| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website https://www.jocmr.org |
Original Article
Volume 13, Number 2, February 2021, pages 101-106
Evaluation of Gut Microbiota in Patients With Vulvovestibular Syndrome
Laura Codaa, Paola Cassisb, d, Stefania Angiolettib, Cristina Angelonib, Stefania Pilonia, c, Cristian Testab, d
aHealth Center Ginecea, Milan, Italy
bFunctional Point srl, Bergamo, Italy
cSan Raffaele Resnati Hospital, Milan, Italy
dCorresponding Author: Cristian Testa, Functional Point srl, Via dell’Industria 7, 24127 Bergamo, Italy; Paola Cassis, Functional Point srl, Via dell’Industria 7, 24127 Bergamo, Italy
Manuscript submitted October 29, 2020, accepted January 5, 2021, published online February 25, 2021
Short title: Role of Gut Dysbiosis in Vulvodynia
doi: https://doi.org/10.14740/jocmr4221
| Abstract | ▴Top |
Background: Vulvovestibular syndrome (VVS) or vulvodynia is a chronic, heterogeneous and multifactorial disease that dramatically affects women’s health and quality of life. Despite important advancements in understanding VVS etiology have been achieved in the past decades, VVS still remains an elusive and complex condition without identifiable causes and effective treatments. In the present observational, retrospective, case-control study, we sought to investigate whether gut dysbiosis developed in patients with VVS.
Methods: To this aim, we compared both bacterial and fungal composition in VVS patients (n = 74; 34.3 ± 10.9 years old) with those of women without gynecological symptoms (n = 13 healthy control; 38.3 ± 10.4 years old). Furthermore, to assess whether gut ecology may have an impact on gut function, the degree of intestinal inflammation (calprotectin levels) and gut permeability (zonulin levels) were also evaluated.
Results: VVS patient developed gut dysbiosis, mainly characterized by a significant increase of Escherichia coli along with increased colonization of mold/yeast compared to healthy controls. Furthermore, fecal levels of zonulin indicated that in VVS patients gut dysbiosis translated into increased gut permeability.
Conclusion: Our preliminary study, by demonstrating that alterations in gut microbiota and intestinal permeability are present in patients with VVS, highlights the novel notion that gut dysbiosis may be considered an important associated factor for VVS. These findings, if confirmed, may be clinically relevant and may help in choosing further diagnostic methods and more effective therapies for these patients.
Keywords: Vulvovestibular syndrome; Gut; Microbiota; Mycobiota; Gut permeability; Dysbiosis
| Introduction | ▴Top |
Vulvovestibular syndrome (VVS) or vulvodynia is a chronic vulvar pain condition lasting at least 3 months, without a clear identifiable cause, and resulting in significant impairment of sexual, relational and psychological functioning of affected women and their romantic partners [1]. The lifetime estimates prevalence of vulvodynia is 8-28% among reproductive-aged women in the general population [2-6]. This high prevalence translates into countless women searching an effective treatment and only 50% of them receive a formal diagnosis of VVS [2].
The pathogenesis of VVS is largely unknown and a wide range of possible etiological factors have been identified including inflammatory, genetic, musculoskeletal, neurosensory and neuropathic factors, making VVS not one disease, but a variety of symptoms of several diseases processes, that diversify the experience and clinical presentation of individual woman. Thus, in 2015, the International Society for the Study of Vulvovaginal Disease, the International Society for the Study of Women’s Sexual Health and the International Pelvic Pain Society adopted a new terminology for VVS by adding “potential associated factor” to the definition of the disease, rendering VVS being likely the result of multifactorial process. Because of the heterogeneity of women suffering from VVS, the identification of a widely accepted therapeutic strategy remains a significant challenge, and probably combining multiple approaches may be maximally beneficial. Up to now, treatment recommendations included pelvic floor therapy, pharmacological and psychological medications, and surgical interventions in some cases, but they are mainly targeted toward managing symptoms rather than toward a specific cause for the pathology [7-9].
