.122, 95% CI: 1.063 - 1.183; and FPG OR = 5.950, 95% CI: 2.525 - 14.020), as shown in Table 3.
 Click to view | Table 3. Logistic Regression Analysis of Influencing Factors of Diabetes Mellitus |
| Discussion | ▴Top |
With the aging, economic development and changes in people’s lifestyle and dietary habits, the incidence and prevalence of diabetes are getting higher and higher. According to a report in 2013 [8], a survey conducted in 2010 by Ruijin Hospital Affiliated to Shanghai Jiao Tong University showed that the prevalence rate of diabetes among Chinese people over the age of 18 was 11.6% (the World Health Organization (WHO)1999 diagnostic criteria were applied), among which the majority was T2DM. Diabetes can cause a variety of acute and chronic complications, and the incidence, prevalence and mortality of complications are also increasing, seriously affecting the quality of life and survival of patients. In addition, high incidence of T2DM causing a huge social and economic burden, has become an urgent public health problem in China and even in the world.
Although current studies agree that exercise has a protective effect on chronic noncommunicable diseases such as diabetes, the mechanisms underlying the health benefits of exercise are not entirely clear. In early 2012, professor Bostrom [1] from Harvard Medical School reported that exercise can induce peroxisome proliferator-activated receptor gamma coactivator-1-alpha (PGC-1α) expression; and activation of PGC-1α can increase skeletal muscle fibronectin type III domain containing 5 (FNDC5) gene expression, and its expression product characteristics for type I membrane protein structure, after cutting and being modified to form a molecular weight of 12 kDa, consisting of 112 amino acid polypeptide, which is called irisin. The FNDC5 gene is released from skeletal muscle mainly after exercise or cold [9, 10]. Irisin can be secreted, activated and transferred to multiple tissues or organs to perform their corresponding physiological functions. For example, it can induce increased expression of uncoupling protein-1, transform white fat into brown fat with catabolic features, increase energy consumption and glucose utilization, effectively improve insulin resistance, and coordinate the treatment of metabolic related diseases such as obesity and type 2 diabetes [11, 12]. Therefore, exercise can improve insulin resistance and play a hypoglycemic effect, which is speculated to be related to exercise enhancing skeletal muscle secretion of irisin.
Our study found that the irisin level of the T2DM group was lower than that of the control group, which was consistent with the results of most studies [4, 5, 13, 14]. As a 1:1 ratio, health cohort members with the same sex and age difference of ± 5 years were selected as the control group, and the possible influence of gender and age was excluded. Although the mechanism of reduced irisin levels in patients with T2DM is not fully understood; however, some scholars believe that reduced PGC-1α activity in the muscle tissue of patients with T2DM can explain the decreased irisin level in patients with T2DM, which has been observed before the discovery of irisin [15]. Reduced activity of PGC-1 in muscular tissue of T2DM patients resulted in reduced FNDC5 synthesis and reduced irisin production. In addition, insulin resistance led to hyperglycemia and an increase in circulating free fatty acids, which also led to a decrease in PGC-1α activity [16]. However, Rana et al [6] found that irisin level was elevated in patients with T2DM. In this study cohort, the BMI of T2DM patients was significantly increased (mean BMI: 31.5 ± 5.4 kg/m2), the average body fat percentage and the percentage of abdominal (trunk) fat were higher, and the irisin level was strongly correlated with obesity (including trunk fat percentage). The level of circulating irisin in their study cohort might be determined by the degree of obesity. Therefore, differences in circulating irisin levels between studies may be at least partly related to these phenotypic variables. Differences such as race and diet may also play a role. It should also be noted that the determination of irisin in biological samples by immunological methods is not entirely without controversy. In fact, the reported level of irisin in serum or plasma varies largely dependent upon the method of detection [17]. Therefore, there is currently no universally accepted reference range for circulating irisin levels in normal healthy people.
Irisin is currently believed to be associated with a range of metabolically related diseases, including obesity, T2DM and cardiovascular disease [18]. Irisin is involved in regulating the mitochondrial function of muscle cells, the expression of FNDC5 gene and other metabolic pathways [19]. In vitro and animal studies have shown that irisin has direct and indirect effects on metabolic pathways, mainly on adipose tissue, muscle and liver. First of all, in adipose tissue, irisin can enhance glucose uptake [20], stimulate fat breakdown, but inhibit fat accumulation [21]. Secondly, in muscular tissue, irisin promotes glucose uptake, lipid uptake and metabolism increase, and reduces glycogen decomposition and gluconeogenesis [22]. In hepatocytes, irisin has been shown to reduce oxidative stress [23], promote sugar production and inhibit gluconeogenesis [24], reduce fat production and lipid accumulation [23]. In addition, studies have shown that irisin can specifically promote the generation of β cells from the pancreas in mice and increase the amount of these cells [25].
