| Journal of Clinical Medicine Research, ISSN 1918-3003 print, 1918-3011 online, Open Access |
| Article copyright, the authors; Journal compilation copyright, J Clin Med Res and Elmer Press Inc |
| Journal website http://www.jocmr.org |
Original Article
Volume 11, Number 9, September 2019, pages 635-641
Change in the Antimicrobial Resistance Profile of Extended-Spectrum β-Lactamase-Producing Escherichia coli
Motoyasu Miyazakia, f, g, Yota Yamadaa, f, Koichi Matsuoa, Yukie Komiyab, Masanobu Uchiyamaa, Nobuhiko Nagatac, Tohru Takatad, Shiro Jimie, Osamu Imakyurea
aDepartment of Pharmacy, Fukuoka University Chikushi Hospital, 1-1-1 Zokumyoin, Chikushino-shi, Fukuoka 818-8502, Japan
bDepartment of Clinical Laboratory, Fukuoka University Chikushi Hospital, 1-1-1 Zokumyoin, Chikushino-shi, Fukuoka 818-8502, Japan
cDepartment of Respiratory Medicine, Fukuoka University Chikushi Hospital, 1-1-1 Zokumyoin, Chikushino-shi, Fukuoka 818-8502, Japan
dDepartment of Infection Control, Fukuoka University Hospital, 7-45-1 Nanakuma, Jonan-ku, Fukuoka 814-0180, Japan
eCentral Laboratory for Pathology and Morphology, Faculty of Medicine, Fukuoka University, 7-45-1 Nanakuma, Jonan-ku, Fukuoka 814-0180, Japan
fThese authors contributed equally to this work.
gCorresponding Author: Motoyasu Miyazaki, Department of Pharmacy, Fukuoka University Chikushi Hospital, 1-1-1 Zokumyoin, Chikushino-shi, Fukuoka 818-8502, Japan
Manuscript submitted July 12, 2019, accepted July 23, 2019
Short title: Antimicrobial Resistance Profile of ESBL-EC
doi: https://doi.org/10.14740/jocmr3928
| Abstract | ▴Top |
Background: This study aimed to investigate the trends and antimicrobial resistance profile of extended-spectrum β-lactamase-producing Escherichia coli (ESBL-EC) clinical isolates.
Methods: A total of 1,303 E. coli isolates from January 2012 to December 2017 at Fukuoka University Chikushi Hospital, Japan, were analyzed. The rate of resistance to cefmetazole (CMZ), flomoxef (FMOX), imipenem (IPM), meropenem (MEPM), amikacin (AMK), gentamicin (GM), minocycline (MINO), ciprofloxacin (CPFX), and levofloxacin (LVFX) was compared between non-ESBL-producing E. coli (non-ESBL-EC) and ESBL-EC.
Results: The proportion of ESBL-EC among all the E. coli isolates was 24.6% (320/1,303), and the proportion remained stable throughout the study period. There was no difference in the rate of resistance to CMZ, FMOX, IPM, MEPM, and AMK between non-ESBL-EC and ESBL-EC; however, the rate of resistance to GM, MINO, CPFX, and LVFX was higher in ESBL-EC than in non-ESBL-EC (17.5% vs. 10.0%, 19.1% vs. 7.7%, 87.5% vs. 24.2%, and 87.5% vs. 23.5%, respectively; P < 0.01). The rate of resistance to CPFX and LVFX in ESBL-EC increased throughout the study course. The rate of E. coli isolates susceptible to all the antibiotics was significantly higher in non-ESBL-EC than in ESBL-EC (68.2% vs. 7.5%; P < 0.01), and this rate decreased significantly from 10.0% in 2012 to 3.8% in 2017 in ESBL-EC (P < 0.01).
Conclusions: Our findings indicate a changing antimicrobial resistance profile of ESBL-EC, particularly to fluoroquinolones. Determination of the prevalence and antimicrobial resistance of ESBL-EC will help physicians in selecting the initial empirical treatment for patients with ESBL-EC infections.
Keywords: Escherichia coli; Extended-spectrum β-lactamase; Antimicrobial resistance profile; Fluoroquinolone resistance
| Introduction | ▴Top |
Extended-spectrum β-lactamases (ESBLs) can hydrolyze penicillins and oxyimino-cephalosporins, such as ceftazidime (CAZ), cefotaxime (CTX), and ceftriaxone, which have reportedly played important roles in the treatment of infections caused by Enterobacteriaceae, including Escherichia coli [1]. Recently, the prevalence of ESBL-producing E. coli (ESBL-EC) has been dramatically increasing worldwide [2-4]. In Japan, CTX-M-type ESBL-EC, which exhibits co-resistance to fluoroquinolones, has been detected more frequently than TEM- or SHV-type ESBL-EC [5, 6]. Infections caused by ESBL-EC are reportedly associated with poor clinical outcomes, inappropriate empirical antibiotic therapy, longer hospital stays, and greater hospital expenses [7-9].