Despite the great progress achieved in understanding the etiology and the underlying pathophysiology of VVS, much more attempts have to be done by clinicians and researchers in order to develop more specific and efficient diagnostic tests and therapies. Recent studies have focused on the analysis of vaginal microbiome, and there are indications of potential difference in vaginal microbiome of women with VVS compared to healthy women [10, 11]. In particular, a poor growth of Lactobacilli and an increased abundance of mould and yeast has been found in vaginal microbiome of patients with VVS [10]. Furthermore, population-based epidemiologic studies have identified an association between a history of vulvovaginal infections and the subsequent development of VVS, indicating that a vaginal insult following an infection may favor an immunological response with abnormal release of pro-inflammatory cytokines and chemokines in “susceptible” individuals [11-16].
In the last decades, many efforts have been made for investigating human microbiomes, and their impact on disease development and progression, and particular attention has been pointed toward gut microbiome. Of note, women suffering from VVS are up to three times more likely than healthy women to have one or more chronic pain conditions, including gastrointestinal disorders [17-20], and similarly, we found that gastrointestinal symptoms (i.e., gaseousness, constipation and colitis) are quite common in our cohort of women with VVS. Based on these evidence and findings that gut microbiota composition regulates gut behavior and may impact other organ functions [21, 22], we hypothesize that alterations in gut microbiota may occur in patients with VVS, and probably may participate in disease development and progression.
In the present study, we evaluated whether alterations in gut microbiota were present in women affected by VVS compared with healthy women, paving the way for considering the gut microbiota a potential associated factor in the pathogenesis of VVS, and thus a new and effective therapeutic target for patients.
| Materials and Methods | ▴Top |
This observational, retrospective and case-control study includes a total of 74 women (34.3 ± 10.9 years old) with clinical diagnosis of VVS who attended clinics at Health Center Ginecea, Milano, Italy. As control, 13 age-matched healthy women (38.3 ± 10.4 years old) with no previous medical history of VVS and no complaints, problems or current or past infectious in the genital tract were recruited (Table 1).
 Click to view | Table 1. Characteristics of VVS Patients |
This study was carried out in accordance with the Helsinki Declaration for research involving human subjects. The study was performed on the anonymized data of patients who signed an informed written consent form allowing for the anonymized use of their clinical data for research purposes. The study was approved by IRB.
The diagnosis of VVS was confirmed through the swab test performed with a cotton swab touched to the vaginal vestibule as previously reported [10]. Patients had dyspareunia treated with different topical or systemic therapies. The quantification of the intensity of the symptoms (vulvovaginal pain, burning pain, vulvovaginal dryness, pelvic floor muscle hypertonicity and vulvovaginal lesions) is determined by using the numeric rating scale (NRS). The severity of the symptoms was indicated by a number from 0 (no symptoms) to 10 (higher score) (Table 1).
The presence of specific comorbidity such as irritable bowel disease, anxiety and bladder pain syndrome (i.e., cystitis, urethritis and trigonitis) has been considered (Table 1).
In this study, the participants had not undergone any FANS, corticosteroids, antibiotics, antiviral and antifungal treatments. We also excluded participants undergoing pharmacotherapy for bacteria, as well as pregnant and lactating women, and women with gynecological diseases other than VVS.
Gut flora was determined by the quantification of bacteria (Lactobacillus spp., Bifidobacterium spp., Escherichia coli and Bacteroides spp.), mould and yeast in stools, as previously described [23]. Stool samples were collected with strikers and inserted into hermetic vials using a specific medium. The microbiota was measured after 48 h of incubation under proper conditions using a selective agar. Further proof of isolation was performed by using bacterial metabolic tests on isolated organisms through the Remel RapID ONE system (REMEL Inc., Santa Fe, USA). The results are expressed in colony-forming units per milliliter (CFU/mL) of stool. The test was performed by Functional Point (Bergamo, Italy), a clinical and virology laboratory that adheres to international quality control standards and is accredited as an official laboratory within the National Health System. The test coefficient of variation was < 9%.
