| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Case Report
Volume 12, Number 7, July 2020, pages 454-457
First Reported Use of Highly Adsorptive Hemofilter in Critically Ill COVID-19 Patients in the USA
Sandeep Anand Padalaa, Anusha Vakitib, John Jason Whitea, Laura Mulloya, Azeem Mohammeda, c
aDivision of Nephrology, Department of Medicine, Medical College of Georgia, Augusta University, 1120 15th St, Augusta, GA 30912, USA
bDivision of Hematology-Oncology, Department of Medicine, Medical College of Georgia, Augusta University, 1120 15th St, Augusta, GA 30912, USA
cCorresponding Author: Azeem Mohammed, Division of Nephrology, Department of Medicine, Medical College of Georgia, Augusta University, 1120 15th St, Augusta, GA 30912, USA
Manuscript submitted May 21, 2020, accepted June 9, 2020, published online June 25, 2020
Short title: Highly Adsorptive Hemofilter in COVID-19 Patients
doi: https://doi.org/10.14740/jocmr4228
| Abstract | ▴Top |
Critically ill patients with coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) develop respiratory failure and septic shock. Extracorporeal blood purification is proposed as an adjuvant therapy for sepsis and aims at controlling the dysregulated autoimmune system. We describe our experience in treating COVID-19 patients with the oXiris® hemofilter which adsorbs both cytokines and endotoxins, provides renal replacement therapy and has anti-thrombogenic properties. It was approved by the US Food and Drug Administration (FDA) under emergency use authorization for COVID-19 patients in April 2020. In our study, the use of the oXiris® filter decreased levels of inflammatory markers including interleukin-6 (IL-6), erythrocyte sedimentation rate (ESR), and C-reactive protein (CRP), and improved clinical outcomes in two out of three patients.
Keywords: Adsorptive hemofilter; oXiris; COVID-19; Coronavirus 2; SARS-CoV-2; Acute kidney injury
| Introduction | ▴Top |
Critically ill patients with coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) develop hypoxic respiratory failure and septic shock [1]. SARS-CoV-2 infection may lead to rapid activation of the innate and adaptive immune systems resulting in cytokine storm and multi-organ dysfunction [2]. Extracorporeal blood purification is being explored as an adjuvant therapy for sepsis, aiming at controlling the dysregulated autoimmune system [3]. We describe the use of a highly adsorptive membrane oXiris® which is different from the traditional filters due to its unique four-in-one properties which include cytokine and endotoxin removal, renal replacement therapy, and anti-thrombogenic feature [3]. The oXiris® filter is designed by Baxter International and it was approved by the US Food and Drug Administration (FDA) in April 2020 under emergency use authorization to treat COVID-19 patients. Herein, we present our experience at the Augusta University Medical Center, and to our knowledge, these are the first reported cases in the USA.
| Case Reports | ▴Top |
Case 1
A 67-year-old African American man was admitted for management of hypoxic respiratory failure and septic shock. His medical history included hypertension, type 2 diabetes mellitus and stage 3 chronic kidney disease (baseline serum creatinine of 1.5 - 1.7 mg/dL). He presented to the emergency room (ER) with persistent fever, worsening respiratory distress and altered mental status of 5-day duration. Upon arrival, vitals included a temperature of 39.5 °C, respiratory rate of 26 breaths per minute, oxygen saturation of 90% on 50% ventimask, heart rate of 112 beats per minute and blood pressure of 160/92 mm Hg. Patient was intubated and transferred to the intensive care unit (ICU). Subsequently he developed septic shock requiring vasopressor support. He was initially treated with azithromycin, hydroxychloroquine and a single dose of tocilizumab. Three days later, he received convalescent plasma. Nephrology was consulted for oliguric acute kidney injury (AKI) with a rise in serum creatinine to 2.62 mg/dL. He was started on continuous veno-venous hemodiafiltration (CVVHDF) with regional citrate anticoagulation. After 7 days, the conventional filter was switched to the oXiris® filter for the next 72 h while continuing CVVHDF. Inflammatory markers including interleukin-6 (IL-6), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), ferritin and D-dimer were collected prior to and during therapy (Table 1). He suffered a cardiac arrest while receiving CVVHDF but was resuscitated successfully. CVVHDF was continued with traditional filter due to the limited supply of the oXiris® filter. Five days later, he suffered another cardiac arrest and could not be resuscitated.