Antimicrobial resistance patterns are often available for monitoring the endemicity of specific clones. In small- and medium-sized hospitals, antimicrobial resistance patterns are particularly useful for empiric therapy because it is difficult to routinely conduct genotyping in clinical laboratories. However, only few studies have been reported on trends and antimicrobial resistance patterns of ESBL-EC in limited regions. Because of the increasing clinical importance of ESBL-EC, we should carefully monitor the prevalence and antimicrobial resistance profile of ESBL-EC at our facility. Thus, the aim of this study was to investigate the trends of the rate of detection and antimicrobial resistance of ESBL-EC at our hospital.
| Methods | ▴Top |
Setting
Fukuoka University Chikushi Hospital is a 310-bed university-affiliated hospital located in the southwestern district of Japan. This study was approved by the Fukuoka University Medical Ethics Review Board (R18-014). This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Collection and identification of isolates
E. coli clinical isolates were recovered from inpatients and outpatients who visited Fukuoka University Chikushi Hospital between January 2012 and December 2017. Only one isolate per patient per year was included in this study. For those patients from whom more than one isolate was recovered, only the first isolate for which the results of antimicrobial susceptibility testing were available was included. The isolates were identified using the automated Vitek-2 system (Sysmex bioMerieux, Tokyo, Japan).
Antimicrobial susceptibility testing
Antimicrobial susceptibility testing was performed using the automated Vitek-2 system, following the manufacturer’s instructions. The breakpoint (susceptible, intermediate, or resistant) was determined according to the M100-S27 performance standards established by the Clinical and Laboratory Standards Institute (CLSI) [10]. Nine antimicrobial agents were used for susceptibility testing: cefmetazole (CMZ), flomoxef (FMOX), imipenem (IPM), meropenem (MEPM), amikacin (AMK), gentamicin (GM), minocycline (MINO), ciprofloxacin (CPFX), and levofloxacin (LVFX).
ESBL detection
ESBL detection was performed by the double disk diffusion using both CTX, CAZ, and cefpodoxime (CPDX) alone and in combination with clavulanic acid. An increase in zone size of greater than or equal to 5 mm for CTX, CAZ, and CPDX with and without clavulanic acid was taken as an indication of ESBL production.
Statistical analysis
Categorical variables were compared using the Chi-squared test or Fisher’s exact test, as appropriate. Temporal trends of antimicrobial resistance were evaluated using linear regression analysis. The JMP software program (version 10, SAS Institute Inc., Cary, NC, USA) was used for all the statistical analyses. P < 0.05 was considered statistically significant.
| Results | ▴Top |
Prevalence of ESBL-EC
A total of 2,146 E. coli clinical isolates were recovered between January 2012 and December 2017. Of those, 1,303 (60.7%) isolates were included in this study (Fig. 1). Further, 983 (75.4%) of the included 1,303 were non-ESBL-EC and 320 (24.6%) were ESBL-EC. The proportion of ESBL-EC among all the E. coli isolates was relatively stable throughout the study period (22.6-27.2%) (Fig. 2).