Intestinal permeability was evaluated as a fecal zonulin concentration (ng/mL) using commercial ELISA kit (Zonulin (Stool) ELISA, DRG Instruments Gmbh, Germany). The normal amount of zonulin in feces of healthy subjects is considered to be < 60 ng/mL.
Calprotectin determination in stool samples was performed by an immunoenzymatic method and measured by Chorus TRIO instrument (DIESSE Diagnostica Senese S.p.A, Italy) according to the manufacturer’s instruction. Calprotectin values < 50 µg/g per stool sample were considered normal.
Statistical analysis
Any differences in variables between control healthy subjects and VVS patients were evaluated by using the non-parametric Mann-Whitney U test. Statistical significance was set at P < 0.05.
| Results | ▴Top |
The results of gut flora are showed in Table 2. Women with VVS had increased Bifidobacterium spp. and Escherichia coli (P < 0.05 and P < 0.0001 versus healthy control women, respectively), whereas Lactobacillus spp. and Bacteroides spp. were comparable among groups (Fig. 1, Table 2). Then, we move to evaluate whether VVS patients had an altered mycobiota composition. Our results showed that microbiological mould and yeast colonization increased significantly in stools of VVS women (P < 0.0001 versus healthy women) (Fig. 1, Table 2), but no differences were found in the development rate of Candida spp. between the two groups (Fig. 1, Table 2).
 Click to view | Table 2. Evaluation of Gut Flora in Healthy Patients (Controls) and VVS Patients |
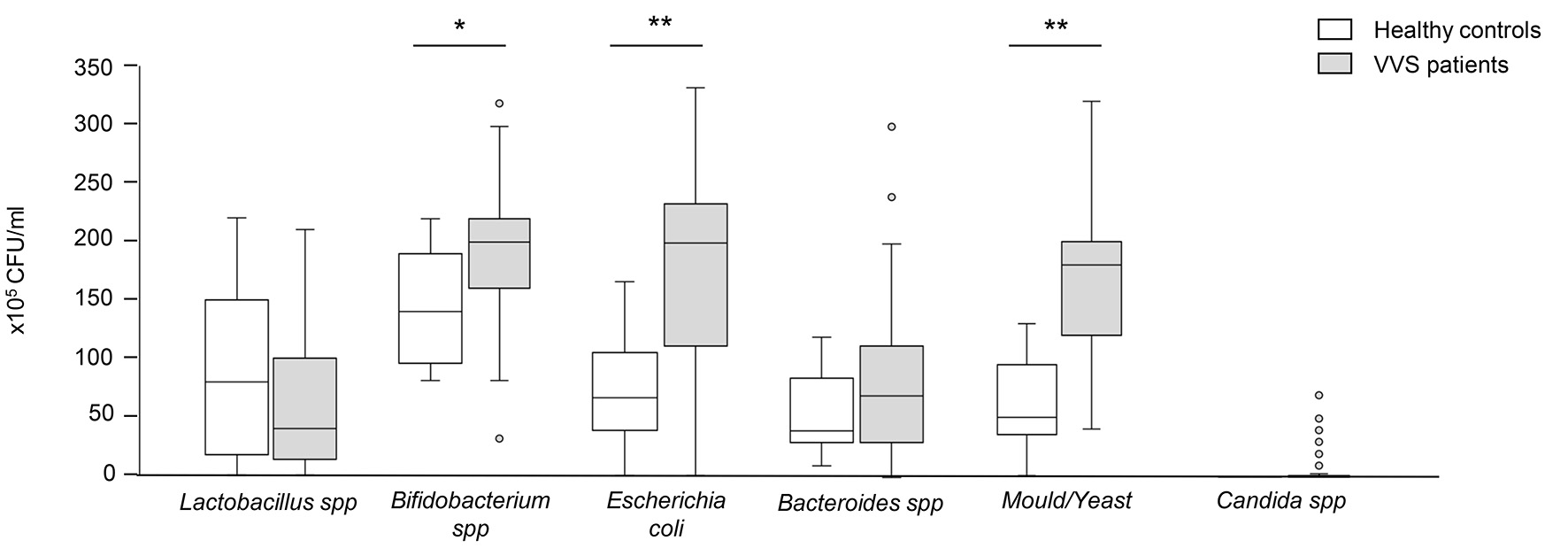 Click for large image | Figure 1. Evaluation of gut flora in healthy controls and VVS patients. The results are expressed in colony-forming units per milliliter (CFU/mL) of stool. Data are expressed as median and IQR, and were analyzed by the non-parametric Mann-Whitney U test. Asterisks indicate a significant difference between the two groups (*P < 0.05; **P < 0.0001). VVS: vulvovestibular syndrome; IQR: interquartile range. |