 Click to view | Table 1. Laboratory Data |
Case 2
A 41-year-old Caucasian man with no prior medical problems was admitted for evaluation of fever, non-productive cough, and diarrhea of 3-day duration. Upon arrival, vitals included a temperature of 38.7 °C, respiratory rate of 22 breaths per minute, oxygen saturation of 85% on room air, heart rate of 90 beats per minute and blood pressure of 126/76 mm Hg. On physical examination, lung sounds were diminished bilaterally with minimal crackles. Nasopharyngeal swab was positive for SARS-CoV-2 via polymerase chain reaction (PCR). Treatment was initiated with azithromycin and hydroxychloroquine. The following day, he received a single dose of tocilizumab. He subsequently developed hemodynamic compromise and was transferred to the ICU for vasopressor support and mechanical ventilation. Convalescent plasma was given upon admission to the unit. Two days later, he was started on empiric CVVHDF using the oXiris® filter for 72 h with effluent rates of 35 mL/kg/h and was maintained on an even fluid balance (0 mL net ultrafiltration rate). Despite using regional citrate anticoagulation, the filter clotted within 3 h after initiation of therapy. This was resolved by initiating systemic heparin and using large pre-filter replacement fluid volume. Inflammatory markers were collected prior to and during therapy (Table 1). Patient developed massive pulmonary embolism and was treated with catheter directed thrombectomy. CVVHDF was terminated due to unavailability of the filter. He was gradually weaned off vasopressors and was extubated 4 days later.
Case 3
A 44-year-old African American woman was admitted for the management of hypoxic respiratory failure and altered sensorium. Her medical history included hypertension and diabetes mellitus. A day prior to admission, she tested positive for SARS-CoV-2 via PCR. Upon arrival to the ER, vitals included a temperature of 39.3 °C, respiratory rate of 30 breaths per minute, oxygen saturation of 89% on a non-rebreather, heart rate of 124 beats per minute and blood pressure of 75/43 mm Hg. Patient was intubated, initiated on vasopressors, and transferred to the ICU and was treated with azithromycin, hydroxychloroquine, and a single dose of tocilizumab. Patient did not receive convalescent plasma as it was not available at that point. Over the course of the next 3 weeks, there was no improvement in her clinical status. She was then started on empiric CVVHDF using the oXiris® filter and was maintained on even fluid balance (0 mL net ultrafiltration rate). Inflammatory markers were collected prior to and during therapy (Table 1). CVVHDF remained uneventful for a period of 72 h after which it was terminated due to unavailability of the filter. Over the next 5 days patient improved clinically and was extubated.
| Discussion | ▴Top |
Approximately 67% of the severely ill patients with COVID-19 may experience multi-organ dysfunction due to cytokine storm [4]. Targeting these pro-inflammatory cytokines with monoclonal antibodies such as tocilizumab is currently being used to mitigate the multi-organ dysfunction from COVID-19 [2].
An alternative therapeutic approach involves removing the inflammatory mediators via extracorporeal blood purification [5]. Although several cytokine or endotoxin removing modalities exist, we used the oXiris® membrane that removes both cytokines and endotoxins. Shum et al reported that Sequential Organ Failure Assessment (SOFA) score decreased significantly by 37% in six patients treated with CVVHDF and oXiris® versus an increase of 3% in 24 patients treated with CVVHDF and a high-flux hemofilter at 48 h interval [6]. Broman et al reported the outcomes of 16 patients requiring continuous renal replacement therapy (CRRT) for septic shock-associated AKI and the use of the oXiris® filter allowed effective removal of endotoxins and IL-6 [7]. Ma et al reported significant decline in the inflammatory markers in three critically ill COVID-19 patients when treated with plasma exchange and the oXiris® filter [8].
To date, oXiris® filter is the only commercially available filter in the USA that can be used to perform multiple blood purification therapies simultaneously along with CRRT. It consists of three layers, and the first layer is composed of polyacrylonitrile copolymer made up of acrylonitrile and methallylsulfonate. The negative charge of the sulfonate helps in adsorbing the cytokines. The second layer is composed of positively charged polyethyleneimine, which adsorbs the endotoxins. The third layer is pregrafted with heparin conferring antithrombogenic property (Fig. 1) [3].