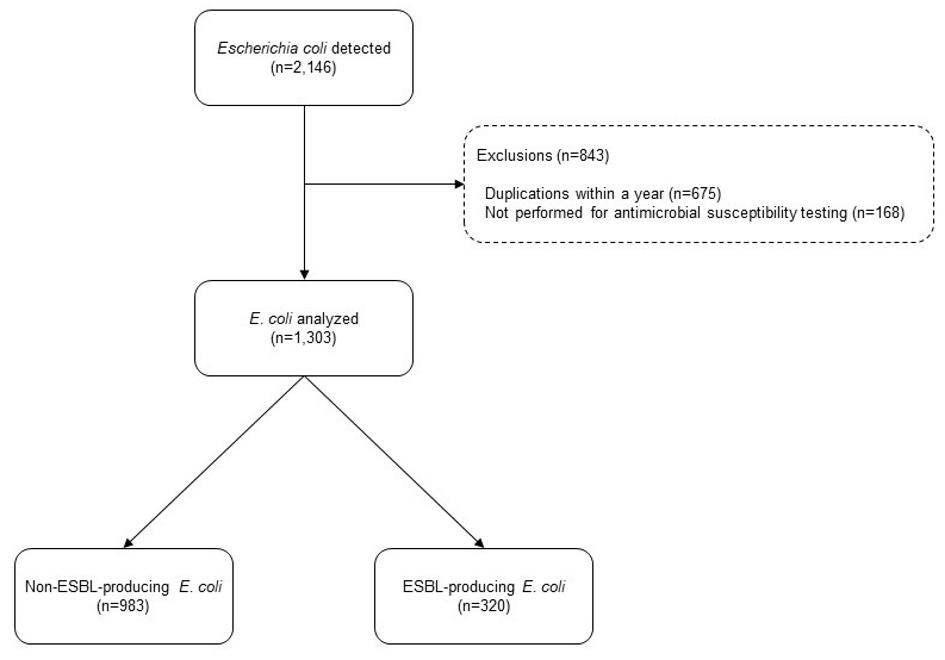 Click for large image | Figure 1. Samples analyzed in the present study. |
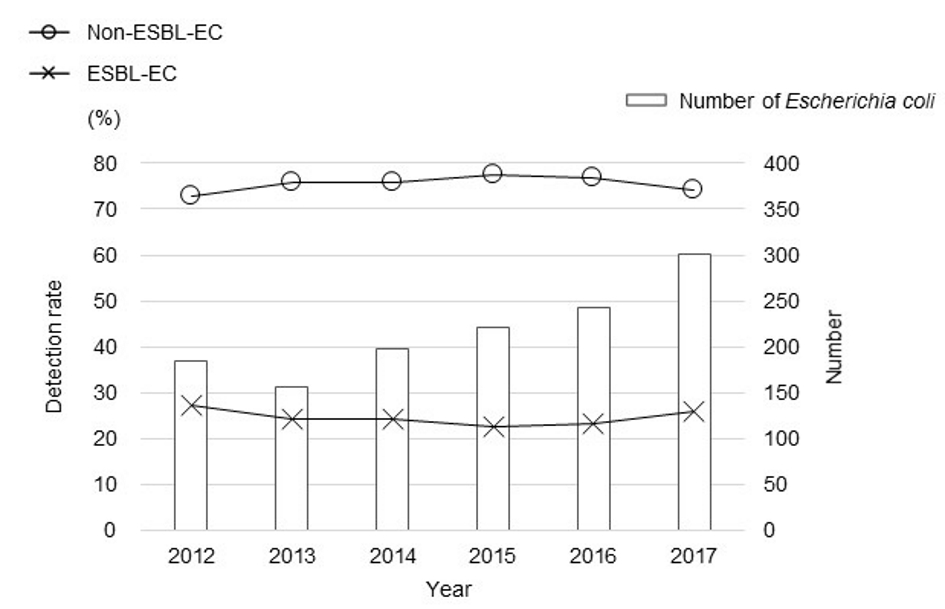 Click for large image | Figure 2. Yearly rate of detection of ESBL-EC among all the E. coli isolates. |
Antimicrobial resistance profile of non-ESBL-EC and ESBL-EC
Almost all the E. coli isolates were susceptible to CMZ, FMOX, IPM, MEPM, and AMK, and there was no difference between non-ESBL-EC and ESBL-EC in the rate of resistance to these antibiotics. However, the rate of resistance to GM, MINO, CPFX, and LVFX was significantly higher in ESBL-EC than in non-ESBL-EC (17.5% vs. 10.0%, 19.1% vs. 7.7%, 87.5% vs. 24.2%, and 87.5% vs. 23.5%, respectively; P < 0.01) (Fig. 3). In ESBL-EC, the rate of resistance to GM and MINO was stable throughout the study period, whereas that to CPFX and LVFX increased over the study period (P = 0.048 and P = 0.062, respectively) (Fig. 4).