Of note, intestinal dysbiosis in VVS women was associated with altered gastrointestinal permeability as indicated by a significant increase of zonulin levels compared with healthy control women (P < 0.05, Fig. 2a). No differences were found in calprotectin levels between the two groups (Fig. 2b).
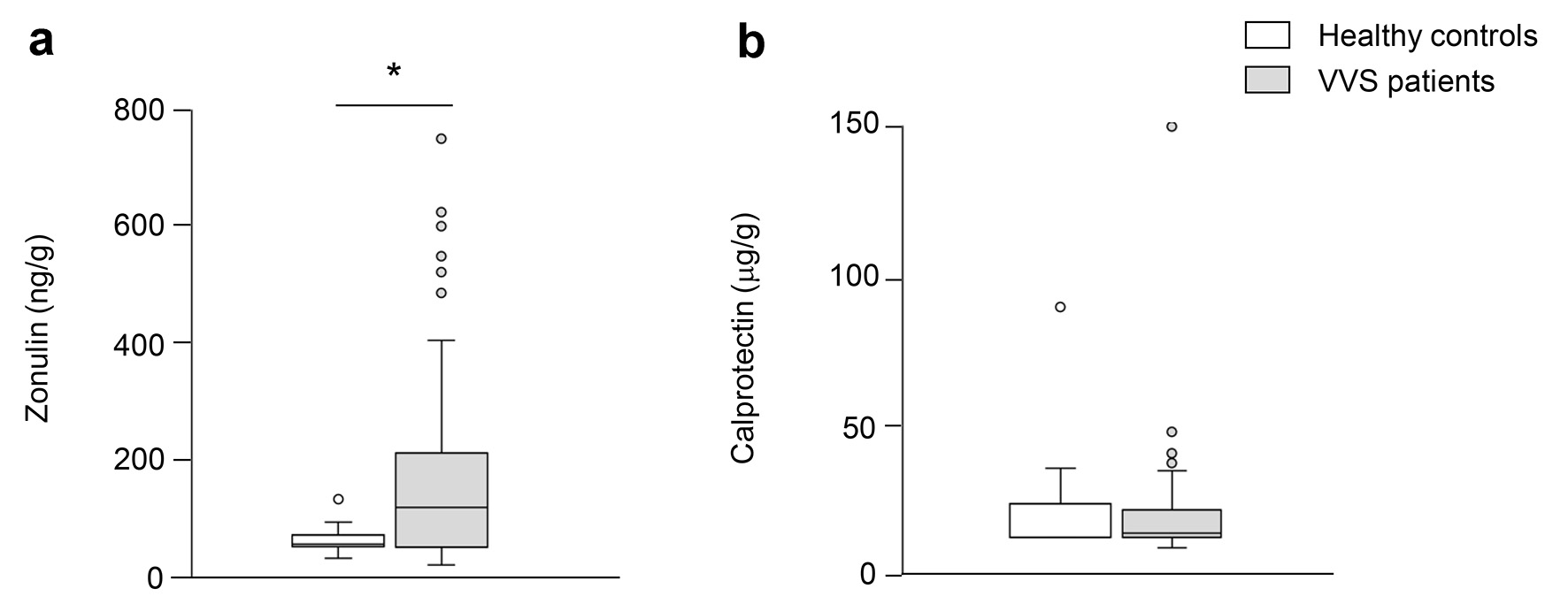 Click for large image | Figure 2. Evaluation of zonulin and calprotectin in healthy controls and VVS patients. Concentration of zonulin (a) and calprotectin (b) in fecal samples of healthy controls and VVS patients. Data are expressed as median and IQR, and were analyzed by the non-parametric Mann-Whitney U test. Asterisk indicates a significant difference between the two groups (P < 0.05). VVS: vulvovestibular syndrome; IQR: interquartile range. |
| Discussion | ▴Top |
In this study, we report for the first time that gut dysbiosis and dysfunction are present in patients with VVS. Specifically, we found that women suffering from VVS had enhanced colonization of opportunistic bacteria and fungi, together with increased intestinal permeability.
VVS is commonly considered an inflammatory disease in which an initial vaginal insult followed by an inflammatory response induces peripheral and central pain sensitization, mucosa nerve fiber proliferation, hypertrophy, hyperplasia and increased local sensitivity [15, 24]. Despite no clear and identifiable causes exists for VVS, a variety of endogenous and environmental risk factors (i.e., pregnancy, lactation, genital tract infection, hormone levels, oral contraceptive, vaginal surgery, etc.), may trigger the chronic stimulation and/or proliferation of nerve fiber that translate into persistent vulvar pain. Considering the close correlation between inflammation and bacteria, one of the current avenue of research in VVS pathogenesis is focused on the analysis of vaginal microbiome. In our previous study, we have demonstrated that vaginal microbiome is altered in women with VVS [10], and other studies have suggested that vaginal dysbiosis could have a causative role in the development of inflammation in VVS patients [11, 25-27].