Majority of current studies showed decreased irisin concentrations in patients with T2DM regardless of the time of diagnosis with or without treatment and even lower concentration in the presence of complications of T2DM [26]. Shoukry et al demonstrated that circulating serum irisin levels were increased in obese nondiabetic subjects, while decreased in T2DM patients. Furthermore, serum irisin levels were correlated with anthropometric and metabolic markers of obesity and T2DM [27]. However, a few studies showed that plasma irisin levels of resting and overnight-fasting were significantly higher in T2DM patients compared with healthy volunteers, suggesting that elevated plasma irisin in T2DM is associated with indices of adiposity, and that irisin may be involved in pro-atherogenic endothelial disturbances accompanying by obesity and T2DM. Irisin may indicate a potentially novel therapeutic approach in the field of obesity and diabetes [6]. Circulating irisin level was reported to increase in the patients with metabolic syndrome compared with healthy controls [28].
FPG is the fastest and simplest test used to diagnose diabetes. It also plays a role in ongoing monitoring of blood glucose control for patients with T2DM [29]. However, FPG is not the best indicator of diabetes risk. Nowadays evidence has shown that irisin is a novel metabolic biomarker [26]. Most published studies showed decreased irisin concentrations in patients with T2DM regardless of the time of diagnosis and whether they are undergoing any treatment, suggesting that irisin may be a novel risk factor of patients with diabetes.
Taken together, considering these beneficial effects, irisin serves as a potential new target for improving insulin resistance and treating T2DM [30, 31]; it may be used in the treatment of T2DM patient with insulin resistance [32]. In our study, logistic regression analysis also indicated that serum irisin level was a protective factor for diabetes mellitus. Decreased irisin level in T2DM population may lead to reduced glucose uptake by muscle, aggravation of lipid metabolism disorder, promotion of lipid production and accumulation, thus resulting in an increase of the cardiovascular risk in T2DM patients.
In conclusion, the benefits of exercise for metabolic diseases such as diabetes and obesity are well recognized, and it is tempting to use the newly discovered irisin pathway to achieve similar results. However, the exact mechanism of irisin regulation and release from muscle and adipose tissue and the multiple roles of circulating irisin in affecting nutrient homeostasis and endothelial function still need to be further elucidated in larger clinical studies and further basic studies, so that irisin may play potential roles in the treatment of obesity and T2DM.
Acknowledgments
This work was supported by Science and Technology Planning Project of Fujian Province (2018D0009, Dr. Xianfa Xuan), and Science and Technology Planning Project of Fujian Province (2019D015, Prof. Yuxin Wang). We would like to thank Xiamen Municipal Government for its financial support from Xiamen Municipal Health Committee.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consents were obtained.
Author Contributions
Xianfa Xuan and Jincheng Lin participated in the design of the study and wrote the manuscript; Yuxin Wang, Hanxiang Jiang and Benhua Zhao performed the statistical analysis and contributed to discussion; Yiqin Zhang, Junlu Jia, Lina Zhou, Liping Xu, Ting Wu and Siyu Zhang participated in the data collection and serum irisin detection; Lianmeng Li, Zhiyang Lin and Qiong Zhu performed blood tests. All authors have read and approved the final manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, et al. A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463-468.
doi pubmed - Choi YK, Kim MK, Bae KH, Seo HA, Jeong JY, Lee WK, Kim JG, et al. Serum irisin levels in new-onset type 2 diabetes. Diabetes Res Clin Pract. 2013;100(1):96-101.
doi pubmed - Arhire LI, Mihalache L, Covasa M. Irisin: a hope in understanding and managing obesity and metabolic syndrome. Front Endocrinol (Lausanne). 2019;10:524.
doi pubmed - Zhang C, Ding Z, Lv G, Li J, Zhou P, Zhang J. Lower irisin level in patients with type 2 diabetes mellitus: a case-control study and meta-analysis. J Diabetes. 2016;8(1):56-62.
doi pubmed - Elizondo-Montemayor L, Gonzalez-Gil AM, Tamez-Rivera O, Toledo-Salinas C, Peschard-Franco M, Rodriguez-Gutierrez NA, Silva-Platas C, et al. Association between Irisin, hs-CRP, and metabolic status in children and adolescents with type 2 diabetes mellitus. Mediators Inflamm. 2019;2019:6737318.
doi pubmed - Rana KS, Pararasa C, Afzal I, Nagel DA, Hill EJ, Bailey CJ, Griffiths HR, et al. Plasma irisin is elevated in type 2 diabetes and is associated with increased E-selectin levels. Cardiovasc Diabetol. 2017;16(1):147.
doi pubmed - Guo X, Xuan X, Zhao B, Wang Y, Zhong S, Su Y, Yu X, et al. Irisin in elderly people with hypertension, diabetes mellitus type 2, and overweight and obesity. Int J Diabetes Dev Ctries. 2020;40:196-202.
doi - Xu Y, Wang L, He J, Bi Y, Li M, Wang T, Wang L, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310(9):948-959.
doi pubmed - Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C, Perron RM, et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19(2):302-309.
doi pubmed - Panati K, Suneetha Y, Narala VR. Irisin/FNDC5 - an updated review. Eur Rev Med Pharmacol Sci. 2016;20(4):689-697.