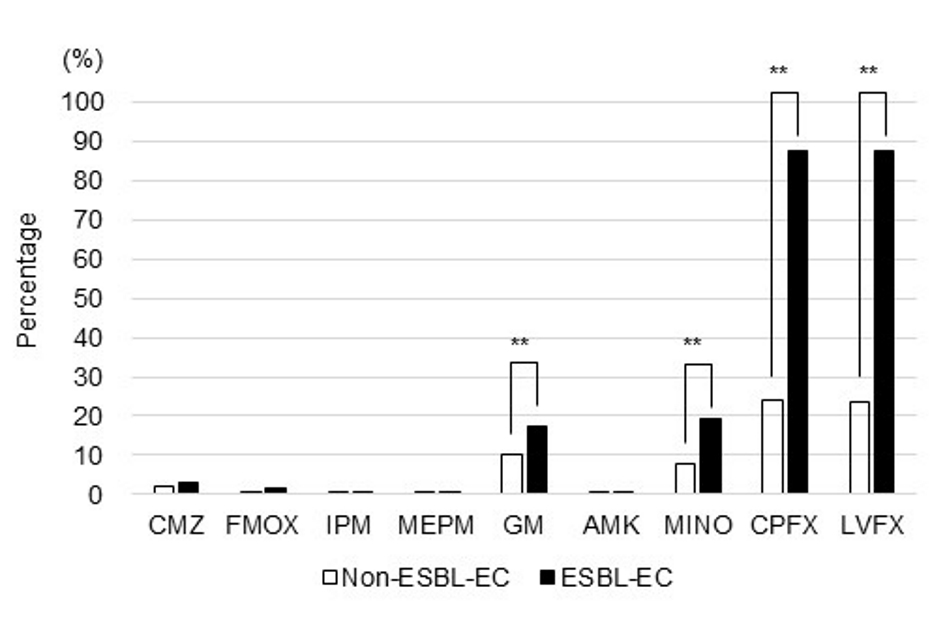 Click for large image | Figure 3. Antimicrobial resistance profiles of non-ESBL-EC and ESBL-EC (**P < 0.01). |
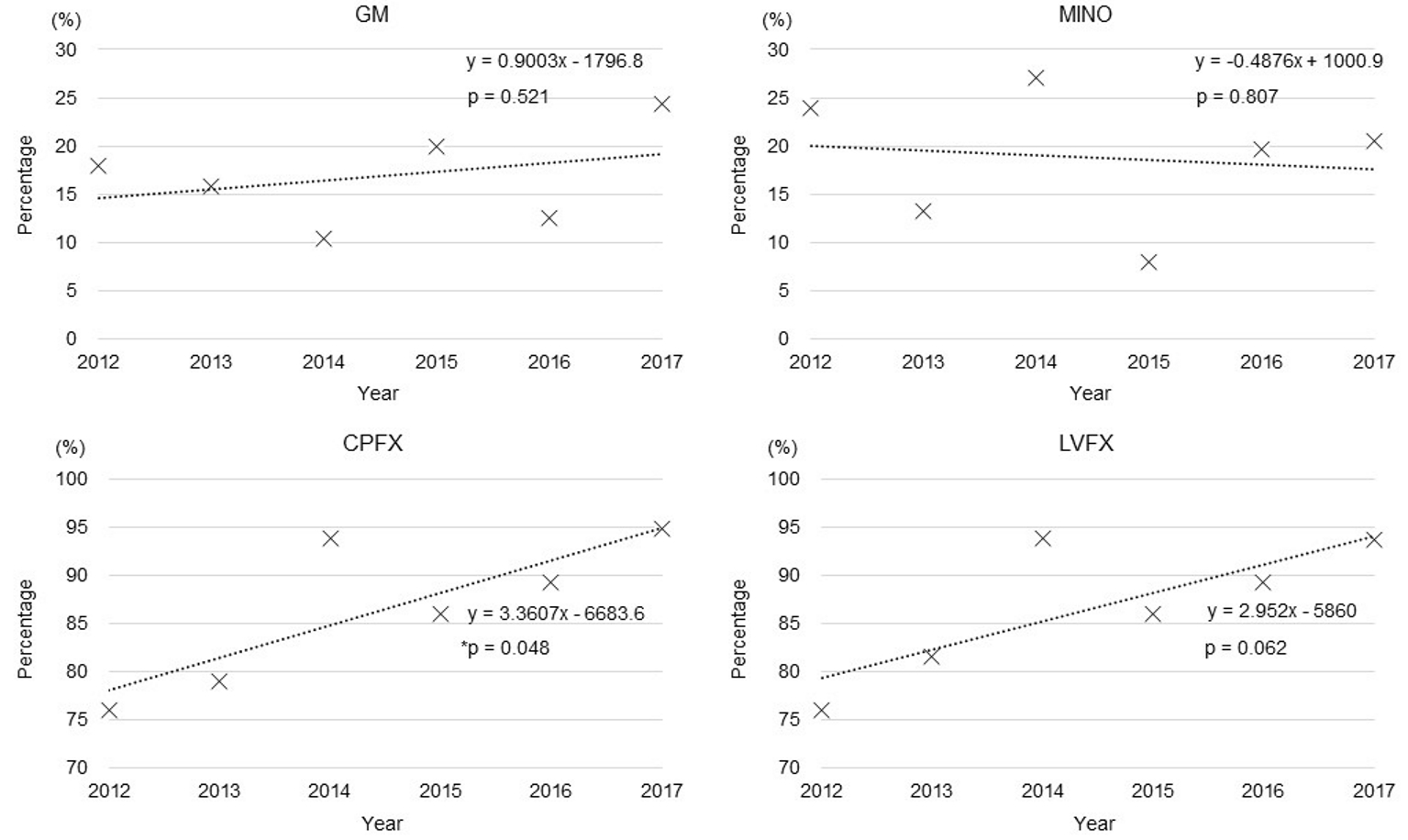 Click for large image | Figure 4. Trends in the rates of resistance of ESBL-EC for GM, MINO, CPFX, and LVFX (*P < 0.05). |
Change in the antimicrobial resistance pattern of ESBL-EC
A total of 34 resistance patterns were identified based on the resistance profile of the nine antibiotics in the E. coli isolates (pattern IDs: Null, I-a-f, II-a-h, III-a-i, IV-a-f, V-a-c, and VII) (Table 1). Of the 983 non-ESBL-EC isolates, the pattern ID Null (susceptible to all nine antibiotics) was the predominant resistance pattern (68.2%), followed by ID II-h (resistance to CPFX and LVFX), ID III-h (resistance to GM, CPFX, and LVFX), and ID I-e (resistance to MINO). Of the 320 ESBL-EC isolates, ID II-h (resistance to CPFX and LVFX) was the predominant resistance pattern (57.2%), followed by ID III-i (resistance to MINO, CPFX, and LVFX), ID III-h (resistance to GM, CPFX, and LVFX), and ID Null (susceptible to all antibiotics).
 Click to view | Table 1. Antimicrobial Resistance Pattern of Non-Extended-Spectrum β-Lactamase-Producing Escherichia coli and Extended-Spectrum β-Lactamase-Producing Escherichia coli |
The yearly rates of detection of these predominant patterns in non-ESBL-EC and ESBL-EC are shown in Figure 5. There was no change in the rate of isolates with pattern ID in non-ESBL-EC. In ESBL-EC, the rate of isolates with ID Null (susceptible to all antibiotics) decreased significantly from 10.0% in 2012 to 3.8% in 2017 (P < 0.01), and the rate of isolates with ID III-h (resistance to GM, CPFX, and LVFX) increased from 4.0% in 2012 to 20.5% in 2017 (P < 0.01).
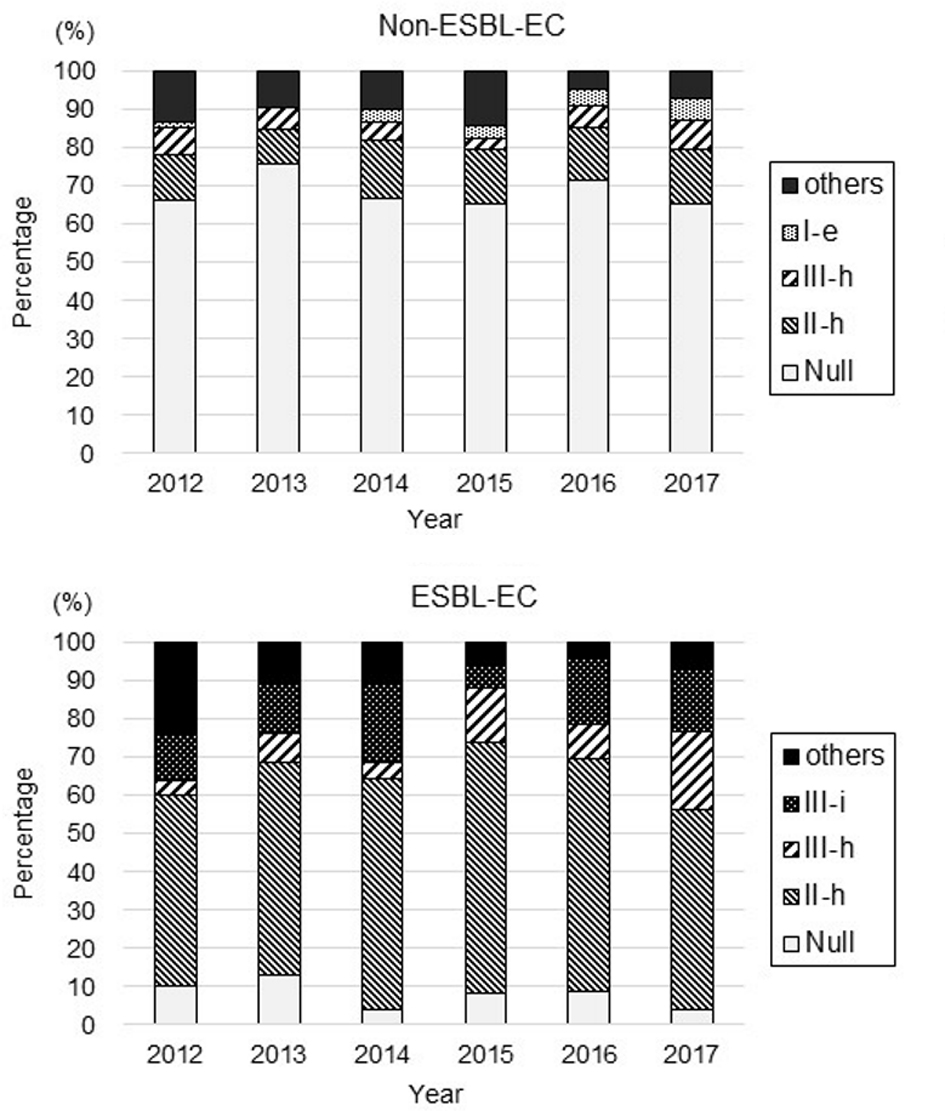 Click for large image | Figure 5. Changes in the antimicrobial resistance patterns of non-ESBL-EC and ESBL-EC. |