In the last decade, a role of gut microbiota in regulating various aspects of host physiology has been robustly emerged [21, 22, 28]. In particular, the brain-gut-microbiota axis has been found to be essential for promoting proper organ functions, to the extent that gut dysbiosis may also dictate the development of neurological and neuroinflammatory pathologies including several visceral pain conditions [29-31]. Evidence that VVS often coexists with other chronic somatic and visceral pain disorders such as irritable bowel syndrome (IBS), suggests the existence of a common etiology or shared mechanisms between these pathologies [17, 19, 31]. Based on this evidence, we sought to investigate whether gut microbiota composition and function are altered also in patients with VVS.
It has been recognized that gut dysbiosis originates from shifts in relative bacterial abundances, and such a perturbation may foster the expansion of otherwise low-abundance and harmful bacteria, thus contributing to disease progression. Our findings show that this phenomenon actually develops in women with VVS. Indeed, an overgrowth of Enterobacterial species such as Escherichia coli occurred in our cohort of VVS patients in face of a poor colonization of Lactobacillus spp. Previous studies have already reported an abnormal presence of intramucosal Escherichia coli or mucosa-associated Escherichia coli with invasive properties in many conditions of gut inflammation and excessive gut fermentation, such as IBS, obesity and celiac disease [32]. In line with this, several gastrointestinal symptoms (i.e., chronic abdominal pain, gaseousness, constipation and colitis) are quite common in our VVS patients.