- Rizk FH, Elshweikh SA, Abd El-Naby AY. Irisin levels in relation to metabolic and liver functions in Egyptian patients with metabolic syndrome. Can J Physiol Pharmacol. 2016;94(4):359-362.
doi pubmed - Jung UJ, Choi MS. Obesity and its metabolic complications: the role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int J Mol Sci. 2014;15(4):6184-6223.
doi pubmed - Liu JJ, Liu S, Wong MD, Tan CS, Tavintharan S, Sum CF, Lim SC. Relationship between circulating irisin, renal function and body composition in type 2 diabetes. J Diabetes Complications. 2014;28(2):208-213.
doi pubmed - Kurdiova T, Balaz M, Vician M, Maderova D, Vlcek M, Valkovic L, Srbecky M, et al. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: in vivo and in vitro studies. J Physiol. 2014;592(5):1091-1107.
doi pubmed - Mootha VK, Lindgren CM, Eriksson KF, Subramanian A, Sihag S, Lehar J, Puigserver P, et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34(3):267-273.
doi pubmed - Gamas L, Matafome P, Seica R. Irisin and myonectin regulation in the Insulin resistant muscle: implications to adipose tissue: muscle crosstalk. J Diabetes Res. 2015;2015:359159.
doi pubmed - Kalayci M. Preanalytical, analytical, and postanalytical errors in the measurement of irisin levels. Pol Arch Intern Med. 2017;127(9):643.
doi pubmed - Polyzos SA, Anastasilakis AD, Efstathiadou ZA, Makras P, Perakakis N, Kountouras J, Mantzoros CS. Irisin in metabolic diseases. Endocrine. 2018;59(2):260-274.
doi pubmed - Perakakis N, Triantafyllou GA, Fernandez-Real JM, Huh JY, Park KH, Seufert J, Mantzoros CS. Physiology and role of irisin in glucose homeostasis. Nat Rev Endocrinol. 2017;13(6):324-337.
doi pubmed - Huh JY, Dincer F, Mesfum E, Mantzoros CS. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int J Obes (Lond). 2014;38(12):1538-1544.
doi pubmed - Xiong XQ, Chen D, Sun HJ, Ding L, Wang JJ, Chen Q, Li YH, et al. FNDC5 overexpression and irisin ameliorate glucose/lipid metabolic derangements and enhance lipolysis in obesity. Biochim Biophys Acta. 2015;1852(9):1867-1875.
doi pubmed - Huh JY, Mougios V, Kabasakalis A, Fatouros I, Siopi A, Douroudos, II, Filippaios A, et al. Exercise-induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle metabolism through AMPK activation. J Clin Endocrinol Metab. 2014;99(11):E2154-2161.
doi pubmed - Park MJ, Kim DI, Choi JH, Heo YR, Park SH. New role of irisin in hepatocytes: The protective effect of hepatic steatosis in vitro. Cell Signal. 2015;27(9):1831-1839.
doi pubmed - Liu TY, Shi CX, Gao R, Sun HJ, Xiong XQ, Ding L, Chen Q, et al. Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3K/Akt pathway in type 2 diabetic mice and hepatocytes. Clin Sci (Lond). 2015;129(10):839-850.
doi pubmed - Liu S, Du F, Li X, Wang M, Duan R, Zhang J, Wu Y, et al. Effects and underlying mechanisms of irisin on the proliferation and apoptosis of pancreatic beta cells. PLoS One. 2017;12(4):e0175498.
doi pubmed - Martinez Munoz IY, Camarillo Romero EDS, Garduno Garcia JJ. Irisin a novel metabolic biomarker: present knowledge and future directions. Int J Endocrinol. 2018;2018:7816806.
doi pubmed - Shoukry A, Shalaby SM, El-Arabi Bdeer S, Mahmoud AA, Mousa MM, Khalifa A. Circulating serum irisin levels in obesity and type 2 diabetes mellitus. IUBMB Life. 2016;68(7):544-556.
doi pubmed - Tabak O, Simsek G, Erdenen F, Sozer V, Hasoglu T, Gelisgen R, Altunoglu E, et al. The relationship between circulating irisin, retinol binding protein-4, adiponectin and inflammatory mediators in patients with metabolic syndrome. Arch Endocrinol Metab. 2017;61(6):515-523.
doi pubmed - Hoyer A, Rathmann W, Kuss O. Utility of HbA1c and fasting plasma glucose for screening of Type 2 diabetes: a meta-analysis of full ROC curves. Diabet Med. 2018;35(3):317-322.
doi pubmed - Chen N, Li Q, Liu J, Jia S. Irisin, an exercise-induced myokine as a metabolic regulator: an updated narrative review. Diabetes Metab Res Rev. 2016;32(1):51-59.
doi pubmed - Leung PS. The potential of irisin as a therapeutic for diabetes. Future Med Chem. 2017;9(6):529-532.
doi pubmed - Gizaw M, Anandakumar P, Debela T. A review on the role of Irisin in insulin resistance and type 2 diabetes mellitus. J Pharmacopuncture. 2017;20(4):235-242.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