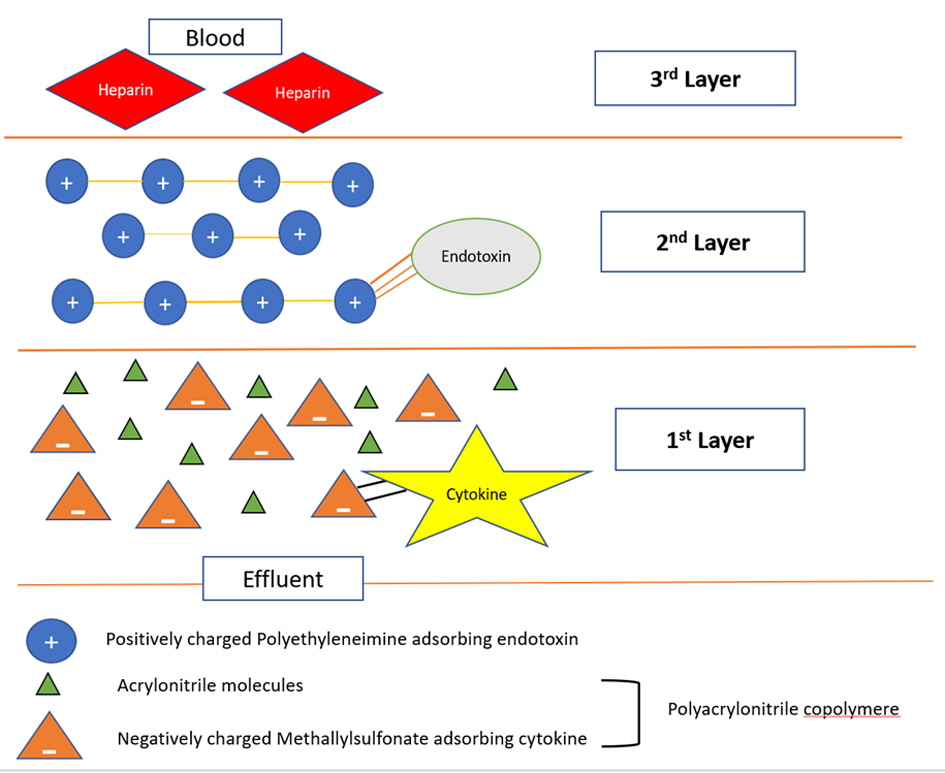 Click for large image | Figure 1. Cross-section of the oXiris filter. The first layer is composed of a polyacrylonitrile copolymer made up of acrylonitrile and methallylsulfonate. The negative charge of the sulfonate helps in adsorbing the cytokines. The second layer is composed of positively charged polyethyleneimine, which adsorbs the endotoxins. The third layer is pregrafted with heparin conferring antithrombogenic property. |
In the present study, despite using conventional therapies for COVID-19, none of the patients showed much clinical improvement. Although the level of IL-6 was not checked prior to initiation of therapy, use of the oXiris® filter resulted in a significant decline in inflammatory markers, especially when used empirically in patients 2 and 3. Patient 2 showed a decline in ESR, CRP and D-dimer levels. Patient 3 showed a decline in CRP and IL-6 levels. After empiric CVVHDF with the oXiris® filter to blunt the immune response, both patients showed gradual improvement in clinical status and were successfully extubated. Patient 1 was on CVVHDF with conventional filter for 1 week and then switched to the oXiris® filter later in the disease process. IL-6 levels initially decreased but rose after cardiac arrest. It is unknown if the clinical outcome would have been different if the oXiris® filter would have been started earlier in patient 1.
None of the patients exhibited untoward side effects to the oXiris® filter. There was a minimal increase in cost with the oXiris filter but with no increase in nursing workload, as compared to a standard CRRT session. A history of heparin allergy is an absolute contraindication to using this filter. The adherence of inflammatory mediators to the filter membrane may lead to increased filter clogging and interruptions in therapy. This can be mitigated by frequent filter changes, large effluent flow rates (35 mL/kg/h of which half was used as pre-filter replacement fluid) and the use of systemic heparin anticoagulation. Despite encouraging findings in our patients, continued use of the filter was limited due to the relative shortage of filters.
Conclusions
Early initiation of CVVHDF with the oXiris® filter in critically ill COVID-19 patients may result in decline in inflammatory markers and prevent multi-organ dysfunction from cytokine storm. In the era of the COVID-19 pandemic, extracorporeal blood purification therapies are of interest and large randomized studies are needed to validate the findings of our study.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consents were obtained with the subjects prior to the therapy.
Author Contributions
SAP, AV and AM contributed to conception and design; SAP, JJW, LM, AV and AM contributed to analysis and interpretation, drafting and critical revision of the article; SAP, JJW, LM, AV and AM were responsible for final approval of the article.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507-513.
doi - Zhang C, Wu Z, Li JW, Zhao H, Wang GQ. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55(5):105954.
doi pubmed - Monard C, Rimmele T, Ronco C. Extracorporeal blood purification therapies for sepsis. Blood Purif. 2019;47(Suppl 3):1-14.
doi - Ronco C, Reis T, De Rosa S. Coronavirus epidemic and extracorporeal therapies in intensive care: si vis pacem para bellum. Blood Purif. 2020;49(3):255-258.
doi pubmed - Rimmele T, Kellum JA. Clinical review: blood purification for sepsis. Crit Care. 2011;15(1):205.
doi pubmed - Shum HP, Chan KC, Kwan MC, Yan WW. Application of endotoxin and cytokine adsorption haemofilter in septic acute kidney injury due to Gram-negative bacterial infection. Hong Kong Med J. 2013;19(6):491-497.
doi pubmed - Broman ME, Hansson F, Vincent JL, Bodelsson M. Endotoxin and cytokine reducing properties of the oXiris membrane in patients with septic shock: A randomized crossover double-blind study. PLoS One. 2019;14(8):e0220444.
doi pubmed - Ma J, Xia P, Zhou Y, Liu Z, Zhou X, Wang J, Li T, et al. Potential effect of blood purification therapy in reducing cytokine storm as a late complication of critically ill COVID-19. Clin Immunol. 2020;214:108408.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