An important finding of our study is that VVS patients had a significant increased growth of mould and yeast, and specifically of Aspergillus, Penicillin, Saccaromyces colonization. At variance, no significant differences in Candida spp. were found between VVS patients and healthy controls. Recent findings have demonstrated that mycobiota composition may regulate strictly gastrointestinal functions by producing mycotoxins that in turn may affect proliferation, differentiation and repair of intestinal epithelial cells [33, 34]. Mycotoxins may also alter the permeability of the gut epithelium by removing specific proteins of the tight junction (i.e., ZO-1, occludin and different claudin isoforms), that are essential for maintaining an adequate transport across the gut barrier [35]. Accordingly, we found that the mould/yeast overgrowth observed in women with VVS was associated with increased intestinal permeability, as indicated by the level of fecal zonulin. Importantly, an altered epithelial barrier integrity may foster toxins, bacterial, fungi and their metabolites to rapidly cross the intestinal barrier into the bloodstream, reach other organs and thus favor the development of systemic inflammation [36-39]. Furthermore, fungal components may directly elicit innate and adaptive immune response with the release of pro-inflammatory chemokines and cytokines, eventually resulting in the exacerbation of systemic inflammation [40]. Based on these findings, it is conceivable that gut dysbiosis and the consequent alterations in gut permeability may participate to the occurrence of vaginal dysbiosis and to the development of chronic submucosa vulvar inflammation in VVS patients [10]. Furthermore, findings that bacterial-derived factors may directly activate sensory neurons by acting on nociceptors [41, 42], suggest that the presence of specific bacteria in contact with visceral organs may evoke vulvar pain in VVS women.
Limitations
The main limitation of this study is that a direct cause-effect relationship between gut dysbiosis and VVS still remains to be demonstrated. Our data show that VVS patients experienced gut dysbiosis, but further studies are needed to undercover the role of gut pathogenic ecology in the development and progression of this pathology.
Conclusions
Here we demonstrate that in women with VVS, an increased opportunistic bacterial and fungal gut colonization occurs, and this translates into an altered gastrointestinal permeability. Collectively our results point out the possibility that gut microbiota dysbiosis may be considered a potential associated factor in the pathogenesis of VVS. If confirmed, our findings may help in identifying even more suitable and effective therapeutic approach for these patients.
Acknowledgments
We are grateful to our patients.
Financial Disclosure
The authors have no financial disclosure.
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
All enrolled patients gave consent for participating in the study.
Author Contributions
CT and LC conceived the study and discussed data. PC performed the experiments, discussed data and wrote the manuscript. SA performed the experiments, the data collection and discussed data. CA helped in data management and collection. SP provided participants’ characteristics. All authors critically commented on the manuscript.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Bornstein J, Goldstein AT, Stockdale CK, Bergeron S, Pukall C, Zolnoun D, Coady D, et al. 2015 ISSVD, ISSWSH, and IPPS consensus terminology and classification of persistent vulvar pain and vulvodynia. J Sex Med. 2016;13(4):607-612.
doi pubmed - Harlow BL, Stewart EG. A population-based assessment of chronic unexplained vulvar pain: have we underestimated the prevalence of vulvodynia? J Am Med Womens Assoc (1972). 2003;58(2):82-88.
- Arnold LD, Bachmann GA, Rosen R, Rhoads GG. Assessment of vulvodynia symptoms in a sample of US women: a prevalence survey with a nested case control study. Am J Obstet Gynecol. 2007;196(2):128 e121-126.
doi pubmed - Reed BD, Harlow SD, Sen A, Legocki LJ, Edwards RM, Arato N, Haefner HK. Prevalence and demographic characteristics of vulvodynia in a population-based sample. Am J Obstet Gynecol. 2012;206(2):170 e171-179.
doi pubmed - Reed BD, Crawford S, Couper M, Cave C, Haefner HK. Pain at the vulvar vestibule: a web-based survey. J Low Genit Tract Dis. 2004;8(1):48-57.
doi pubmed - Harlow BL, Kunitz CG, Nguyen RH, Rydell SA, Turner RM, MacLehose RF. Prevalence of symptoms consistent with a diagnosis of vulvodynia: population-based estimates from 2 geographic regions. Am J Obstet Gynecol. 2014;210(1):40 e41-48.