| Discussion | ▴Top |
In this study, we examined the trends of the rate of detection and antimicrobial resistance of ESBL-EC. The rate of detection of ESBL-EC has been 24.6% in the recent 6 years, which is in line with that reported by previous studies conducted in Japan [11-13]. However, at our hospital, the rate of detection of ESBL-EC exceeded 20% in 2012, and this value was higher than that during the same period at other facilities [3, 11, 14, 15]. A study reported that of 135 Japanese travelers, 55 (40.7%) carried ESBL after returning to Japan and that the majority of these carriers were returning from eastern and central Asia [16]. The Fukuoka Prefecture receives many immigrants and travelers from Southeast Asian countries with high carrier rates of ESBL-producing bacteria, which may contribute to the higher rate of detection of ESBL-EC at our hospital.
The rate of resistance of ESBL-EC to fluoroquinolones, such as CPFX and LVFX, was significantly higher than that of non-ESBL-EC, and this is consistent with the findings of a recent study conducted in Japan [13]. The resistance of ESBL-EC to fluoroquinolones is closely related to the genotype. There are three ESBL types: CTX-M, TEM, and SHV [1]. The CTX-M type is classified into five major groups: CTX-M-1, CTX-M-2, CTX-M-8, CTX-M-9, and CTX-M-25 [17]. CTX-M-9 has been the predominant group in Japan since 2000 [6, 18]. A previous report has suggested that 75% of the 24 strains in the CTX-M-14 and CTX-M-27 groups, which belong to the CTX-M-9 group, are resistant to LVFX [3]. In addition, a recently disseminated lineage of E. coli designated sequence type ST131 according to multilocus sequence typing (MLST) is associated with CTX-M-15, which belongs to the CTX-M-1 group, that is usually fluoroquinolone resistant [19], and this type of ESBL-EC has been reported in Japan [3, 6]. In the antimicrobial resistance pattern