doi pubmed - Rosen NO, Dawson SJ, Brooks M, Kellogg-Spadt S. Treatment of vulvodynia: pharmacological and non-pharmacological approaches. Drugs. 2019;79(5):483-493.
doi pubmed - Goldstein AT, Pukall CF, Brown C, Bergeron S, Stein A, Kellogg-Spadt S. Vulvodynia: assessment and treatment. J Sex Med. 2016;13(4):572-590.
doi pubmed - Sadownik LA. Etiology, diagnosis, and clinical management of vulvodynia. Int J Womens Health. 2014;6:437-449.
doi pubmed - Vadala M, Testa C, Coda L, Angioletti S, Giuberti R, Laurino C, Palmieri B. Vulvovestibular syndrome and vaginal microbiome: a simple evaluation. J Clin Med Res. 2018;10(9):688-692.
doi pubmed - Ventolini G, Gygax SE, Adelson ME, Cool DR. Vulvodynia and fungal association: a preliminary report. Med Hypotheses. 2013;81(2):228-230.
doi pubmed - Pathak D, Agrawal S, Dhali TK. Prevalences of and risk factors for vulvar diseases in Nepal: a hospital-based study. Int J Dermatol. 2011;50(2):161-167.
doi pubmed - Edgardh K, Abdelnoor M. Vulvar vestibulitis and risk factors: a population-based case-control study in Oslo. Acta Derm Venereol. 2007;87(4):350-354.
doi pubmed - Nguyen RH, Swanson D, Harlow BL. Urogenital infections in relation to the occurrence of vulvodynia. J Reprod Med. 2009;54(6):385-392.
- Akopians AL, Rapkin AJ. Vulvodynia: The role of inflammation in the etiology of localized provoked pain of the vulvar vestibule (Vestibulodynia). Semin Reprod Med. 2015;33(4):239-245.
doi pubmed - Falsetta ML, Foster DC, Woeller CF, Pollock SJ, Bonham AD, Haidaris CG, Stodgell CJ, et al. Identification of novel mechanisms involved in generating localized vulvodynia pain. Am J Obstet Gynecol. 2015;213(1):38 e31-38 e12.
doi pubmed - Reed BD, Harlow SD, Sen A, Edwards RM, Chen D, Haefner HK. Relationship between vulvodynia and chronic comorbid pain conditions. Obstet Gynecol. 2012;120(1):145-151.
doi pubmed - Leusink P, Kaptheijns A, Laan E, van Boven K, Lagro-Janssen A. Comorbidities among women with vulvovaginal complaints in family practice. J Sex Med. 2016;13(2):220-225.
doi pubmed - Drummond J, Ford D, Daniel S, Meyerink T. Vulvodynia and irritable bowel syndrome treated with an elimination diet: a case report. Integr Med (Encinitas). 2016;15(4):42-47.
- Lamvu G, Nguyen RH, Burrows LJ, Rapkin A, Witzeman K, Marvel RP, Hutchins D, et al. The Evidence-based Vulvodynia Assessment Project. A national registry for the study of vulvodynia. J Reprod Med. 2015;60(5-6):223-235.
- Grenham S, Clarke G, Cryan JF, Dinan TG. Brain-gut-microbe communication in health and disease. Front Physiol. 2011;2:94.
doi pubmed - Martin CR, Osadchiy V, Kalani A, Mayer EA. The Brain-Gut-Microbiome Axis. Cell Mol Gastroenterol Hepatol. 2018;6(2):133-148.
doi pubmed - Pasini E, Aquilani R, Testa C, Baiardi P, Angioletti S, Boschi F, Verri M, et al. Pathogenic gut flora in patients with chronic heart failure. JACC Heart Fail. 2016;4(3):220-227.