analysis of ESBL-EC conducted in this study, the ESBL-EC pattern ID III-h, which shows resistance to GM as well as fluoroquinolones, increased from 4.0% in 2012 to 20.5% in 2017. Ender et al reported the transmission of an ESBL-EC ST131 producing CTX-M-15 strain that is resistant to GM, trimethoprim-sulfamethoxazole, and fluoroquinolones between a father and his daughter [20]. It has been suggested that specific ESBL-EC clones, such as isolates with ST131, CTX-M-14, CTX-M-15, or CTX-M-27, are transmitted in our hospital or the area surrounding our hospital.
Particularly for urinary tract infections, fluoroquinolones are still recommended as the initial treatment. However, in the present study, approximately 90% of ESBL-EC and 25% of non-ESBL-EC were resistant to fluoroquinolones, and fluoroquinolones as the initial treatment should be used with caution. Particularly in cases wherein infection with ESBL-EC is suspected, initial treatment with non-fluoroquinolone drugs should be considered. In previous studies, the use of immunosuppressive drugs or corticosteroids, the use of quinolones prior to isolation, nursing home-associated infections, and antibiotic administration within the preceding 30 days were the independent predictors associated with ESBL-EC bacteremia [13, 21]. Carbapenems may be recommended as the initial treatment for infectious cases with these factors [22]. In the present study, the sensitivity of ESBL-EC and non-ESBL-EC to CMZ and FMOX was as high as that previously reported in Japan [13, 21]. According to a previous multicenter retrospective study conducted using a propensity score, CMZ and FMOX were not inferior to carbapenems in the empirical and definitive treatment of ESBL-EC bacteremia regarding the 30-day mortality rates and clinical success; hence, these cephems may be effective alternatives to carbapenems in the treatment of ESBL-EC bacteremia [23].
There are several limitations to this study. First, this study was conducted at a single center; hence, it is not clear if our results reflect the trend of ESBL-EC in Japan or in only the Fukuoka Prefecture. Second, we did not routinely conduct antimicrobial sensitivity tests for tazobactam/piperacillin (TAZ/PIPC) against E. coli. Because TAZ/PIPC, like carbapenems, is recommended for the empirical treatment of infections with suspected urosepsis, it is necessary to consider routine sensitivity testing at our hospital. Third, we have not stored the strain nor determined the ESBL type using polymerase chain reaction and the ESBL-EC strain type using MLST in the E. coli isolates. However, there were only few reports in Japan that compared the antimicrobial resistance profile between ESBL-EC and non-ESBL-EC, and there were no reports concerning the trends in the resistance patterns.
In conclusion, the rate of detection of ESBL-EC has been 24.6% in the recent 6 years. Almost all the E. coli isolates were susceptible to CMZ, FMOX, IPM, MEPM, and AMK; however, the rates of resistance to GM, MINO, CPFX, and LVFX were significantly higher in ESBL-EC than in non-ESBL-EC. In ESBL-EC, the rates of the isolates susceptible to all antibiotics decreased significantly during the study period, and the rates of the isolates resistant to GM, CPFX, and LVFX increased over the study period. ESBL-EC is an important resistant strain for infection control or infection treatment, and it is necessary to carefully monitor the trends in its resistance profile and genotype in the future.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
Not applicable.
Author Contributions
MM, YY and OI contributed to the concept and design of the study; MM, YY, KM, and YK conducted the study; MM, YY, MU, and NN were involved in data analysis and interpretation of the results; MM and YY drafted the manuscript; TT, SJ, and OI supervised the entire project and reviewed the manuscript; All the authors approved the final version of the manuscript.
| References | ▴Top |
- Paterson DL, Bonomo RA. Extended-spectrum beta-lactamases: a clinical update. Clin Microbiol Rev. 2005;18(4):657-686.
doi pubmed - Pitout JD, Laupland KB. Extended-spectrum beta-lactamase-producing Enterobacteriaceae: an emerging public-health concern. Lancet Infect Dis. 2008;8(3):159-166.
doi - Hara T, Sato T, Horiyama T, Kanazawa S, Yamaguchi T, Maki H. Prevalence and molecular characterization of CTX-M extended-spectrum beta-lactamase-producing Escherichia coli from 2000 to 2010 in Japan. Jpn J Antibiot. 2015;68(2):75-84.
- Nicolas-Chanoine MH, Bertrand X, Madec JY. Escherichia coli ST131, an intriguing clonal group. Clin Microbiol Rev. 2014;27(3):543-574.
doi pubmed - Kuroda H, Yano H, Hirakata Y, Arai K, Endo S, Kanamori H, Yamamoto H, et al. Molecular characteristics of extended-spectrum beta-lactamase-producing Escherichia coli in Japan: emergence of CTX-M-15-producing E. coli ST131. Diagn Microbiol Infect Dis. 2012;74(2):201-203.
doi pubmed - Yano H, Uemura M, Endo S, Kanamori H, Inomata S, Kakuta R, Ichimura S, et al. Molecular characteristics of extended-spectrum beta-lactamases in clinical isolates from Escherichia coli at a Japanese tertiary hospital. PLoS One. 2013;8(5):e64359.
doi pubmed - Haruki Y, Hagiya H, Haruki M, Sugiyama T. Clinical characteristics and outcome of critically ill patients with bacteremia caused by extended-spectrum beta-lactamase-producing and non-producing Escherichia coli. J Infect Chemother. 2018;24(11):944-947.
doi pubmed - Lee CC, Wang JL, Lee CH, Hung YP, Hong MY, Chang CM, Ko WC. Age-Related Trends in Adults with Community-Onset Bacteremia. Antimicrob Agents Chemother. 2017;61(12):e01050-17.
doi pubmed - Maslikowska JA, Walker SA, Elligsen M, Mittmann N, Palmay L, Daneman N, Simor A. Impact of infection with extended-spectrum beta-lactamase-producing Escherichia coli or Klebsiella species on outcome and hospitalization costs. J Hosp Infect. 2016;92(1):33-41.
doi pubmed - Clinical and Laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing: 27th informational supplement. CLSI M100-S27. 2017.