doi pubmed - Bohm-Starke N. Medical and physical predictors of localized provoked vulvodynia. Acta Obstet Gynecol Scand. 2010;89(12):1504-1510.
doi pubmed - Havemann LM, Cool DR, Gagneux P, Markey MP, Yaklic JL, Maxwell RA, Iyer A, et al. Vulvodynia: What We Know and Where We Should Be Going. J Low Genit Tract Dis. 2017;21(2):150-156.
doi pubmed - Donders GGG, Bellen G, Ruban KS. Abnormal vaginal microbioma is associated with severity of localized provoked vulvodynia. Role of aerobic vaginitis and Candida in the pathogenesis of vulvodynia. Eur J Clin Microbiol Infect Dis. 2018;37(9):1679-1685.
doi pubmed - Farmer MA, Taylor AM, Bailey AL, Tuttle AH, MacIntyre LC, Milagrosa ZE, Crissman HP, et al. Repeated vulvovaginal fungal infections cause persistent pain in a mouse model of vulvodynia. Sci Transl Med. 2011;3(101):101ra191.
doi pubmed - Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev. 2010;90(3):859-904.
doi pubmed - Dworsky-Fried Z, Kerr BJ, Taylor AMW. Microbes, microglia, and pain. Neurobiol Pain. 2020;7:100045.
doi pubmed - Defaye M, Gervason S, Altier C, Berthon JY, Ardid D, Filaire E, Carvalho FA. Microbiota: a novel regulator of pain. J Neural Transm (Vienna). 2020;127(4):445-465.
doi pubmed - Guo R, Chen LH, Xing C, Liu T. Pain regulation by gut microbiota: molecular mechanisms and therapeutic potential. Br J Anaesth. 2019;123(5):637-654.
doi pubmed - Zeng MY, Inohara N, Nunez G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017;10(1):18-26.
doi pubmed - Bennett JW, Klich M. Mycotoxins. Clin Microbiol Rev. 2003;16(3):497-516.
doi pubmed - Grenier B, Applegate TJ. Modulation of intestinal functions following mycotoxin ingestion: meta-analysis of published experiments in animals. Toxins (Basel). 2013;5(2):396-430.
doi pubmed - McLaughlin J, Padfield PJ, Burt JP, O'Neill CA. Ochratoxin A increases permeability through tight junctions by removal of specific claudin isoforms. Am J Physiol Cell Physiol. 2004;287(5):C1412-1417.
doi pubmed - Fukata T, Sasai K, Baba E, Arakawa A. Effect of ochratoxin A on Salmonella typhimurium-challenged layer chickens. Avian Dis. 1996;40(4):924-926.
doi pubmed - Kubena LF, Phillips TD, Witzel DA, Heidelbaugh ND. Toxicity of ochratoxin A and penicillic acid to chicks. Bull Environ Contam Toxicol. 1984;32(6):711-716.
doi pubmed - Viggiano D, Ianiro G, Vanella G, Bibbo S, Bruno G, Simeone G, Mele G. Gut barrier in health and disease: focus on childhood. Eur Rev Med Pharmacol Sci. 2015;19(6):1077-1085.
- Underhill DM, Iliev ID. The mycobiota: interactions between commensal fungi and the host immune system. Nat Rev Immunol. 2014;14(6):405-416.
doi pubmed - Iliev ID, Leonardi I. Fungal dysbiosis: immunity and interactions at mucosal barriers. Nat Rev Immunol. 2017;17(10):635-646.
doi pubmed - van Thiel IAM, Botschuijver S, de Jonge WJ, Seppen J. Painful interactions: Microbial compounds and visceral pain. Biochim Biophys Acta Mol Basis Dis. 2020;1866(1):165534.
doi pubmed - Chiu IM, Heesters BA, Ghasemlou N, Von Hehn CA, Zhao F, Tran J, Wainger B, et al. Bacteria activate sensory neurons that modulate pain and inflammation. Nature. 2013;501(7465):52-57.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