- Takesue Y, Kusachi S, Mikamo H, Sato J, Watanabe A, Kiyota H, Iwata S, et al. Antimicrobial susceptibility of pathogens isolated from surgical site infections in Japan: Comparison of data from nationwide surveillance studies conducted in 2010 and 2014-2015. J Infect Chemother. 2017;23(6):339-348.
doi pubmed - Takesue Y, Kusachi S, Mikamo H, Sato J, Watanabe A, Kiyota H, Iwata S, et al. Antimicrobial susceptibility of common pathogens isolated from postoperative intra-abdominal infections in Japan. J Infect Chemother. 2018;24(5):330-340.
doi pubmed - Komatsu Y, Kasahara K, Inoue T, Lee ST, Muratani T, Yano H, Kirita T, et al. Molecular epidemiology and clinical features of extended-spectrum beta-lactamase- or carbapenemase-producing Escherichia coli bacteremia in Japan. PLoS One. 2018;13(8):e0202276.
doi pubmed - Matsumura Y, Nagao M, Iguchi M, Yagi T, Komori T, Fujita N, Yamamoto M, et al. Molecular and clinical characterization of plasmid-mediated AmpC beta-lactamase-producing Escherichia coli bacteraemia: a comparison with extended-spectrum beta-lactamase-producing and non-resistant E. coli bacteraemia. Clin Microbiol Infect. 2013;19(2):161-168.
doi pubmed - Chong Y, Shimoda S, Yakushiji H, Ito Y, Miyamoto T, Kamimura T, Shimono N, et al. Community spread of extended-spectrum beta-lactamase-producing Escherichia coli, Klebsiella pneumoniae and Proteus mirabilis: a long-term study in Japan. J Med Microbiol. 2013;62(Pt 7):1038-1043.
doi pubmed - Mizuno Y, Miura Y, Yamaguchi T, Matsumoto T. Extended-spectrum beta-lactamase-producing Enterobacteriaceae colonisation in long-term overseas business travellers. Travel Med Infect Dis. 2016;14(6):561-567.
doi pubmed - Woerther PL, Burdet C, Chachaty E, Andremont A. Trends in human fecal carriage of extended-spectrum beta-lactamases in the community: toward the globalization of CTX-M. Clin Microbiol Rev. 2013;26(4):744-758.
doi pubmed - Suzuki S, Shibata N, Yamane K, Wachino J, Ito K, Arakawa Y. Change in the prevalence of extended-spectrum-beta-lactamase-producing Escherichia coli in Japan by clonal spread. J Antimicrob Chemother. 2009;63(1):72-79.
doi pubmed - Johnson JR, Johnston B, Clabots C, Kuskowski MA, Castanheira M. Escherichia coli sequence type ST131 as the major cause of serious multidrug-resistant E. coli infections in the United States. Clin Infect Dis. 2010;51(3):286-294.
doi pubmed - Ender PT, Gajanana D, Johnston B, Clabots C, Tamarkin FJ, Johnson JR. Transmission of an extended-spectrum-beta-lactamase-producing Escherichia coli (sequence type ST131) strain between a father and daughter resulting in septic shock and Emphysematous pyelonephritis. J Clin Microbiol. 2009;47(11):3780-3782.
doi pubmed - Namikawa H, Yamada K, Fujimoto H, Oinuma KI, Tochino Y, Takemoto Y, Kaneko Y, et al. Clinical characteristics of bacteremia caused by Extended-spectrum Beta-lactamase-producing Escherichia coli at a Tertiary Hospital. Intern Med. 2017;56(14):1807-1815.
doi pubmed - Tamma PD, Han JH, Rock C, Harris AD, Lautenbach E, Hsu AJ, Avdic E, et al. Carbapenem therapy is associated with improved survival compared with piperacillin-tazobactam for patients with extended-spectrum beta-lactamase bacteremia. Clin Infect Dis. 2015;60(9):1319-1325.
doi - Matsumura Y, Yamamoto M, Nagao M, Komori T, Fujita N, Hayashi A, Shimizu T, et al. Multicenter retrospective study of cefmetazole and flomoxef for treatment of extended-spectrum-beta-lactamase-producing Escherichia coli bacteremia. Antimicrob Agents Chemother. 2015;59(9):5107-5113.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Journal of Clinical Medicine Research is published by Elmer Press Inc.


